Abstract
Plant natriuretic peptides (PNPs) are a class of extracellular, systemically mobile molecules that elicit a number of plant responses important in homeostasis and growth. The bacterial citrus pathogen, Xanthomonas axonopodis pv. citri, also contains a gene encoding a PNP-like protein, XacPNP, that shares significant sequence similarity and identical domain organization with plant PNPs but has no homologues in other bacteria. We have expressed and purified XacPNP and demonstrated that the bacterial protein alters physiological responses including stomatal opening in plants. Although XacPNP is not expressed under standard nutrient rich culture conditions, it is strongly induced under conditions that mimic the nutrient poor intercellular apoplastic environment of leaves, as well as in infected tissue, suggesting that XacPNP transcription can respond to the host environment. To characterize the role of XacPNP during bacterial infection, we constructed a XacPNP deletion mutant. The lesions caused by this mutant were more necrotic than those observed with the wild-type, and bacterial cell death occurred earlier in the mutant. Moreover, when we expressed XacPNP in Xanthomonas axonopodis pv. vesicatoria, the transgenic bacteria caused less necrotic lesions in the host than the wild-type. In conclusion, we present evidence that a plant-like bacterial PNP can enable a plant pathogen to modify host responses to create conditions favorable to its own survival.
Keywords: bacterial plant pathogenesis, plant natriuretic peptides
Natriuretic Peptide (NP) systems have been identified in many vertebrates and are commonly associated with organs involved in cardiac and osmoregulatory homeostasis (1). In higher plants, NPs (PNPs) that are heterologues of animal NPs elicit a number of responses that are essential in homeostasis and growth (2). These include cGMP dependent stomatal guard cell movements and thus, plant gas exchange (3), regulation of net water uptake (4), and tissue specific ion movements (5). By searching public databases, we found that Xanthomonas axonopodis pv. citri, the bacteria that causes citrus canker (6), has a PNP-like protein (XacPNP) that shares significant sequence similarity, identical domain organization, and conserved residues, within the active domain, with an Arabidopsis thaliana PNP (AtPNP-A) (7) (Fig. 1A). Because no significant similarity between the X. axonopodis pv. citri protein and other bacterial proteins from GenBank was found, we proposed that the XacPNP may have been acquired by bacteria in an ancient lateral gene transfer event (7).
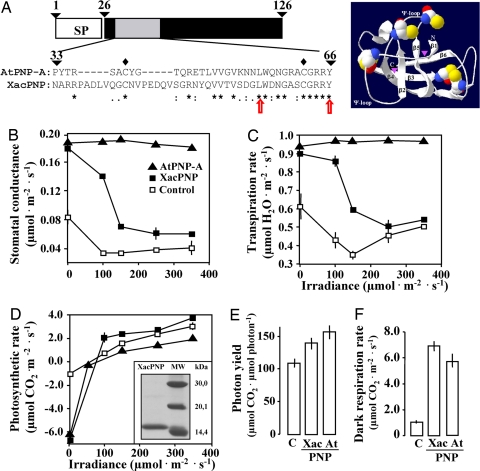
Fig. 1.
Structure and physiological testing of XacPNP. (A) The N-terminal contains the signal peptide (SP) that directs the protein into the extracellular space. In AtPNP-A, amino acids 33 to 66 convey the homeostasis regulating biological activity (in gray) and are aligned with X. axonopodis pv. citri PNP (XacPNP). Red arrows delineate the smallest tested recombinant PNP fragment that has significant biological activity. Asterisks (*) signify identical amino acids; colons (:) are conservative replacements; full stops (.) are semiconservative replacements; and lozenges ( ) are cysteine residues in AtPNP-A that can form disulphide bridges. In the right, a fold model of XacPNP with six stranded double-ψ β barrel structure is shown. Conserved cysteine residues are represented with balls. (B) Stomatal conductance, (C) transpiration rates, and (D) photosynthetic rates of the youngest, fully expanded leaves of Plectranthus ecklonii Benth. over increasing irradiances, 0–350 μmol/m2s in the presence or absence of 2 μg of recombinant XacPNP or AtPNP-A. (E) In the inset, SDS/PAGE of the purified recombinant XacPNP stained with Coomassie blue. (E) Apparent photon yields and (F) dark respiration rates, in the presence or absence of XacPNP and AtPNP-A. The values represent the means of at least three experiments, with standard error bars.
) are cysteine residues in AtPNP-A that can form disulphide bridges. In the right, a fold model of XacPNP with six stranded double-ψ β barrel structure is shown. Conserved cysteine residues are represented with balls. (B) Stomatal conductance, (C) transpiration rates, and (D) photosynthetic rates of the youngest, fully expanded leaves of Plectranthus ecklonii Benth. over increasing irradiances, 0–350 μmol/m2s in the presence or absence of 2 μg of recombinant XacPNP or AtPNP-A. (E) In the inset, SDS/PAGE of the purified recombinant XacPNP stained with Coomassie blue. (E) Apparent photon yields and (F) dark respiration rates, in the presence or absence of XacPNP and AtPNP-A. The values represent the means of at least three experiments, with standard error bars.
