Abstract
Levamisole-sensitive acetylcholine receptors (L-AChRs) are ligand-gated ion channels that mediate excitatory neurotransmission at the neuromuscular junctions of nematodes. They constitute a major drug target for anthelminthic treatments because they can be activated by nematode-specific cholinergic agonists such as levamisole. Genetic screens conducted in Caenorhabditis elegans for resistance to levamisole toxicity identified genes that are indispensable for the biosynthesis of L-AChRs. These include 5 genes encoding distinct AChR subunits and 3 genes coding for ancillary proteins involved in assembly and trafficking of the receptors. Despite extensive analysis of L-AChRs in vivo, pharmacological and biophysical characterization of these receptors has been greatly hampered by the absence of a heterologous expression system. Using Xenopus laevis oocytes, we were able to reconstitute functional L-AChRs by coexpressing the 5 distinct receptor subunits and the 3 ancillary proteins. Strikingly, this system recapitulates the genetic requirements for receptor expression in vivo because omission of any of these 8 genes dramatically impairs L-AChR expression. We demonstrate that 3 α- and 2 non-α-subunits assemble into the same receptor. Pharmacological analysis reveals that the prototypical cholinergic agonist nicotine is unable to activate L-AChRs but rather acts as a potent allosteric inhibitor. These results emphasize the role of ancillary proteins for efficient expression of recombinant neurotransmitter receptors and open the way for in vitro screening of novel anthelminthic agents.
Keywords: anthelminthic drug, recombinant receptor expression
Soil-transmitted helminth infections are a public health problem of great importance. In the developing world, >1 billion people are infected by various intestinal nematode parasites (1). Among the different anthelmintic drugs, cholinergic agonists such as levamisole are widely used against intestinal nematodes (2). They activate ligand-gated acetylcholine receptors present on muscle cell membranes and cause spastic paralysis of the parasites. Levamisole can be used in mammals because it does not activate the AChRs of the infected host (3), yet the molecular basis of this specificity remains unknown.
Molecular identification of the levamisole target was achieved in the nonparasitic nematode Caenorhabditis elegans (4). Acetylcholine is the main excitatory neurotransmitter in C. elegans, and >29 genes encoding AChR subunits are predicted from its genome sequence (5). Despite this complexity, genetic screens were able to identify the genes coding for the subunits of levamisole-sensitive AChRs (L-AChRs). Levamisole causes body-wall muscle hypercontraction, paralysis, and ultimately death of C. elegans at high concentrations. By screening for mutant animals that survive exposure to levamisole, mutations in 5 genes encoding AChR subunits were found to confer partial or complete insensitivity to levamisole (4). These include 2 non-α-subunits (LEV-1 and UNC-29) and 3 α-subunits (LEV-8, UNC-38, UNC-63) as defined by the presence of a vicinal dicysteine in the primary sequence (6–8). Consistently, electrophysiological analysis demonstrated a drastic reduction of levamisole-elicited currents in the muscle cells of these mutants. In addition, these experiments identified a second subtype of muscle AChR activated by nicotine (N-AChR) but insensitive to levamisole (9). This receptor contains the subunit ACR-16, which is closely related to the vertebrate α7 gene. ACR-16 forms functional homomeric AChRs when expressed in Xenopus oocytes (10). L-AChRs and N-AChRs are partially redundant because disruption of either receptor causes no or weak locomotory defects, whereas disruption of both receptors causes almost complete paralysis of the animal (11, 12).
In addition to AChR subunits, genetic screens identified 3 ancillary proteins, RIC-3, UNC-50, and UNC-74, that are absolutely required for the expression of L-AChRs in vivo. RIC-3 is an endoplasmic reticulum transmembrane protein required for the expression of at least 4 distinct AChRs in C. elegans, including L-AChRs and N-AChRs in muscle (13, 14). RIC-3 is thought to act as a chaperone promoting AChR folding, assembly, or maturation (reviewed in ref. 15). unc-74 was identified in early screens for resistance to levamisole (4). It is predicted to encode a thioredoxin closely related to the human TMX3 protein and seems to be solely required for the expression of L-AChRs (D.C.W. and E. M. Jorgensen, unpublished data; and ref. 16). unc-50 encodes a transmembrane protein that localizes mostly to the Golgi apparatus and interacts with an ARF-GEF (guanine nucleotide exchange factor for ADP-ribosylation factor GTPases) (17). In the absence of UNC-50, L-AChRs but not N-AChRs are targeted to lysosomes after they exit the endoplasmic reticulum and are degraded. unc-50 is evolutionarily conserved in most eukaryotes, including yeast, plants, and mammals. However, its role for AChR expression has not been tested so far in nonnematode species.
Despite extensive study of the L-AChR in C. elegans, this receptor remains poorly characterized at the pharmacological level, and the molecular basis for the action of levamisole is still unknown. Such analysis was complicated by the inability to express recombinant L-AChRs in a controlled heterologous system. Here, we demonstrate that L-AChRs can be expressed in Xenopus oocytes by providing not only the 5 receptor subunits but also the 3 ancillary factors RIC-3, UNC-50, and UNC-74. This expression system was used to characterize the biophysical and pharmacological properties of the L-AChR.
