Abstract
Sex differences in parenting are common in species where both males and females provide care. Although there is a considerable body of game and optimality theory for why the sexes should differ in parental care, genetics can also play a role, and no study has examined how genetic influences might influence differences in parenting. We investigated the extent that genetic variation influenced differences in parenting, whether the evolution of differences could be constrained by shared genetic influences, and how sex-specific patterns of genetic variation underlying parental care might dictate which behaviors are free to evolve in the burying beetle Nicrophorus vespilloides. Females provided more direct care than males but did not differ in levels of indirect care or the number of offspring they were willing to rear. We found low to moderate levels of heritability and evolvability for all 3 parenting traits in both sexes. Intralocus sexual conflict was indicated by moderately strong intersex genetic correlations, but these were not so strong as to represent an absolute constraint to the evolution of sexual dimorphism in care behavior. Instead, the pattern of genetic correlations between parental behaviors showed sex-specific tradeoffs. Thus, differences in the genetic correlations between parental traits within a sex create sex-specific lines of least evolutionary resistance, which in turn produce the specific patterns of sex differences in parental care. Our results therefore suggest a mechanism for the evolution of behavioral specialization during biparental care if uniparental and biparental care behaviors share the same genetic influences.
Keywords: behavior genetics, genetic architecture, lines of least evolutionary resistance, parental care, sexual dimorphism
Parental care, although taxonomically widespread, is relatively rare. Nevertheless, it represents one of the main areas of research in behavioral ecology (1). Within the animal kingdom there is variation in the sex that provides care (2), but even when both parents provide care, males and females tend to specialize, often with a clear division of labor between the sexes (1, 3, 4). Specialization is possible because parental care typically involves several behaviors, such as incubation of eggs, defense of young, nest construction and maintenance, and provisioning of food to developing offspring (1). Thus, one sex may specialize in the incubation of eggs and the other in provisioning offspring, as is the case in the majority of hawks and eagles (5). This specialization can be total, with one sex performing one behavior exclusively, or partial, with both sexes performing all behaviors associated with care but at different levels (1).
There are 3 fundamental yet unanswered questions with regard to parental care specialization: Why should males and females specialize, which behaviors should they specialize in, and how is the evolutionary trajectory of specialization influenced or constrained by patterns of genetic variation? Current theory and empirical research typically examine potential sex differences in the costs and benefits of care to address specialization (6–8). Although this approach provides insights into the predicted strength and direction of selection, addressing the first 2 issues, understanding the evolutionary process also requires quantitative genetic information (9–11). Information on genetics is critical for understanding the evolution of sexual dimorphism in traits because males and females share a common genome, and this can constrain and bias the evolution of differences between the sexes (10–12). Yet, information on patterns of genetic variation (e.g., heritabilities and evolvabilities) and covariation (e.g., genetic correlations) is almost completely lacking for traits associated with parental care, with only a few estimates of heritability (13, 14) and no estimates of the genetic correlations between different behaviors within or between the sexes. Measuring genetic variation underlying parental care should provide additional insights into sex differences in patterns of care observed in nature.
Behavioral specialization makes quantifying genetic influences on parental care difficult under biparental conditions because genetic variation cannot be quantified if the trait is not (or is rarely) expressed. However, in a number of species, both sexes will display the full range of parental behaviors following the loss of the partner (i.e., when the other sex dies or is removed) (3, 6). These systems provide an opportunity for quantifying the genetic variation underlying parental care behaviors in both sexes and assess whether or not this variation differs. Such studies are important because the evolution of parental care as a multivariate trait will be influenced not only by genetic variation but also genetic covariation between different traits within a sex and the same trait between the sexes.
In this study we measured the genetic variation and covariation within and between the sexes in parental care in the burying beetle, Nicrophorus vespilloides. These beetles express elaborate forms of parental care under uniparental male and female and under biparental conditions (15–18). Burying beetles breed on vertebrate carcasses, which are used as a food resource for their developing larvae. Complex parental care is apparently universal in this Silphid beetle subfamily (Nicrophorinae) (16, 17). Offspring obtain food both through direct provisioning of predigested carrion from the parent and by self-feeding from the carcass, which is maintained and prepared by the parent. Thus, parenting involves 2 distinct forms of care: direct care, whereby parents predigest the carcass and feed offspring carrion directly through regurgitation (Fig. 1 A and B), and indirect care, whereby the parents remove fungus and bacteria from the carcass and cover it with anal secretions to slow decomposition and extend the usable lifespan of the resource available to offspring (16, 17). Parents also determine family size via filial cannibalism (16), resulting in the attending parent controlling the number of offspring that will receive care. Under biparental conditions there is specialization, with females providing the majority of the direct care while the sexes provide similar levels of indirect care (15). Under uniparental conditions, however, males provide direct care at levels similar to those of females while maintaining high levels of indirect care (15).