Here, we report that recombinant XacPNP is biologically active and can alter physiological responses in plants. We also investigate its role during host pathogen interactions and examine whether the pathogen may manipulate host responses to create conditions favorable to its own survival.
Results
Effects of Recombinant XacPNP In Planta.
To test whether the bacterial protein can induce PNP-like responses in plants, we cloned, expressed, and purified a 115-aa XacPNP without the predicted signal peptide (Fig. 1 A and D). The purified protein was tested for effects on plant photosynthetic responses in an indigenous Cape sage, a species that has previously been used to test responses to native and recombinant PNPs from different species (8). Treatment with either XacPNP or AtPNP-A resulted in rapid and significant increase in stomatal conductance (Fig. 1B) and, hence, stomatal opening occurred. In the case of the bacterial PNP, the effect was light intensity dependent and less pronounced at higher irradiances. The increase in stomatal conductance caused by XacPNP and AtPNP-A concur with higher leaf transpiration rates (Fig. 1C). These increases also correspond to XacPNP- and AtPNP-A dependent increases in leaf photosynthetic rates (Fig. 1D). In addition, the efficiency of light utilization during photosynthetic CO2 fixation was enhanced, as evident in the higher apparent photon yield (Fig. 1E), while leaf dark respiration rates were 4-fold higher after XacPNP, as well as AtPNP-A, application (Fig. 1F).
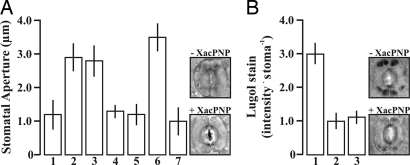
Moreover, to elucidate whether XacPNP elicits similar responses as AtPNP-A, we analyzed both peptides for their ability to promote stomatal opening (Fig. 2) and net water uptake (supporting information (SI) Fig. S1). Stomatal aperture evaluated in orange leaves that were incubated in the presence of XacPNP or AtPNP-A showed mean stomatal apertures of 3 μm, whereas apertures of 1.2 μm were observed in the control (Fig. 2A). These XacPNP- or AtPNP-A-induced aperture changes were prevented by the guanylate cyclase inhibitor, methylene blue, suggesting cGMP dependent signaling. As a positive control for opening the auxin analogue, naphthalene acetic acid (NAA) was used, and stomata closed with abscisic acid (ABA) (Fig. 2A). Differences were statistically significant among XacPNP, AtPNP-A, and NAA in comparison with buffer, methylene blue, and ABA treatments (P < 0.01). To determine whether XacPNP induces changes in stomatal aperture because of solute accumulation, we measured starch degradation in guard cells of detached abaxial leaf epidermis. Marked XacPNP and AtPNP-A dependent starch reduction occurred in guard cell chloroplasts (Fig. 2B), was statistically different from the control incubation with buffer (P < 0.01), and coincided with stomatal opening. In protoplasts, net water uptake causes swelling in response to the cGMP analogue, 8-Br-cGMP, and it is also promoted by XacPNP and AtPNP-A. Swelling responses to AtPNP-A and XacPNP are shown to be cGMP-dependent because they are inhibited by guanylate cyclase inhibitors (Fig. S1A). In addition, swelling caused by both the plant and the bacterial PNP is strongly inhibited in the presence of cycloheximide, indicating that de novo protein synthesis is required for the response (Fig. S1B).
Fig. 2.
Stomatal guard cell assay and analysis of starch. (A) Quantification of stomatal apertures in epidermal peels of C. sinensis plants incubated with control Buffer (1), 5 μM XacPNP or AtPNP-A (2 and 3, respectively), 5 μM XacPNP or AtPNP-A with 10 μM methylene blue (4 and 5, respectively); and controls with NAA (6) or ABA (7). Bars are the means ± SEM of apertures of >60 stomata, and the results are representative of three independent experiments. The significance of the differences was P < 0.01. (Right) Representative images of stomatal apertures, with or without XacPNP, are shown. (B) Quantification of Lugol staining of the starch present in cells incubated with control Buffer (1), and 5 μM XacPNP or AtPNP-A (2 and 3, respectively). Data were analyzed and represented as A. (Right) Representative stomata stained with Lugol's in the presence or absence of XacPNP.
Expression of XacPNP In Planta.