Results
Eight Genes Are Required to Reconstitute L-AChRs in Xenopus Oocytes.
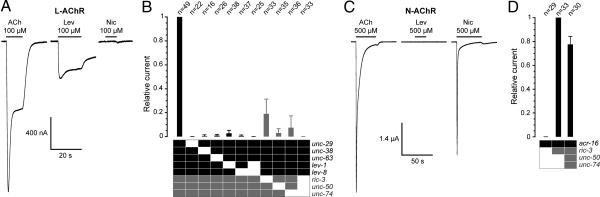
Because 8 genes are required in vivo for L-AChR expression, we reasoned that the same set of genes may be necessary for functional expression in a heterologous system. Xenopus oocytes are particularly well suited to express multimeric receptors because complex cRNA mixtures can be directly injected into the oocyte cytoplasm. We injected in vitro-transcribed cRNAs corresponding to the 5 L-AChR subunit genes lev-1, lev-8, unc-29, unc-38, and unc-63 and the 3 ancillary factors ric-3, unc-50, and unc-74. Robust expression of L-AChR was obtained 1 or 2 days after the injection. Perfusion of 100 μM acetylcholine elicited large inward currents in the 100 nA to several μA range, with fast activation and deactivation kinetics (limited by the speed of the solution exchange), as expected for a receptor containing a ligand-gated ion channel (Fig. 1A). The initial fast-desensitizing component of the response was likely caused by the activation of calcium-activated chloride channels endogenously expressed in Xenopus oocytes (see below and Fig. 2A). Levamisole elicited responses similar to ACh although of lower amplitude, whereas nicotine was surprisingly ineffective, a feature that we later characterized. Altogether, these data indicate that recombinant L-AChRs can be efficiently expressed in Xenopus oocytes.
Fig. 1.
Expression of functional AChRs from C. elegans in X. laevis oocytes. (A) A single oocyte coinjected with the 8 cRNAs unc-29, unc-38, unc-63, lev-1, lev-8, ric-3, unc-50, and unc-74 displays large inward currents elicited by ACh (100 μM) or levamisole (Lev, 100 μM) but not by nicotine (Nic, 100 μM). (B) Coexpression of 5 receptor subunits (black squares) and 3 ancillary factors (gray squares) is mandatory for robust expression of the L-AChR. Currents were measured at the peak. Average peak current for coinjection of all 8 cRNAs was 4.1 ± 3.7 μA (n = 49). (C) A single oocyte coinjected with the acr-16 and ric-3 cRNAs displays large transient inward currents elicited by ACh (500 μM) or nicotine (500 μM), but not by levamisole (500 μM). (D) Functional expression of the homopentameric ACR-16 nicotine-sensitive AChR requires the ancillary factor RIC-3 but not UNC-50 or UNC-74. Currents were measured at the peak. Average peak current for coinjection of acr-16 and ric-3 cRNAs was 6 ± 4.2 μA (n = 33). All recordings were made with 1 mM external CaCl2. Numbers above bars represent the number of oocytes recorded for each condition.
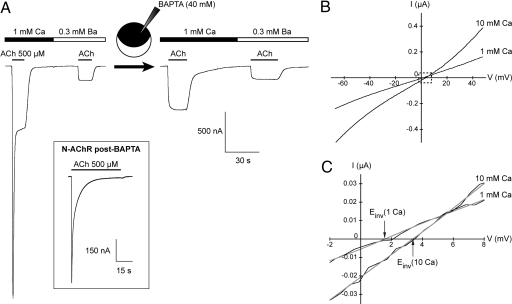
Fig. 2.
The L-AChR is permeable to calcium and shows no macroscopic desensitization. (A) All traces are from a single oocyte expressing L-AChRs. In 1 mM external CaCl2, the response to ACh displays a preeminent inward peak current. Chelating intracellular calcium by injection of BAPTA or replacing extracellular calcium by a low amount of barium (0.3 mM) eliminates this peak and results in stable responses upon continuous application of ACh. (Inset) N-AChRs display profound macroscopic desensitization upon continuous application of ACh (500 μM) even after BAPTA injection (n = 6). (B) Comparison of the I/V relationships of L-AChR responses elicited by 100 μM ACh in the presence of 1 mM or 10 mM extracellular CaCl2. Note that the L-AChR is potentiated by 10 mM extracellular calcium and that this potentiation occurs over the whole voltage range (n = 5, BAPTA-loaded oocytes). (C) Magnified view of the dash-boxed region in B showing the rightward shift of the reversal potential induced by switching from 1 mM to 10 mM external Ca2+ (1.6 mV to 3.4 mV for this cell).