Fig. 1.
Family life in the burying beetle (N. vespilloides). (A) A mother engaged in direct care by regurgitating predigested carrion to the larvae, which are begging from the mouse carcass. (B) Larvae self-feeding on predigested carrion within the crater (an excavation in the surface of the mouse carcass created by the parents) (photographs by A.J.M.).
Burying beetles provide an excellent experimental system to examine how genetic variation underlying parental care behavior might influence short-term (and therefore potential long-term) evolutionary trajectories in the 2 sexes. Our ability to rear large numbers in specific breeding designs under natural conditions allows us to use quantitative genetic approaches to study genetic influences on behavior in this species (19). Quantitative genetic data allow us to explicitly address whether and how such sexual dimorphism in parental care might evolve (10, 11). First, is there sufficient genetic variation for further evolution of parental care? Second, are there intralocus conflicts or other constraints to evolution? Finally, if there are no constraints, are there sex-specific lines of least evolutionary resistance (12) predisposing the sexes to invest in different parental traits when rearing young?
Results and Discussion
Sex Differences in Parental Care.
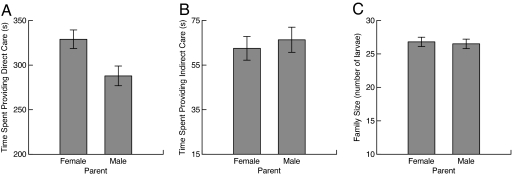
Males and females differed in their expression of parental traits under uniparental conditions (Fig. 2). Females provided more direct care than males (Fig. 2A), but there were no differences between the sexes in levels of indirect care or in family size (Fig. 2 B and C). This finding is similar to previous research using removal experiments with a substantially smaller sample size than this study demonstrating that males and females provide similar levels of care under uniparental conditions, with a trend toward females providing more direct care and males more indirect care (15). In addition, the sex differences we observed under uniparental conditions are similar to, although more subtle than, the patterns observed under biparental conditions (15).
Fig. 2.
Components of care by females and males presented as means and standard errors. (A) Females provide more direct care than males (1-way ANOVA, F1,479 = 7.32, P = 0.007), but the sexes did not differ in the levels of (B) indirect care (1-way ANOVA, F1,479 = 0.247, P = 0.619) or (C) family size (1-way ANOVA, F1,479 = 0.071, P = 0.790).
Males were phenotypically more variable than females for direct care, approaching statistically significant differences (Bartlett test χ2 = 3.411, P = 0.065), even though they expressed a lower mean level of direct care (Table 1). This is unexpected, because means and variances tend to be positively correlated in biological data. Males and females did not differ in the level of phenotypic variation in indirect care (χ2 = 0.006, P = 0.940) or the extent of phenotypic variation in family size (χ2 = 0.007, P = 0.931).
Table 1.
Descriptive statistics, variance components, narrow-sense heritability (h2), and evolvability (CVA) related to parental care in N. vespilloides
| Trait | Sex | Mean | VP | VA | h2 | SE | CVA |
|---|---|---|---|---|---|---|---|
| Direct care | Female | 329.0 | 24623 | 3260 | 0.13 | 0.20 | 16.68 |
| Male | 288.0 | 31269 | 6547 | 0.21 | 0.18 | 27.45 | |
| Indirect care | Female | 62.5 | 7295 | 3316 | 0.47 | 0.27 | 92.17 |
| Male | 66.3 | 7368 | 1178 | 0.16 | 0.17 | 51.76 | |
| Family size | Female | 26.8 | 113 | 57 | 0.51 | 0.23 | 28.10 |
| Male | 26.5 | 118 | 15 | 0.14 | 0.19 | 14.71 |
Values are based on 256 female offspring or 225 male offspring derived from a full-sib/half-sib breeding design of 30 sires each mated to 3 dams, with 2–3 male and female offspring scored for each family.