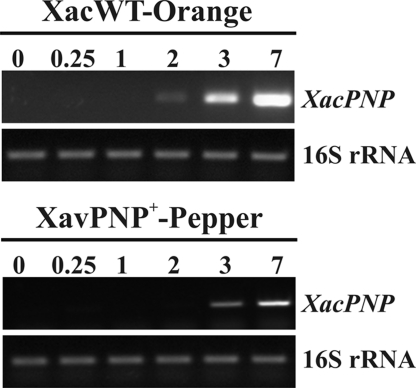
A search for signatures in the XacPNP promoter has revealed that it contains an imperfect PIP box (ATCGC-N15-TTCGC) at a conserved distance from the −10 promoter motif. PIP boxes are plant inducible promoter elements (PIP) that are recognized by the product of the hrpX gene, which regulates the expression of hrp genes involved in pathogenicity (9). We analyzed XacPNP expression in rich and minimal media and observed expression induction in only XVM2, a nutrient poor medium that simulates conditions in the apoplastic space to induce expression of virulence genes (9) (Fig. S2). Having established that XacPNP expression is induced in XVM2, we evaluated its expression in plant-pathogen interactions by using RT-PCR. RNA was obtained from bacteria recovered from C. sinensis infected leaves at different times of infection. During infection, XacPNP expression was barely detected 2 days after X. axonopodis pv. citri infiltration and increased over the time monitored (Fig. 3). To answer the question of whether or not a PNP-like molecule is functional in bacteria other than X. axonopodis pv. citri, we cloned XacPNP under the control of its own promoter in the plasmid pBBR1MCS-5 (pBBR1XacPNP). This plasmid was conjugated in X. axonopodis pv. vesicatoria, a pathogen that causes pepper bacterial spot, rendering it XavPNP+. Expression of XacPNP in X. axonopodis pv. vesicatoria in pepper leaves was hardly detectable at the beginning of the infection process and subsequently increased to the highest transcript levels at 7 days post-inoculation (Fig. 3). As a control for constitutive bacterial expression, a fragment of 16S rRNA was simultaneously amplified (Fig. 3), and to ascertain the absence of plant RNA in bacterial samples, controls with plant actin primers were carried out (data not shown).
Fig. 3.
Analysis of the expression of XacPNP in planta. RT-PCR of XacPNP with RNA obtained from either X. axonopodis pv. citri wild-type (XacWT) that were recovered from inoculated orange leaves or X. axonopodis pv. vesicatoria that were recovered from inoculated pepper leaves, and which were both conjugated with the vector pBBR1XacPNP (XavPNP+) and analyzed at different times of infection (0, 0.25, 1, 2, 3, and 7 days). As constitutive controls, a fragment of 16S rRNA was amplified by using the same RT-PCR conditions (bottom gels).
Analyses of XacPNP Mutant Strains in Citrus Canker.
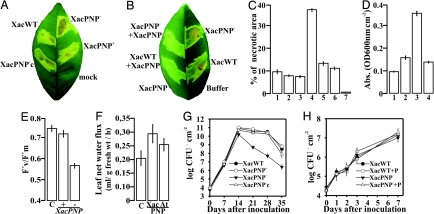
To further elucidate the role of the X. axonopodis pv. citri PNP-like gene in citrus canker, a XacPNP− mutant was generated by marker exchange, and it was genetically verified (Fig. S3). The XacPNP− mutant strain was tested for its ability to trigger disease in citrus leaves. Both wild-type bacteria and XacPNP− induced disease upon infiltration at a concentration of 107 cfu/ml. No differences were observed in the onset of lesion formation or lesion size. However, large necrotic areas only appeared on the lesions produced by the XacPNP− (Fig. 4A), and the percentage of necrotic area was three times larger than that caused by the wild-type bacteria (P < 0.001) (Fig. 4C). Similar results were obtained at lower concentrations of bacteria (104, 105, and 106 cfu/ml), as well as after infiltrating other tissues, such as mature fruit tissue (data not shown). XacPNP− was complemented by conjugating pBBR1XacPNP into this mutant (XacPNP−c). We also coinfiltrated XacPNP− with an XacPNP purified protein. In both cases, the lesions reverted to a significantly less necrotic phenotype (Fig. 4 B and C). Infiltrations with the X. axonopodis pv. citri wild-type carrying pBBR1XacPNP (XacPNP+) results in similar expression levels as the wild-type (Fig. S3C) and the X. axonopodis pv. citri coinfiltrated with a XacPNP protein, which both cause the same symptoms and similar percentages of necrotic area (Fig. 4 A–C). The lack of significant differences among coinfiltrated protein strains, XacPNP+, and wild-type infiltrations may be attributed to protein saturation, at which time no further enhancement of biological effects will occur. We also complemented an XacPNP− strain with an AtPNP-A gene, carried in a replicative plasmid, and obtained similar results as with the XacPNP complemented mutant, suggesting that similar biological responses are triggered by both proteins (Fig. S4). To further quantify the leaf necrotic areas, we performed Evans blue staining, which allows reproducible quantification of the stain that is retained by dead cells. The results show that necrotic orange tissue, caused by XacPNP− infection, absorbed more stain than tissue from wild-type infection, confirming a larger number of dead cells in XacPNP−-induced lesion (Fig. 4D). Other diagnostic techniques to examine necrotic areas, such as trypan blue staining, ion leakage, and enzymatic activity, were also used (Fig. S5) and have confirmed the larger necrotic areas produced by XacPNP− infection. It is noteworthy that, in leaves infiltrated with XacPNP−, the photosystem II maximum efficiency was significantly reduced (P < 0.001) (Fig. 4E), and that this reduction was prevented by bacteria carrying the XacPNP gene. In addition, net water flux through the leaf was significantly (P < 0.01) increased in the presence of both XacPNP and AtPNP-A (Fig. 4F). Such increased fluxes under unchanged atmospheric conditions are the consequence of lower leaf water potential, as confirmed by leaf xylem water potential measurements (results not shown). When wild-type, XacPNP−, and XacPNP−c bacterial growth on citrus leaves was analyzed, growth was ≥1010 cfu/cm2 at 14 days, after infiltration, for all of the strains tested (Fig. 4G). After 14 days, growth curves showed that XacPNP− dependent cell death was significantly different from that of other strains (P < 0.05) (Fig. 4G), arguably because of the fact that bacteria could not grow on the large area of dead tissue that covers almost the entire lesion at this stage. Growth curves of wild-type and XacPNP−, coinfiltrated with recombinant protein, showed that the presence of XacPNP does not alter bacterial growth (Fig. 4H).