To test the relative contribution of each gene for the functional expression of L-AChRs, we removed cRNAs from the injection mixture, either singularly or in combination. Similar to the in vivo situation, functional receptors were virtually eliminated when 1 or more of the 5 receptor subunits were omitted, causing >97% reduction of ACh-induced currents (Fig. 1B). Strikingly, coinjection of the 5 receptor subunits never yielded any measurable current when the 3 ancillary proteins RIC-3, UNC-50, and UNC-74 were absent. The requirement for individual ancillary proteins was then tested by injecting the 5 L-AChR subunits and only 2 of the 3 ancillary factors. Removal of UNC-50 or UNC-74 reduced response amplitudes to <10% of the response obtained with the full set of cRNAs, whereas removal of RIC-3 gave slightly larger and more variable responses (Fig. 1B). Hence, these data demonstrate the critical requirement of these ancillary proteins for the expression of functional L-AChRs in Xenopus oocytes.
Because the expression requirement of L-AChRs in Xenopus oocytes resembles the in vivo situation, we tested whether this would also be the case for the expression of N-AChRs. Injection of ACR-16 cDNA alone was reported to produce functional AChRs in Xenopus oocytes, yet yielding only small currents (usually <200 nA at −100 mV) (10, 18). Because ric-3 was demonstrated to be essential in vivo for N-AChR expression (13), we coinjected acr-16 and ric-3 cRNAs. After only 1 day of expression, we recorded large acetylcholine-induced currents (>1 μA at −60 mV) (Fig. 1C). These recombinant receptors had the expected properties of N-AChRs because they were activated by nicotine and insensitive to levamisole. Strikingly, we could never measure any significant current when the ric-3 cRNA was omitted (Fig. 1D). Because UNC-50 and UNC-74 enhance the expression of L-AChRs in Xenopus oocytes, they might also improve N-AChR expression. However, coinjection of unc-50 and unc-74 cRNAs together with ric-3 did not further enhance the N-AChR expression. This result is consistent with the in vivo situation because mutations in unc-50 and unc-74 do not affect N-AChR expression in C. elegans (17) (J.E.R. and E. M. Jorgensen, unpublished data). Altogether, these results demonstrate that robust expression of C. elegans AChRs can be achieved in Xenopus oocytes by providing C. elegans ancillary proteins in addition to the subunits of the AChR. Strikingly, the expression requirement in the Xenopus oocyte recapitulates the genetics of AChR expression in C. elegans.
L-AChRs Are Permeable to Calcium and Do Not Show Macroscopic Desensitization.
In vertebrates, AChRs form nonselective cation channels with differing permeability to calcium ions. Whereas muscle nAChRs are only weakly permeable to calcium, neuronal AChRs display significantly higher calcium permeabilities that vary depending on subunit composition (19–22). In C. elegans, calcium permeability of L-AChRs remains unexplored.
To test whether calcium can permeate recombinant L-AChRs, we replaced external Ca2+ (1 mM) by Ba2+ (0.3 mM) and observed a strong reduction in the current amplitude together with a complete disappearance of the initial transient peak response (Fig. 2A). Because Xenopus oocytes are known to express chloride channels that are activated by Ca2+ but not Ba2+ (23), our results suggest that in 1 mM Ca2+ a large fraction of the peak current evoked by ACh is carried by endogenous chloride channels that are activated secondary to the entry of Ca2+ flowing through L-AChR channels. This hypothesis was confirmed by demonstrating that intracellular injection of the calcium chelator BAPTA totally eliminates the peak response in 1 mM external Ca2+ (Fig. 2A). Next, the relative Ca2+ permeability of L-AChRs was quantified by measuring changes in the reversal potential of ACh-induced currents when increasing the external Ca2+ concentration from 1 mM to 10 mM. In these conditions, reversal potentials shifted from 1.7 ± 0.3 mV (n = 4) to 3.7 ± 0.5 mV (n = 4) (Fig. 2 B and C). According to the constant-field theory, this shift of the reversal potential corresponds to a permeability ratio PCa/PNa of ≈0.6 (see Materials and Methods), a value intermediate between that of embryonic vertebrate muscle receptors (0.1–0.3) and neuronal AChRs [≥1–2 (22, 24)].
Inspection of the current traces also revealed that L-AChR responses are enhanced by external calcium in a voltage-independent manner (Fig. 2B). This modulatory effect is reminiscent of the positive allosteric effect exerted by calcium on vertebrate neuronal AChRs (20, 21). An additional property of the L-AChR is its absence of apparent macroscopic desensitization. Indeed, steady responses are recorded upon ACh application in conditions where Ca2+-activated chloride currents are silenced by BAPTA injection (Fig. 2A). This is in striking contrast with the profound desensitization observed with N-AChRs even after BAPTA injection (Fig. 2A Inset).
Pharmacology of the L-AChR.