Heritability of Parental Traits.
The heritability and evolvability of direct care were higher in males than in females, despite higher levels of phenotypic variation, whereas heritability and evolvability of indirect care and family size were higher in females (Table 1). Genetic variation was low to moderate, as expected for behavioral traits, whereas evolvability was moderate to high, suggesting that there is scope for evolution in all traits related to parental care. Males in general were more susceptible to environmental rather than genetic influences, because they had lower heritabilities for 2 of the 3 measured traits despite similar levels of phenotypic variation.
Genetic and Phenotypic Correlations Within and Between the Sexes.
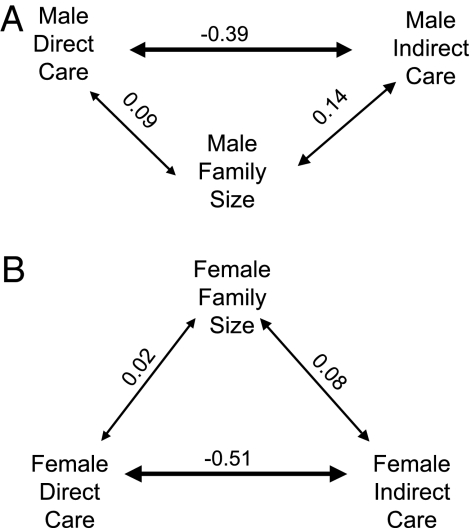
Phenotypic correlations between parenting traits within a sex were all low (Fig. 3). In contrast, genetic correlations varied in strength and direction (Fig. 4). Intersexual genetic correlations were moderately strong for direct and indirect care and for unity (1.0) between male and female family size. All intersexual genetic correlations were positive. Thus, any selection on one sex would result in a similar response for the same trait in the other sex. Although all traits will tend to evolve together, only the correlation for male and female family size represents an absolute constraint to the evolution of sex differences (10, 11). However, selection for a sex difference in family size is not expected, because we expect no sexual conflict for this trait and the number of offspring to be maximized (i.e., under positive directional selection) for both sexes.
Fig. 3.
Phenotypic correlations between parental traits within a sex. (A) Phenotypic correlations among parental care traits in males (n = 225). (B) Phenotypic correlations among the same traits in females (n = 256). The width of the arrows reflects the strength of the correlation. Only the correlations between male indirect care and male family size (P = 0.036), between male direct care and male indirect care (P < 0.001), and between female direct care and female indirect care (P < 0.001) are significant.
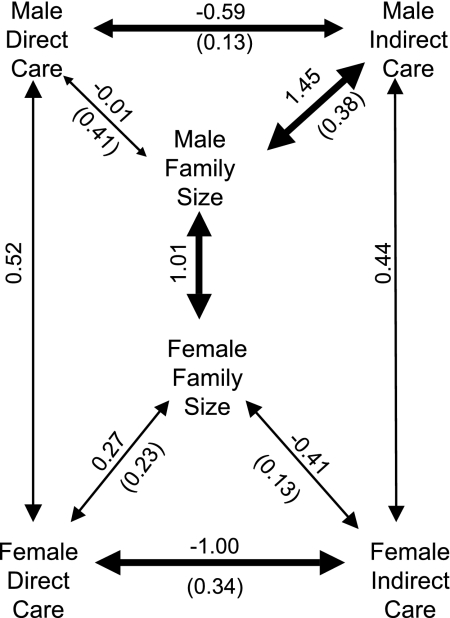
Fig. 4.
Genetic architecture underlying parental care behavior in males and females. Arrows between the same behaviors expressed in the different sexes indicate intersexual genetic correlations, whereas arrows between different behaviors within a sex indicate intrasexual genetic correlations. The width of the arrows reflects the strength of the genetic correlation, the estimated value is given above the arrows, and a jackknifed standard error (40) is presented in parentheses below the arrow.
The patterns of intrasexual genetic correlations differed for males and females (Fig. 4). The genetic correlation between family size and direct care was moderate and positive for females, but indistinguishable from zero for males. The genetic correlation between family size and indirect care was moderate and negative for females but strongly positive (indistinguishable from unity) for males. For both males and females there was a strong negative genetic correlation between direct and indirect care.