Fig. 4.
Effects of the XacPNP mutant, and expression of XacPNP, on pathogenicity. (A) X. axonopodis pv. citri wild-type (XacWT), knocked out strain XacPNP−, and complemented knocked out strain XacPNP−c and XacPNP+ were inoculated at 107 cfu/ml into the intercellular spaces of fully expanded orange leaves. A representative leaf is shown seven days after inoculation. (B) Orange leaves were coinfiltrated with 5 μM XacPNP purified protein and either XacWT or XacPNP−. A representative leaf is shown seven days after inoculation. (C) Means of the percentages of necrotic areas of orange leaves infiltrated with: XacWT (1), XacPNP+ (2), XacWT coinfiltrated with XacPNP protein (3), XacPNP− (4), XacPNP−c (5), XacPNP− coinfiltrated with XacPNP protein (6), and XacPNP protein (7). Values represent means of 40 infected leaves photographed and analyzed; error bars represent standard deviations. (D) Evans Blue staining of necrotic areas of orange leaves infiltrated with 10 mM MgCl2 (1), XacWT (2), XacPNP− (3) and XacPNP+ (4). In each case, values represent means of three samples; error bars represent standard deviations. (E) Photosystem II maximum efficiency (F′v/F′m) was measured in leaves infiltrated with 10 mM MgCl2 (C), XacWT and XacPNP−. In each case, values represent means of three samples; error bars represent standard deviations. (F) H2O net flux was measured in orange leaves in the presence of 20 μl of 50 mM Tris/HCl (pH 8.0) (C), 2 μg of XacPNP or AtPNP-A in 20-μl buffer, spread on the lower leaf surface. Each experiment was done with four leaves per treatment, and the data are mean ± SEM of three independent experiments. (G) Bacterial growth of XacWT, XacPNP−, XacPNP−c, and XacPNP+ in orange leaves inoculated as described in (A). (H) Growth of XacWT bacteria, XacPNP− bacteria, and these same two bacteria, coinfiltrated with XacPNP protein (XacWT+P and XacPNP−+P, respectively) in orange leaves.
Role of XacPNP in Pepper Bacterial Spot.
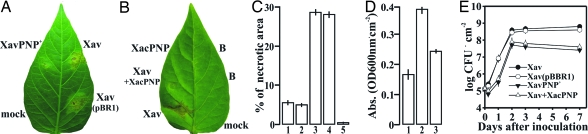
To answer the question of whether or not PNP function in Xanthomonas is restricted to a particular and exclusive host, we investigated the role of XacPNP in a different compatible interaction. To this end, we infiltrated pepper plants with X. axonopodis pv. vesicatoria that were expressing XacPNP (XavPNP+) and wild-type bacteria. We observed that XavPNP+ caused significantly smaller lesions, with less necrosis, than either the wild-type bacteria or the control conjugated with the empty vector pBBR1-MCS5 (P < 0.001), thus making the response similar to the X. axonopodis pv. citri response (Fig. 5A, C, and D and Fig. S5). We also observed the same XacPNP effect when we coinfiltrated X. axonopodis pv. vesicatoria with purified XacPNP protein (Fig. 5 B and C). Growth, in planta, of XavPNP+, as well as wild-type coinfiltrated with XacPNP protein, was significantly reduced (P < 0.05) by at least one order of magnitude as compared with wild-type bacteria and Xav (pBBR1-MCS5) (Fig. 5E), while all strains showed the same growth kinetics in culture media (SB and XVM2, data not shown). These results show that XacPNP is able to modify host responses to the benefit of the pathogen, even in a different plant host; but, in this case, the aggressive colonizing strategy is compromised.
Fig. 5.
Effects of XacPNP expression on the pathogenicity of X. axonopodis pv. vesicatoria. (A) X. axonopodis pv. vesicatoria wild-type (Xav), XavPNP+, and Xav(pBBR1-MCS5) [named in the figure as Xav(pBR1)] were inoculated, 107 cfu/ml, into the intercellular space of fully expanded pepper leaves. A representative leaf is shown two days after inoculation. (B) Pepper leaves were coinfiltrated with Xav and 5 μM XacPNP purified protein, and, as controls, 50 mM Tris/HCl pH 8.0 (B) and 10 mM MgCl2 (mock). A representative leaf is shown two days after inoculation. (C) Means of the percentages of necrotic areas of pepper leaves infected with: XavPNP+ (1), Xav coinfiltrated with XacPNP protein (2), Xav (3), Xav(pBR1) (4), and XacPNP protein (5). Values represent means of 40 infected leaves photographed and analyzed; error bars represent standard deviations. (D) Evans Blue staining of necrotic areas of pepper leaves infiltrated with 10 mM MgCl2 (1), Xav (2), and XavPNP+ (3). In each case, values represent means of three samples; error bars represent standard deviations. (E) Bacterial growth of Xav, XavPNP+, Xav(pBBR1-MCS5), and Xav coinfiltrated with XacPNP protein in pepper leaves inoculated as described in A. Values represent means of three samples, and error bars are standard deviations.