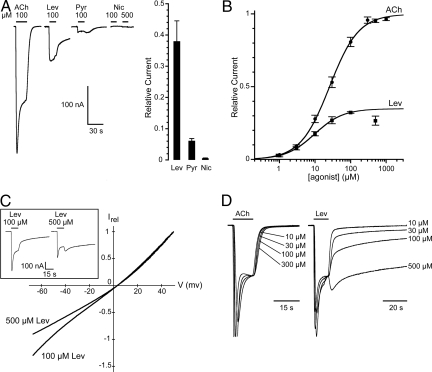
The pharmacological profile of recombinant L-AChRs was characterized by using cholinergic agonists and antagonists. Levamisole (100 μM) induced inward currents in all cells that were tested for their responsiveness to ACh (Fig. 3A). However, responses to levamisole were consistently smaller than the responses obtained in the same oocytes with 100 μM ACh, suggesting that levamisole may not be as efficient as ACh in activating L-AChRs. Pyrantel, another anthelminthic known to act as a cholinergic agonist, was also able to activate L-AChRs. However, the currents obtained with 100 μM pyrantel were much smaller than those induced by the same concentrations of levamisole or ACh (Fig. 3A). Surprisingly, nicotine, the prototypical agonist of ionotropic AChRs, had almost no agonistic effect on the L-AChR when applied at 100 or 500 μM (Figs. 1A and 3A). Nicotine-elicited currents were only detectable in oocytes expressing very high levels of L-AChRs. When ACh-evoked peak currents reached 7–15 μA, nicotine-induced responses were on average <1% of the ACh-induced responses (0.55 ± 0.21%, n = 4).
Fig. 3.
Agonist pharmacology of the L-AChR. (A) (Left) Acetylcholine, levamisole, and pyrantel, but not nicotine, activate the L-AChR. Concentrations of agonist are indicated above each application. Traces are from a single oocyte. (Right) Current relative to 100 μM ACh (plateau values): 100 μM levamisole 38 ± 6% (n = 6), 100 μM pyrantel 6.1 ± 0.7% (n = 6), 500 μM nicotine 0.55 ± 0.21% (n = 4). (B) Dose–response curves for ACh and levamisole. The value at 500 μM levamisole has been excluded from the fit because of voltage-dependent block at this concentration. (C) High concentrations of levamisole cause voltage-dependent channel block of L-AChR. BAPTA-injected oocytes were subjected to voltage ramps in the presence of 100 μM or 500 μM levamisole (n = 5). (Inset) Representative traces of L-AChR responses evoked by 100 μM and 500 μM levamisole. The rebound of the current after washout of 500 μM levamisole probably occurs because unbinding of levamisole from its pore-blocking site is faster than unbinding of levamisole from its agonist site. (D) Concentration-dependent washout kinetics of levamisole-evoked responses. ACh or levamisole traces obtained from a single oocyte at different agonist concentrations were first normalized to the current level measured just before agonist washout and then superimposed. Note that although ACh-evoked responses display classical concentration-independent washout kinetics, the washout time course of levamisole-evoked responses increases with increasing levamisole concentrations.
To compare the relative sensitivities of L-AChRs to acetylcholine and levamisole, full dose-response experiments were performed (Fig. 3B). Acetylcholine EC50 was found to be 26.0 ± 3.2 μM (n = 13) and the Hill coefficient 1.05 ± 0.06. This EC50 value was very similar to that of N-AChRs [31 ± 0.5 μM (n = 6); supporting information (SI) Fig. S1], yet the Hill coefficient was estimated to be significantly higher for N-AChRs (2.4; Fig. S1). The levamisole EC50 was 10.1 ± 1.8 μM (n = 6), indicating that levamisole was slightly more potent than ACh in activating L-AChRs. However, its efficacy was markedly lower than that of ACh. At a saturating levamisole concentration of 100 μM, levamisole-induced current amplitudes were on average only 35 ± 2% (n = 6) of those recorded at saturating ACh (Fig. 3B). At a very high levamisole concentration (500 μM), an additional antagonistic effect of levamisole could be unmasked (Fig. 3B). This inhibition likely resulted from open-channel block by the positively charged levamisole molecule (10, 25, 26). To test this hypothesis, we performed voltage ramps and demonstrated that 500 μM levamisole produced a slight voltage-dependent block of L-AChR responses (Fig. 3C). However, no channel block was seen at 100 μM levamisole, indicating that channel block alone cannot account for the partial efficacy of levamisole on L-AChRs. Another striking difference between ACh and levamisole responses was their washout kinetics (Fig. 3D). Although ACh-evoked responses displayed classical concentration-independent washout kinetics, the washout time course of levamisole-evoked responses became increasingly slower with increasing levamisole concentrations. Noticeably, at 500 μM levamisole, current washout was extremely slow, requiring minutes for complete recovery. These long-lasting effects of levamisole are suggestive of complex mode(s) of interaction of the levamisole molecule on L-AChRs (see Discussion).
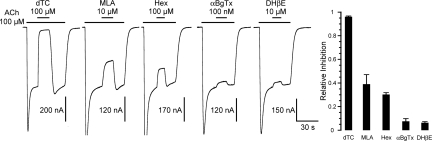
To determine the antagonist spectrum of L-AChR, we tested 5 compounds that inhibit ACh responses of different classes of AChRs. d-Tubocurarine (dTC), methyllycaconitine (MLA), and hexamethonium (Hex) each inhibited ACh-triggered responses (Fig. 4). In contrast, α-bungarotoxin (α-BgTx) and dihydro-β-erythroidine (DHβE) had very little antagonistic effects, similar to endogenous L-AChRs in vivo (9). Even long applications of α-BgTx (100 nM, 30-min incubation), which may be required to reach binding equilibrium, were ineffective at inhibiting L-AChR responses [7.6 ± 1% (n = 7)].