This sex-specific genetic architecture has consequences for short-term evolution of male and female behavior. Family size, or number of offspring reared for a given resource, is directly related to fitness. We therefore expect this trait to be under strong directional selection to maximize the number of offspring reared. The consequence of such selection is that indirect care of males will evolve through correlated selection as a result of the strong positive genetic correlation. Direct care by males is uncorrelated with family size, but it is negatively genetically correlated with male indirect care, and so it should decrease as a result of correlated evolution. In contrast, in females there is a positive genetic correlation between direct care and family size and a negative genetic correlation between indirect care and family size. Thus, selection for larger family sizes will result in a correlated increase in direct care and a decrease in indirect care by females. This is further reinforced by the strong negative genetic correlation between female direct care and indirect care. Thus, the genetic architecture of parental care suggests that females should provide more direct care than males while males should provide more indirect care than females. There are no absolute genetic constraints to the evolution of sex differences (Fig. 4); the intersex genetic correlations are all moderate and positive, and so they only slow rather than prevent the evolution of sex differences in care (10, 11).
Sex Differences in Selection Acting on Parental Care.
The above explanation does not consider the effects of direct selection on care behavior of males and females. Although parental care is clearly positively associated with offspring fitness, especially in the first 24 h after hatching (19, 20), our data suggest that there are also subtle sex differences in selection acting on parental care through its effect on offspring performance. Because of our sample sizes we can examine how the natural range of variation in care influences offspring. Average offspring mass, which is positively related to offspring survival (19), increased with increasing amounts of total parental care (direct care plus indirect care) provided by females (average mass = 149.0 + 0.017 × care; R2 = 0.02, F1,254 = 4.26, P = 0.040) but not males (average mass = 151.3 + 0.004 × care; R2 = 0.004, F1,223 = 0.850, P = 0.358). Examining direct and indirect care separately, there was no effect of variation in either type of care on average larval mass in either sex (direct care: female parent, average mass = 152.6 + 0.010 × direct care; R2 = 0.007, F1,254 = 1.80, P = 0.18; male parent, average mass = 152.0 + 0.005 × direct care; R2 = 0.003, F1,223 = 0.666, P = 0.42; indirect care: female parent, average mass = 155.1 + 0.011 × indirect care; R2 = 0.003, F1,254 = 0.649, P = 0.42; male parent, average mass = 153.3 + 0.002 × indirect care; R2 = 0.0001, F1,224 = 0.024, P = 0.88). Thus, females are experiencing directional selection to increase the total amount of parental care they provide, whereas males are not. Selection was weak, as might be expected, but detectable given our large sample sizes.
Conclusions.
Detailed examination of the quantitative genetic variation underlying parental care provides us with an explanation for sex differences in care under uniparental conditions and the specialization that is manifest during biparental care (15). Evolution of differences between the sexes can be constrained because of intralocus sexual conflict (opposing selection on the loci influencing the same traits in the 2 sexes; refs. 10 and 11). Although there was evidence of shared genetic influences, the intersexual genetic correlations were only moderately strong, so that the evolution of sex differences might be slowed but there is no absolute constraint. However, we found differences in the genetic correlations between behaviors within the sexes that would create sex-specific lines of least evolutionary resistance. The sex-specific genetic correlations predict different outcomes for the 2 sexes resulting from correlated evolution from selection for maximal family size because different behaviors are free to respond to selection in males and females. Furthermore, we found direct selection for increased parental care in females but not males. Taken together with the pattern of genetic correlations between traits, selection acting only on total amounts of female care should therefore result in direct care being the only behavior to differ between males and females, with higher levels of direct care expressed in females than in males. Thus, the pattern of expected and observed differences in selection, along with the patterns of genetic correlations that differ between the sexes, predict that females should show higher levels of direct care, with males more inclined toward providing indirect care or less total care.
Assuming uniparental care is ancestral, as it appears to be in other taxa (2), and that the genetic influences on care behavior are the same for uniparental and biparental conditions, the specialization observed when the sexes cooperate seems likely to have arisen because of differences in the innate parental tendencies. This highlights the importance of estimates of genetic correlations between parental behaviors within a sex and between the same behaviors in males and females, a crucial piece of information that is lacking in the few other studies examining genetic variation underlying parental care (13, 14, 19, 21–26). Such information is essential to our understanding of the evolution of parental care, given that care involves suites of behaviors, and thus the total response to selection depends not only on the genetic variation in the traits of interest but also on their genetic covariation within and between the sexes (10–12, 27).