Discussion
Plant natriuretic peptides induce many physiological responses, including the modulation of homeostatic processes such as K+, Na+, and H+ net fluxes (5), and protoplast H2O uptake (4). Recently, it has been shown that, in Arabidopsis, significant increases in AtPNP-A expression occur in response to both abiotic (e.g., osmotic stress and K+ starvation) and biotic stimuli (10). The finding that X. axonopodis pv. citri, but not any other bacteria, has a PNP-like gene raises the question of the advantage that the bacteria might gain from this acquisition.
Infections cause changes in host carbohydrate metabolism, and biotrophic pathogens have been reported to trigger down-regulation of photosynthetic genes. However, host measures are aimed at starving the pathogen without weakening defense responses, at which time the pathogen will attempt to manipulate host carbohydrate metabolism to its own advantage (11). There are a number of indications that point to a role of XacPNP in host homeostasis regulation. First, the “late” induction of the gene in the minimal medium and the plant leaf suggests sensing of, and adapting to, low nutrients in the host environment. Second, we show that, in the presence of XacPNP, photosynthesis is sustained during infection, and, with it, the generation of assimilates. Thirdly, we provide direct evidence that XacPNP causes starch degradation in guard cells with a consequent rise in solute content, which, in guard cells, causes stomatal opening and can lead to increases in net water flux through the leaf. It has been shown previously that PNPs promote radial water movements from the xylem to the surrounding tissue (12) and that PNPs cause net H2O uptake into the protoplast (4, 13). Accordingly, we prove that XacPNP promotes water uptake into protoplasts in a cGMP-dependent manner, similar to what occurs with AtPNP-A, suggesting that XacPNP employs a similar biological mechanism operating via the same second messenger as AtPNP-A. Our results are, thus, compatible with the idea that X. axonopodis pv. citri bearing XacPNP, as well as the purified bacterial peptide, can influence cell turgor and draw water to the infected tissue. Furthermore, the performance of the photosystem II depends on the leaf water status, and leaf drying causes reduction in quantum yield (14). We, therefore, argue that the significantly improved performance of the photosystem II in the presence of XacPNP is, at least in part, because of the improved water status.
Having established that XacPNP can influence host homeostasis, we were interested to see effects on host responses and in particular tissue necrosis. Homeostatic perturbations caused by infections are likely to shorten the life of the plant leaf (15, 16) to the detriment of the pathogen, particularly in biotrophs. When expressing XacPNP, X. axonopodis pv. citri reduces the damage to the host, at least, in part, through the maintenance of photosynthesis and PNP dependent net H2O flux, and, thus, favors pathogen survival. Depending on their lifestyle, pathogens can either suppress or promote host cell death to induce disease susceptibility (17). In X. axonopodis pv. citri, a biotrophic pathogen, suppression of cell death will enable prolonged infection and, thus, prolonged survival. Conversely, in X. axonopodis pv. vesicatoria that has to switch to a necrotrophic lifestyle to enhance pathogen multiplication and dispersal (18), the suppression of tissue necrosis, observed by the expression of XacPNP, may limit its growth.
Because AtPNP-A has been implicated in abiotic and biotic stress responses in Arabidopsis, and AtPNP-A expression is correlated with defensive gene expression and is up-regulated in mutants with elevated salicylic acid levels (10), we cannot rule out the possibility that XacPNP can cause a direct suppression of plant defense responses to favor pathogen survival and tissue colonization. However, when we have analyzed the expression levels of several genes involved in defense responses in orange leaves incubated with XacPNP, we have observed no changes as compared with the controls (Garavaglia et al. unpublished results). These findings suggest that the bacterial protein is principally involved in the regulation of homeostasis.
The origin of the PNP-like gene in X. axonopodis pv. citri is difficult to establish with certainty; however, the incongruous phylogeny is compatible with the hypothesis of horizontal gene transfer. While horizontal gene transfer is the predominant mechanism of genome ‘innovation’ and variation in both viruses and bacteria, the sources of the acquired genes in the latter are typically either different strains or species rather than host organisms (19). Legionella pneumophila appears to be an exception (20, 21); however, the biological roles of its eukaryotic-like genes remain a matter of speculation. In addition, there is phylogenetic evidence for horizontal gene transfer from filamentous ascomycete fungi to the distantly related oomycetes, and, specifically, to pathogens, so as to modify genomes dynamically and, thereby, facilitate responses to changing environmental conditions and help invasion of host organisms (22). An example of molecular mimicry was recently reported in another plant-pathogen interaction where the nematode Heterodera glycines used a polypeptide, similar to a CLAVATA3/ESR-related peptide, to control the balance between meristem cell proliferation and differentiation, thus enabling it to modify plant cell differentiation to improve its food supply (23).