Fig. 4.
Antagonist pharmacology of the L-AChR. L-AChRs activated by 100 μM ACh are inhibited by 100 μM dTC (96 ± 1%; n = 7), 10 μM MLA (39 ± 8%; n = 5), and 100 μM Hex (30 ± 2%; n = 5). In contrast, 100 nM α-BgTx and 10 μM DHβE produce only very weak inhibition (7 ± 1%, n = 5; and 6 ± 1%, n = 5; respectively).
These results indicate that the L-AChR has a unique pharmacological profile and that levamisole has very distinct agonist properties compared with the natural agonist acetylcholine.
Nicotine Is an Allosteric Inhibitor of L-AChRs.
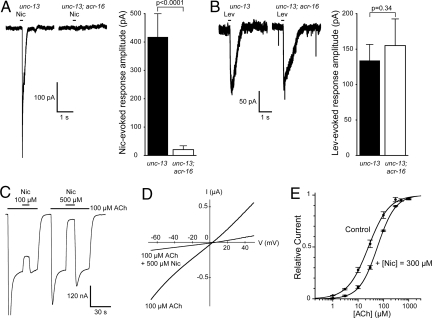
In the experiments presented above, we observed that nicotine was unable to activate recombinant L-AChRs (Figs. 1A and 3A). To test whether this was an intrinsic characteristic of L-AChRs and not a peculiar feature of our recombinant receptors, we analyzed the nicotine response of L-AChRs on C. elegans muscle cells. We used a double-mutant strain containing an acr-16-null mutation, which removes nicotine-sensitive N-AChRs (12), and a mutation in the unc-13 gene, which blocks presynaptic vesicle release (27). We expect that in such a double-mutant strain, any current recorded upon nicotine application can only be attributed to postsynaptic L-AChR activation and not to activation of presynaptic AChRs. When muscle cells of double-mutant animals were recorded, no measurable response was detected after nicotine application (Fig. 5A). In contrast, the response to levamisole application was indistinguishable from the levamisole response in control animals (Fig. 5B; see also refs. 11 and 26). These results demonstrate that native and recombinant L-AChRs share the inability to be activated by nicotine.
Fig. 5.
Nicotine acts mainly as an allosteric inhibitor of the L-AChR. (A) Nicotine fails to activate L-AChR significantly in vivo. In whole-cell recordings from C. elegans body-wall muscles, a strain lacking both acr-16 and unc-13 shows no response to nicotine, unlike unc-13 mutants [mean amplitudes of the response to 500 μM nicotine were 21 ± 11 pA (n = 4) and 420 ± 80 pA (n = 4), respectively]. (B) In contrast, responses to levamisole are indistinguishable between these 2 strains [mean amplitudes of the response to 500 μM levamisole were 155 ± 37 pA (n = 4) and 134 ± 22 pA (n = 4), respectively]. (C) Responses elicited by ACh are inhibited by nicotine in a dose-dependent manner. Percentage inhibition of responses elicited by 100 μM ACh were 26 ± 2% (n = 6) for 100 μM nicotine and 93 ± 3% (n = 6) for 500 μM nicotine. (D) Nicotine inhibition is not voltage-dependent. Voltage ramps (−70 to +50 mV) in 100 μM ACh with or without 500 μM nicotine. Note that nicotine inhibition occurs over the whole voltage range (n = 10, BAPTA-injected oocytes). (E) Nicotine has a modest effect on ACh sensitivity. ACh dose–response curves were performed with or without 300 μM nicotine. In the absence of nicotine, EC50 and nH for ACh are 26 ± 3 μM and 1.05 ± 0.06 (n = 6–13), respectively. In the presence of nicotine, EC50 and nH for ACh are 58 ± 3 μM and 1.23 ± 0.06 (n = 5), respectively.
We next tested the possibility that nicotine might act as an L-AChR antagonist. Strikingly, 500 μM nicotine had a potent inhibitory effect on currents evoked by ACh applications (Fig. 5C). This inhibition appears to be almost entirely voltage-independent (Fig. 5D), suggesting that it is not the result of direct channel block. Alternatively, nicotine might act as a competitive antagonist of ACh. However, performing acetylcholine dose–response experiments in the presence of 300 μM nicotine revealed that nicotine has little effect on the apparent affinity for ACh (<2-fold increase in ACh EC50 by 300 μM nicotine; Fig. 5E). Taken together, these results indicate that nicotine inhibits L-AChRs mainly through a negative allosteric mechanism.
Discussion
We demonstrate here that the C. elegans heteromeric levamisole-sensitive AChR can be readily expressed in Xenopus oocytes by providing 3 ancillary C. elegans proteins in addition to 5 distinct AChR subunits. Our experiments led to the surprising conclusion that 5 different subunits assemble into the same receptor complex because removal of any of these 5 subunits reduces expression by >97%. In contrast to earlier attempts that only used a subset of the subunits and none of the ancillary proteins (7), L-AChR expression was efficient and robust in our system. ACh-elicited currents, ranging from hundreds of nA to several μA, could be measured in almost all injected oocytes.