Wider Implications.
We doubt that our results are unique to this species and predict that sex differences in genetic architecture underlying parental care will be present and influence the evolution of sexual dimorphism in parenting in other species. There are a number of species in which this prediction can be tested. For example, we predict that asymmetries in the reproductive strategies of males and females, which result in sex-specific genetic variation in parental responses to offspring solicitation (28), will also reflect sex differences in patterns of genetic covariation. Preliminary evidence that this may well be the case comes from studies on several species of birds demonstrating differences between the sexes in the repeatability of provisioning (13, 29) and a study of great tits (Parus major) reporting a genetic correlation between parental response and offspring begging (21). It now would be valuable to test whether males and females in these species differ with respect to genetic correlations between different parental behaviors and whether these differences match any behavioral specializations in parental care. Many species, including birds, fishes, and other insects, show flexibility and variation in the sex that cares (3, 30). These species provide taxonomically diverse candidates in which to examine how male and female parental roles are influenced by the genetic architecture underlying parental care.
The demonstration of sex differences in the genetic variation underlying parental care also has consequences for theories of parent–offspring coadaptation (31–34). There is a growing interest in the potential evolution of genetic correlations between parental provisioning and offspring solicitation as a sign of parent–offspring coadaptation (32, 34). Studies have now demonstrated both positive (19, 21, 24) and negative (22, 26) genetic correlations between female parents and their offspring. The only study to test for coadaptation between male parents and their offspring found no correlation between male care and offspring begging, despite a positive correlation between female care and offspring solicitation (21). The results of our experiment suggest that this difference in coadaptation will reflect differences in genetic architecture underlying care in the 2 sexes as also suggested by the authors of this study (21). This hypothesis for differences in parent–offspring coadaptation within a species could be tested in burying beetles, as we now predict that there will be no genetic correlation between male provisioning (direct care) and offspring solicitation because of the difference in the genetic architecture influencing direct care in male and female burying beetles.
Methods
Source and Maintenance of Beetles.
Our goal was to use beetles that reflected the genetic makeup and behavior of natural populations as closely as possible, so we established a short-term laboratory population specifically for this study. We collected 700 individuals trapped from 2 deciduous forests [Kennel Vale (latitude 50°11′ N and longitude 5°09′ W) and Devichoys (latitude 50°11′ N and longitude 5°07′ W)] in Cornwall, U.K., in July and September 2006 by using Japanese beetle traps filled with 3 cm soil and baited with a small piece of salmon. We brought these beetles back to our laboratory and maintained them in a temperature-controlled room at 20°C under a 16:8-h light–dark cycle, where all rearing and experiments took place. We kept all beetles individually in clear plastic (8 × 8 × 3.5 cm) containers filled to a depth of 2.5 cm with soil. We fed adult beetles decapitated mealworms (Tenebrio) ad libitum twice weekly.
Our experimental stocks were derived from these wild-caught beetles. We allowed females to reproduce using stored sperm or mated to wild-caught males if a female failed to reproduce. We bred beetles by placing mated females into larger (17 × 11 × 5 cm) breeding boxes filled to a depth of ≈2 cm with moist soil and provisioned with a previously frozen mouse carcass (supplied by Livefoods Direct, Sheffield, U.K.). For subsequent generations, we ensured all beetles were outbred by always mating completely unrelated individuals. We initiated our experiments after the third generation of mating, so that third- and fourth-generation beetles were used in this study.
Experimental Breeding Design.
We used a standard full-sib/half-sib breeding design with 30 unrelated sires mated to 3 randomly selected unrelated virgin females to produce a total of 268 female and 268 male beetles of known relatedness. We placed sires and dams in breeding boxes (as above) and provided a mouse carcass for 24 h. After this point, we removed sires and left dams to rear offspring for the duration of larval development on the carcass. We mated sires to 1 dam per week.
We placed larvae into individual clear plastic pots with soil as above when they dispersed from the carcass—typically when the carcass was completely consumed. We then checked larvae daily for survival to pupae and then adult eclosion. We fed them decapitated mealworms twice weekly as above once they reached adulthood and held them in these containers until they reached sexual maturity. We then mated these adults and conducted behavioral observations under uniparental male or uniparental female care.