Taking into account that X. axonopodis pv. citri is not free-living, we suggest that (i) XacPNP is acquired by the bacteria and subsequently acquires and/or modifies its promoter, and (ii) XacPNP indeed possesses a conserved PIP sequence (24) to enable expression during plant-pathogen interaction and, thus, contribute to the optimal adaptation of this strain to the specific host environment. The fact that XacPNP can be induced in different bacteria suggests that its promoter is not dependent on highly specific signals from either one particular host or closely related hosts. Taken together, our results demonstrate that X. axonopodis pv. citri uses a PNP-like gene to modulate host responses that improve its survival conditions in the plant tissue, supporting a hypothesis of molecular mimicry that we will test in the future.
Materials and Methods
Detailed descriptions of protein purification, RNA preparation, RT-PCR, construction of bacterial variants, and cell death measurement assays are given in SI Text.
Bacterial Strains, Culture Conditions and Media. E. coli cells were cultivated at 37°C in Luria Bertani (LB) medium. X. axonopodis pv. citri Xcc99–1330 and X. axonopodis pv. vesicatoria Xcv Bv5–4a strains were grown at 28°C in Silva Buddenhagen (SB) (25), or XVM2 (9). Antibiotics were used as previously reported (25).
Recombinant DNA and Microbiological Techniques.
All DNA manipulations were performed with standard techniques (26), unless otherwise specified. Genomic DNA from X. axonopodis pv. citri was isolated by using the cetyltrimethylammonium bromide procedure (27), and bacterial conjugation was performed as previously described (28).
Protein Purification.
The coding region for the mature XacPNP was amplified by PCR with NPNPB (5′ ATCAGGATCCGACATCGGTACAATTAGTT 3′) and CPNPH (5′ ATACAAGCTTTTAAATATTTGCCCAGGGCG 3′) oligonucleotides, cloned into a pET28a vector (Novagen), and expressed in E. coli BL21(DE3)pLys as an His-tag N-terminal fusion protein. The protein was purified by using Ni-NTA agarose resin (QIAGEN). Recombinant AtPNP-A (At2g18660) was prepared as described previously (29).
Determination of Physiological Parameters.
Plectranthus ecklonii Benth. was grown in potting soil inside an atmospherically controlled greenhouse with day-light conditions. Then, 5 μM solutions of either XacPNP or AtPNP-A were applied and spread on the adaxial and abaxial leaf surfaces. Photosynthesis was quantified as previously detailed (30) in the youngest, fully expanded leaf of each replicate (n = 3). Readings were taken with a portable infrared gas analyzer (LCA-Pro, ADC, Herts SG12 9TA). Photosynthetic light-response curves were determined by varying the light source on the instrument between 0–1,600 μmol/m2s. Photosynthetic water-use efficiency (A/E) was calculated where A was the photosynthetic rate and E was the leaf transpiration rate. The apparent photon yield was the slope of the light-limiting part of the light response curve. Responses were analyzed with ANOVA, and, for differences between treatments, the means were separated by using a post hoc Student Newman Kuehls (SNK) multiple range test. Photosystem II maximum efficiency (F′v/F′m) in control and infected leaves was determined as detailed previously (31), and analyzed by using a one-way ANOVA with the Bonferroni multiple comparison test. Gravimetric measurements of net water flux were done on single leaves, with their petioles in water, in sealed containers. PNPs in 20 μl of H2O were added and spread on lower leaf surfaces with a micropipette tip. Transpiration at ambient light, 25°C, and after a 1-h treatment, was quantified and analyzed with one-way ANOVA. Each experiment had four replicate leaves and was repeated three times.
Stomatal Guard Cell Assay and Analysis of Starch.
C. sinensis plants were grown in a growth chamber in incandescent light at 28°C with a photoperiod of 16 h. Segments of abaxial epidermis from leaves kept in darkness were floated on Buffer A (10 mM Mes, 10 mM KOH, pH 6.15) for 2 h, at 25°C, and in darkness. Epidermis was transferred to Buffer A containing 50 mM KCl and 100 μM CaCl2, with or without treatment, and exposed under incandescent light (λ = 430 nm at 35W m−2) at 25°C for an additional 2 h. Treatments included the addition of 1 μM naphthalene acetic acid (NAA), 50 μM abscisic acid (ABA), or 5 μM either XacPNP or AtPNP-A, with or without 10 μM methylene blue (MB). Pore widths of >20 stomata from three separate segments of each treatment were measured under the microscope with a calibrated ocular micrometer. Analysis of starch stained with 10% Lugol's iodine solution was done as described previously (15). The results were analyzed by using a one-way ANOVA and Bonferroni multiple comparison test.
RNA Preparation and RT-PCR.
X. axonopodis pv. citri, cultured in either SB or XVM2, were harvested, and total RNA was isolated by using TRIzol reagent (Invitrogen). RNA preparations of bacteria from inoculated leaves, at different post infection times, were done as described previously (32). After treatment with DNase (Promega), cDNA was synthesized from 1 μg of total RNA by using MMLV RT (Promega) and the oligonucleotide, dN6; and PCR was done by using NPNPB and CPNPH oligonucleotides.