C. elegans RIC-3, UNC-50, and UNC-74 are critical for the expression of L-AChRs in Xenopus oocytes. Although these 3 proteins are evolutionarily conserved, our results suggest they may be absent or expressed at insufficient levels in oocytes. Alternatively, species-specific determinants contained in L-AChRs may require the presence of nematode ancillary proteins for efficient expression. The orthologs of RIC-3 have been characterized in insects and vertebrates. They are proposed to act as chaperones that directly interact with various AChR or 5-HT3A subunits and promote maturation and assembly of the receptors (28). The human hRIC-3 is able to enhance the expression of the C. elegans DES-2/DEG-3 AChR, demonstrating interspecies functionality (14). Hence, endogenous expression of RIC-3 in Xenopus oocytes might explain why low but significant levels of L-AChRs could be expressed when the 2 other factors UNC-50 and UNC-74 were overexpressed. UNC-50 was also conserved during evolution, and an UNC-50 ortholog can be readily identified in Xenopus laevis (17). Yeast, nematode, and human UNC-50 proteins are all able to interact with the yeast ARF-GEFs Gea1p, yet the human unc-50 ortholog is unable to rescue an unc-50 mutant in C. elegans (S. Eimer and J.-L.B., unpublished data). Because C. elegans UNC-50 promotes L-AChR expression in oocytes, it might be able to interact with the vertebrate trafficking machinery but provide a nematode-specific interface required for proper L-AChR trafficking to the plasma membrane. The requirement of such ancillary factors might be worth considering when recombinant receptor expression fails. For example, there is no system reported so far to express native insect AChRs [except very inefficient expression of the locust αL1 (29)]. The fact that certain Drosophila AChR α-subunits can only be expressed in Xenopus when they are coinjected with vertebrate β-subunits (30, 31) may indicate that vertebrate β-subunits are needed to recruit vertebrate ancillary factors and promote receptor expression in the absence of Drosophila-specific ancillary proteins.
In C. elegans, both levamisole- and nicotine-sensitive AChRs are found at neuromuscular junctions [(11, 12, 32); M. Gendrel and J.-L.B., unpublished data]. Based on the EC50 estimated from our dose–response experiments, L-AChRs and N-AChRs seem equally sensitive to their endogenous ligand ACh (26 μM and 31 μM, respectively). However, their biophysical and pharmacological differences are dramatically different. First, L-AChRs remain fully active during our longest agonist application (40 s), whereas ACR-16 homopentamers desensitize rapidly and profoundly (>97% within 30 s). Second, even though L-AChRs contain 3 α-subunits that could contribute 3 ACh binding sites per receptor molecule, there is no (or little) apparent ACh binding cooperativity as suggested by the Hill coefficient close to 1. This contrasts with the Hill coefficient of 2.4 for ACR-16 homomers. Third, the calcium permeability of L-AChRs is modest (PCa/PNa = 0.6), a value slightly higher than that found for embryonic muscle AChRs at vertebrate neuromuscular junctions. The calcium permeability of N-AChRs has not been measured. However, ACR-16 resembles the neuronal α7-like homomer-forming subunits of vertebrates that are much more permeable to calcium than heteromeric channels (19, 22, 24). Fourth, L-AChRs are activated by levamisole, inhibited by nicotine, and insensitive to DHβE, whereas N-AChRs are activated by nicotine, partially inhibited by levamisole, and blocked by DHβE. Finally, in vivo, both receptor subtypes colocalize at neuromuscular junctions, yet distinct molecular machineries are involved in the synaptic clustering of L- and N-AChRs (11, 32). It is tempting to hypothesize that these receptors might contribute different neuromuscular signaling depending on locomotory regimes, but this still needs to be experimentally demonstrated.
Expression of L-AChRs provides a means to analyze the action of levamisole in greater detail. This drug has been described as a potent agonist of nematode AChRs. However, our dose–response experiments demonstrate that levamisole is only a partial agonist, ≈3-fold less efficient than ACh. At 500 μM, levamisole behaves as an open-channel blocker, as recently suggested by single-channel recording of L-AChRs from C. elegans muscle membranes (ref. 26; see Fig. 3C). However, the effectiveness of levamisole on parasitic nematodes might be explained by at least 2 reasons. First, L-AChRs do not desensitize and should stay open as long as the drug is present in the extracellular environment. Second, at high levamisole concentrations, washout kinetics are very slow, complete recovery taking minutes compared with a few seconds for ACh-evoked responses. Because levamisole is membrane-permeable, it is possible that these very slow deactivation kinetics do not result from an inherently tight ligand–receptor interaction but rather from slow levamisole departure from a nonaqueous reservoir. Such prolonged effects are reminiscent of the activation of GABAA receptors by neurosteroids. These compounds are thought to partition and accumulate into the plasma membrane from where they access a binding site on the receptor. The rate-limiting factor for deactivation may then be reservoir emptying rather than the intrinsic kinetics of ligand dissociation (33). We propose that similar mechanisms may underlie some of the distinctive effects of levamisole on L-AChRs. Interestingly, the very slow-washout kinetics that we observed are consistent with earlier reports that demonstrated that levamisole caused a much more prolonged contraction of isolated Ascaris muscle than ACh (34). This property likely contributes to the anthelminthic efficacy of the drug.