Construction of Bacterial Variants.
The XacPNP deletion mutant (XacPNP−) was created by marker exchange mutagenesis as described previously (25). XacPNP− mutants were verified by PCR and Southern blot (Fig. S3). Complementation of XacPNP− was done by amplifying the XacPNP and its promoter region by PCR and cloning the product in the broad-host-range vector, pBBR1MCS-5 (25), rendering pBBR1XacPNP. This plasmid was transferred to the XacPNP− strain, rendering XacPNP−c. The same plasmid was conjugated to X. axonopodis pv. citri and X. axonopodis pv. Vesicatoria, rendering XacPNP+ and XavPNP+, respectively.
Plant Material, Inoculations, and Cell Death Measurements.
Orange (Citrus sinensis cv. Valencia) was used as the host plant for X. axonopodis pv. citri, and pepper (Capsicum annuum cv. grossum) as host plant for X. axonopodis pv. vesicatoria. All plants were grown in a growth chamber in incandescent light at 28°C with a photoperiod of 16 h. Bacterial infiltrations and in planta growth assays were performed as described previously (25) and were analyzed by using multifactorial ANOVA and a Tukey multiple comparison test. The percentages of necrotic areas in lesions were calculated as necrotic area per infected area. Areas were measured from digitalized images of forty infected leaves, using Adobe Photoshop software, and analyzed by using one-way ANOVA with a Bonferroni multiple comparison test. For evaluation of viability, lesioned tissue was stained with Evans Blue as previously described (33). Leaf disks from one-week infiltrated orange, or pepper, leaves were submerged in 1 ml of 0.25% Evans Blue and incubated for 20 min. Then, they were washed several times with water to remove excess and unbound dye, and after disk homogenization, bound dye was extracted with 1% SDS. The extracted dye was measured spectrophotometrically at 600 nm. The results were analyzed by using a one-way ANOVA and Bonferroni multiple comparison test.
Supplementary Material
Acknowledgments.
We thank Catalina Anderson (INTA Concordia, Argentina) and Gastón Alanis and Rubén Díaz Vélez (Proyecto El Alambrado) for the citrus plants. N.G., E.G.O. and J.O. are staff members of and B.S.G. and L.D.D. are fellows of the Consejo Nacional de Investigaciones Científicas y Técnicas. This work was supported by Agencia Nacional de Promoción Científica y Tecnológie (ANPCyT) Grants PICT01–12783 (to E.G.O.), PICT2006–00678 (to J.O.), and PICT2006–1073 (to N.G.) and the South African National Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810107105/DCSupplemental.
References
- 1.Takei Y. Does the natriuretic peptide system exist throughout the animal and plant kingdom? Comp Biochem Physiol B Biochem Mol Biol. 2001;129:559–573. doi: 10.1016/s1096-4959(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 2.Gehring CA, Irving HR. Natriuretic peptides–a class of heterologous molecules in plants. Int J Biochem Cell Biol. 2003;35:1318–1322. doi: 10.1016/s1357-2725(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 3.Pharmawati M, Maryani MM, Nikolakopoulos T, Gehring CA, Irving HR. Cyclic GMP modulates stomatal opening induced by natriuretic peptides and immunoreactive analogues. Plant Physiol Biochem. 2001;39:385–394. [Google Scholar]
- 4.Maryani MM, Bradley G, Cahill DM, Gehring CA. Natriuretic peptides and immunoreactants modify the osmoticum-dependent volume changes in Solanum tuberosum L. mesophyll cell protoplasts. Plant Sci. 2001;161:443–452. [Google Scholar]
- 5.Ludidi N, Morse M, Sayed M, Wherrett T, Shabala S, Gehring C. A recombinant plant natriuretic peptide causes rapid and spatially differentiated K+, Na+ and H+ flux changes in Arabidopsis thaliana roots. Plant Cell Physiol. 2004;45:1093–1098. doi: 10.1093/pcp/pch113. [DOI] [PubMed] [Google Scholar]
- 6.Graham JH, Gottwald TR, Cubero J, Achor DS. Xanthomonas axonopodis pv. citri: Factors affecting successful eradication of citrus canker. Mol Plant Pathol. 2004;5:1–15. doi: 10.1046/j.1364-3703.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 7.Nembaware V, Seoighe C, Sayed M, Gehring C. A plant natriuretic peptide-like gene in the bacterial pathogen Xanthomonas axonopodis may induce hyper-hydration in the plant host: A hypothesis of molecular mimicry. BMC Evol Biol. 2004;4:10. doi: 10.1186/1471-2148-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rafudeen S, et al. A role for plant natriuretic peptide immuno-analogues in NaCl- and drought-stress responses. Physiol Plant. 2003;119:554–562. [Google Scholar]