Analysis of recombinant L-AChRs also revealed that nicotine, the prototypical agonist of ionotropic acetylcholine receptors, is an antagonist of L-AChRs. Only vertebrate α9* AChR have been reported to be nicotine-insensitive (35). On these receptors, nicotine actually behaves as a potent competitive antagonist. The situation is different with L-AChRs because high concentrations of nicotine do not dramatically affect ACh EC50. We also demonstrated that this inhibition is not caused by open-channel block. Hence, we concluded that nicotine is mainly a noncompetitive L-AChR antagonist, possibly acting through allosteric inhibition. Such a mechanism has been proposed for picrotoxin at GABAA receptors (36), and for some AChR noncompetitive antagonists such as the steroid promegestone that efficiently inhibits Torpedo AChRs most probably by increasing the fraction of desensitized receptors (37).
In conclusion, the expression system that we have characterized here will provide a means to identify the molecular determinants of the unique L-AChR pharmacology. Understanding the molecular basis for the agonist selectivity of levamisole on nematode but not vertebrate AChRs might open the way for the rational design of novel anthelminthic drugs.
Materials and Methods
For more detailed information, see SI Materials and Methods.
General Procedures and Strains.
C. elegans strains were grown on nematode growth medium by using standard conditions according to (38). The following strains were used for electrophysiological recordings: BC168 unc-13(s69)I and EN1645 unc-13(s69)I;acr-16(ok789)V.
Electrophysiological Studies in X. laevis Oocytes.
X. laevis oocytes were prepared, injected, voltage-clamped, and superfused as described in ref. 39 except that gentamycin was omitted from the conservation medium because prolonged treatments with this antibiotic can inhibit AChRs expressed in Xenopus oocytes (40).
Electrophysiological Studies in C. elegans.
Electrophysiological recordings on C. elegans muscle cells were performed according to ref. 12.
Supplementary Material
Acknowledgments.
We thank Stuart Edelstein for critical reading of the manuscript. We thank Erik Jorgensen for providing critical reagents (University of Utah, Salt Lake City). This work was supported by a European Molecular Biology Organization Long-Term Fellowship and Institut National de la Santé et de la Recherche Médicale (INSERM) Junior Contract (to T.B.), a Ministère de la Recherche fellowship (to M.G.), a genetics training grant (to D.C.W.), Association Française Contre les Myopathies and ANR-07-NEURO-032-01 grants (to J.-L.B.), and INSERM and Agence Nationale de la Recherche funding (for P.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806933105/DCSupplemental.
References
- 1.Bethony J, et al. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: Systematic review and meta-analysis. J Am Med Assoc. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 3.Rayes D, De Rosa MJ, Bartos M, Bouzat C. Molecular basis of the differential sensitivity of nematode and mammalian muscle to the anthelmintic agent levamisole. J Biol Chem. 2004;279:36372–36381. doi: 10.1074/jbc.M403096200. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JA, Wu CH, Berg H, Levine JH. The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics. 1980;95:905–928. doi: 10.1093/genetics/95.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones AK, Sattelle DB. Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. Bioessays. 2004;26:39–49. doi: 10.1002/bies.10377. [DOI] [PubMed] [Google Scholar]
- 6.Culetto E, et al. The Caenorhabditis elegans unc-63 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor α-subunit. J Biol Chem. 2004;279:42476–42483. doi: 10.1074/jbc.M404370200. [DOI] [PubMed] [Google Scholar]
- 7.Fleming JT, et al. Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J Neurosci. 1997;17:5843–5857. doi: 10.1523/JNEUROSCI.17-15-05843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Towers PR, Edwards B, Richmond JE, Sattelle DB. The Caenorhabditis elegans lev-8 gene encodes a novel type of nicotinic acetylcholine receptor α-subunit. J Neurochem. 2005;93:1–9. doi: 10.1111/j.1471-4159.2004.02951.x. [DOI] [PubMed] [Google Scholar]
- 9.Richmond JE, Jorgensen EM. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballivet M, Alliod C, Bertrand S, Bertrand D. Nicotinic acetylcholine receptors in the nematode Caenorhabditis elegans. J Mol Biol. 1996;258:261–269. doi: 10.1006/jmbi.1996.0248. [DOI] [PubMed] [Google Scholar]
- 11.Francis MM, et al. The ROR receptor tyrosine kinase CAM-1 is required for ACR-16-mediated synaptic transmission at the C. elegans neuromuscular junction. Neuron. 2005;46:581–594. doi: 10.1016/j.neuron.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Touroutine D, et al. acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J Biol Chem. 2005;280:27013–27021. doi: 10.1074/jbc.M502818200. [DOI] [PubMed] [Google Scholar]
- 13.Halevi S, et al. The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 2002;21:1012–1020. doi: 10.1093/emboj/21.5.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halevi S, et al. Conservation within the RIC-3 gene family: Effectors of mammalian nicotinic acetylcholine receptor expression. J Biol Chem. 2003;278:34411–34417. doi: 10.1074/jbc.M300170200. [DOI] [PubMed] [Google Scholar]
- 15.Millar NS. RIC-3: A nicotinic acetylcholine receptor chaperone. Br J Pharmacol. 2008;153(Suppl 1):S177–S183. doi: 10.1038/sj.bjp.0707661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haugstetter J, Blicher T, Ellgaard L. Identification and characterization of a novel thioredoxin-related transmembrane protein of the endoplasmic reticulum. J Biol Chem. 2005;280:8371–8380. doi: 10.1074/jbc.M413924200. [DOI] [PubMed] [Google Scholar]
- 17.Eimer S, et al. Regulation of nicotinic receptor trafficking by the transmembrane Golgi protein UNC-50. EMBO J. 2007;26:4313–4323. doi: 10.1038/sj.emboj.7601858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raymond V, Mongan NP, Sattelle DB. Anthelmintic actions on homomer-forming nicotinic acetylcholine receptor subunits: Chicken α7 and ACR-16 from the nematode Caenorhabditis elegans. Neuroscience. 2000;101:785–791. doi: 10.1016/s0306-4522(00)00279-7. [DOI] [PubMed] [Google Scholar]
- 19.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain α7: A nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulle C, Lena C, Changeux JP. Potentiation of nicotinic receptor response by external calcium in rat central neurons. Neuron. 1992;8:937–945. doi: 10.1016/0896-6273(92)90208-u. [DOI] [PubMed] [Google Scholar]
- 21.Vernino S, Amador M, Luetje CW, Patrick J, Dani JA. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992;8:127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 22.Bertrand D, Galzi JL, Devillers-Thiery A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal α7 nicotinic receptor. Proc Natl Acad Sci USA. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miledi R, Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Robertson SJ, Martin RJ. Levamisole-activated single-channel currents from muscle of the nematode parasite Ascaris suum. Br J Pharmacol. 1993;108:170–178. doi: 10.1111/j.1476-5381.1993.tb13458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian H, Robertson AP, Powell-Coffman JA, Martin RJ. Levamisole resistance resolved at the single-channel level in Caenorhabditis elegans. FASEB J. 2008 doi: 10.1096/fj.08-110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richmond JE, Davis WS, Jorgensen EM. UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat Neurosci. 1999;2:959–964. doi: 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng A, Bollan KA, Greenwood SM, Irving AJ, Connolly CN. Differential subcellular localization of RIC-3 isoforms and their role in determining 5-HT3 receptor composition. J Biol Chem. 2007;282:26158–26166. doi: 10.1074/jbc.M703899200. [DOI] [PubMed] [Google Scholar]
- 29.Amar M, Thomas P, Wonnacott S, Lunt GG. A nicotinic acetylcholine receptor subunit from insect brain forms a nondesensitising homo-oligomeric nicotinic acetylcholine receptor when expressed in Xenopus oocytes. Neurosci Lett. 1995;199:107–110. doi: 10.1016/0304-3940(95)12033-z. [DOI] [PubMed] [Google Scholar]
- 30.Bertrand D, et al. Physiological properties of neuronal nicotinic receptors reconstituted from the vertebrate β2-subunit and Drosophila α-subunits. Eur J Neurosci. 1994;6:869–875. doi: 10.1111/j.1460-9568.1994.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 31.Ihara M, et al. Diverse actions of neonicotinoids on chicken α7, α4β2, and Drosophila–chicken SADβ2 and ALSβ2 hybrid nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Neuropharmacology. 2003;45:133–144. doi: 10.1016/s0028-3908(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 32.Gally C, Eimer S, Richmond JE, Bessereau JL. A transmembrane protein required for acetylcholine receptor clustering in Caenorhabditis elegans. Nature. 2004;431:578–582. doi: 10.1038/nature02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu HJ, et al. Slow actions of neuroactive steroids at GABAA receptors. J Neurosci. 2004;24:6667–6675. doi: 10.1523/JNEUROSCI.1399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aceves J, Erlij D, Martinez-Maranon R. The mechanism of the paralysing action of tetramisole on Ascaris somatic muscle. Br J Pharmacol. 1970;38:602–607. doi: 10.1111/j.1476-5381.1970.tb10601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verbitsky M, Rothlin CV, Katz E, Elgoyhen AB. Mixed nicotinic–muscarinic properties of the α9 nicotinic cholinergic receptor. Neuropharmacology. 2000;39:2515–2524. doi: 10.1016/s0028-3908(00)00124-6. [DOI] [PubMed] [Google Scholar]
- 36.Newland CF, Cull-Candy SG. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat. J Physiol. 1992;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- 38.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paoletti P, Neyton J, Ascher P. Glycine-independent and subunit-specific potentiation of NMDA responses by extracellular Mg2+ Neuron. 1995;15:1109–1120. doi: 10.1016/0896-6273(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 40.Amici M, Eusebi F, Miledi R. Effects of the antibiotic gentamicin on nicotinic acetylcholine receptors. Neuropharmacology. 2005;49:627–637. doi: 10.1016/j.neuropharm.2005.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.