- 9.Wengelnik K, Bonas U. HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria. J Bacteriol. 1996;178:3462–3469. doi: 10.1128/jb.178.12.3462-3469.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meier S, Bastian R, Donaldson L, Murray S, Bajic V, Gehring C. Co-expression and promoter content analyses assign a role in biotic and abiotic stress responses to plant natriuretic peptides. BMC Plant Biol. 2008;8:24. doi: 10.1186/1471-2229-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger S, Benediktyová Z, Matous K, Bonfig K, Mueller MJ, Roitsch T. Visualization of dynamics of plant-pathogen interaction by novel combination of chlorophyll fluorescence imaging and statistical analysis: Differential effects of virulent and avirulent strains of P. syringae and of oxylipins on A. thaliana. J Exp Bot. 2007;58:797–806. doi: 10.1093/jxb/erl208. [DOI] [PubMed] [Google Scholar]
- 12.Suwastika IN, Gehring CA. Natriuretic peptide hormones promote radial water movements from the xylem of Tradescantia shoots. Cell Mol Life Sci. 1998;54:1161–1167. [Google Scholar]
- 13.Wang YH, Gehring C, Cahill DM, Irving HR. Plant natriuretic peptide active site determination and effects on cGMP and cell volume regulation. Functional Plant Biology. 2007;34:653. doi: 10.1071/FP06316. [DOI] [PubMed] [Google Scholar]
- 14.Brodribb TJ, Holbrook NM. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol. 2003;132:2166–2173. doi: 10.1104/pp.103.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guimaraes RL, Stotz HU. Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol. 2004;136:3703–3711. doi: 10.1104/pp.104.049650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prats E, Gay AP, Mur LA, Thomas BJ, Carver TL. Stomatal lock-open, a consequence of epidermal cell death, follows transient suppression of stomatal opening in barley attacked by Blumeria graminis. J Exp Bot. 2006;57:2211–2226. doi: 10.1093/jxb/erj186. [DOI] [PubMed] [Google Scholar]
- 17.Abramovitch RB, Martin GB. Strategies used by bacterial pathogens to suppress plant defenses. Curr Opin Plant Biol. 2004;7:356–364. doi: 10.1016/j.pbi.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell PJ, Jones JB, Antoine FR, Ciardi J, Klee HJ. Ethylene-dependent salicylic acid regulates an expanded cell death response to a plant pathogen. Plant J. 2001;25:315–323. doi: 10.1046/j.1365-313x.2001.00968.x. [DOI] [PubMed] [Google Scholar]
- 19.Pallen MJ, Wren BW. Bacterial pathogenomics. Nature. 2007;449:835–842. doi: 10.1038/nature06248. [DOI] [PubMed] [Google Scholar]
- 20.Cazalet C, et al. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat Genet. 2004;36:1165–1173. doi: 10.1038/ng1447. [DOI] [PubMed] [Google Scholar]
- 21.de Felipe KS, et al. Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol. 2005;187:7716–7726. doi: 10.1128/JB.187.22.7716-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards TA, Dacks JB, Jenkinson JM, Thornton CR, Talbot NJ. Evolution of filamentous plant pathogens: Gene exchange across eukaryotic kingdoms. Curr Biol. 2006;16:1857–1864. doi: 10.1016/j.cub.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, et al. A parasitism gene from a plant-parasitic nematode with function similar to CLAVATA3/ESR (CLE) of Arabidopsis thaliana. Mol Plant Pathol. 2005;6:187–191. doi: 10.1111/j.1364-3703.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 24.Koebnik R, Kruger A, Thieme F, Urban A, Bonas U. Specific binding of the Xanthomonas campestris pv. vesicatoria AraC-type transcriptional activator HrpX to plant-inducible promoter boxes. J Bacteriol. 2006;188:7652–7660. doi: 10.1128/JB.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunger G, et al. Xanthan is not essential for pathogenicity in citrus canker but contributes to Xanthomonas epiphytic survival. Arch Microbiol. 2007;188:127–135. doi: 10.1007/s00203-007-0227-8. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunger G, Arabolaza LN, Gottig N, Orellano EG, Ottado J. Participation of Xanthomonas axonopodis pv. citri hrp cluster in citrus canker and in non-host plants responses. Plant Pathol. 2005;54:781–788. [Google Scholar]
- 29.Morse M, Pironcheva G, Gehring C. AtPNP-A is a systemically mobile natriuretic peptide immunoanalogue with a role in Arabidopsis thaliana cell volume regulation. FEBS Lett. 2004;556:99–103. doi: 10.1016/s0014-5793(03)01384-x. [DOI] [PubMed] [Google Scholar]
- 30.Valentine A, Osborne B, Mitchell D. Form of inorganic nitrogen influences mycorrhizal colonization and photosynthesis of cucumber. Sci Hort. 2002;92:229–239. [Google Scholar]
- 31.Baker NR, Rosenqvist E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J Exp Bot. 2004;55:1607–1621. doi: 10.1093/jxb/erh196. [DOI] [PubMed] [Google Scholar]
- 32.Mehta A, Rosato YB. A simple method for in vivo expression studies of Xanthomonas axonopodis pv. citri. Curr Microbiol. 2003;47:400–403. doi: 10.1007/s00284-003-4051-3. [DOI] [PubMed] [Google Scholar]
- 33.Baker CJ, Mock NM. An improved method for monitoring cell death in cell suspension and leaf disc assays by using evans blue. Plant Cell, Tissue and Organ Culture. 1994;39:7–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.