Observations of Parental Care.
Because burying beetles bury a carcass and rear offspring underground, where conditions are likely to be more uniform and stable, we believe we can create a reasonable approximation of natural conditions in our laboratory, with the exception that there are no predators and likely to be fewer parasites. Where other researchers and we have compared behavior in the laboratory and the field, the behavioral patterns are identical (35, 36). The mating system of burying beetles is varied, with uniparental female care, uniparental male care, biparental care, and even communal breeding (16, 17). In N. vespilloides, uniparental care is by far the most common (37, 38). In addition, males and females exhibit the same parental care behaviors under all conditions (refs. 15 and 18; C.A.W. and A.J.M., unpublished data).
We paired and bred focal male or female individuals produced by the breeding design with an unrelated stock beetle. Breeding was initiated by allowing a male and female pair access to a previously frozen mouse carcass (mean ± SD = 22.7 ± 0.1 g mass) in a breeding box filled to a depth of 2 cm with moist soil as above. The range of carcass masses we used was very small, and the carcass mass we provided did not differ between female and male parents (22.7 ± 0.1 g (females), 22.7 ± 0.1 g (males); one-way ANOVA, F1,533 = 0.033, P = 0.900) to control for the effect of variation in resources. We removed the random stock beetle that we used as a mate 72 h after placing both on the carcass, before any eggs hatched. We then checked breeding boxes twice daily, morning and evening, for the presence of newly hatched larvae on the carcass. Once larvae arrived on the carcass, we began behavioral observations 24 to 36 h later, the period during which parental care is at a maximum (39). We recorded parental behaviors continuously for a period of 10 min. The extent that a parent engages in parental care, especially direct care, changes over time (15, 39), but the rank order of parenting is stable over time (i.e., high-care parents provide relatively high levels of care over the entire duration of parental care, and low-care parents provide relatively low care over the entire period of parental care; M. Gibbs and A.J.M., unpublished data).
We measured the total amount of time parents spent providing direct care, indirect care, and nonparental behaviors. We define direct care as mouth-to-mouth contact with larvae or carrion within the crater, indicating regurgitation of food to the larvae, manipulation of carrion, or regurgitation of carrion within the crater. We defined indirect care as manipulation of the surface of the carcass outside of the crater, removing fungus and bacteria and preventing rotting, excavation of the crypt (depression in the soil within which the carcass was buried), and movement of the carcass within the crypt. We have also called indirect care “carcass maintenance” in other studies (15, 18, 19, 39). We scored all other behavior that occurred as nonparental behavior, which usually involved the parent sitting and moving its mouthparts, self-grooming, or locomotion without performing any of the above behavior, and nonparental behavior was not analyzed further.
Males were significantly more likely to be completely absent from the carcass for the entire observation period (43 of 268 males versus 12 fo 268 females absent, Fisher exact test, P < 0.0001). We could not record parental or any other behavior for these individuals, and so they are not included in any analyses. Our final sample sizes, therefore, are 256 females and 225 males.
Immediately following the observation period we counted the number of larvae in a family to determine family size. We then checked carcasses twice daily (morning and evening) for larval dispersal, defined as the point at which all larvae crawled away from the crypt and any remaining carcass (19). We washed, dried, and then weighed to within 0.0001 g each larva at dispersal using an electronic balance to calculate the average mass of larvae within a family. At this point the parent was removed, and larvae were placed in individual pots as above. Once larvae disperse, they do not feed again until they are adults. Therefore, larval weight at dispersal closely reflects adult size and mass, which are both closely related to survival (19). Dispersal weight is therefore a useful measure of offspring performance (19).
Statistical Analysis.
All data were checked for normality and homogeneity of variances. We analyzed our data using JMP Professional release 5.0.1a or S-Plus. We calculated genetic parameters from our data with restricted maximum likelihood ANOVA, using an S-Plus routine provided by Derek Roff (40). We calculated estimates of variances and associated narrow sense heritability values (h2) and additive genetic correlations from these programs.
We also calculated the coefficient of additive genetic variation to estimate evolvability (CVA). We calculated this as the percent of the additive genetic variance standardized by the trait mean (41). This measure of evolvability provides a potentially more useful comparative measure of the extent that a population can respond to selection (41).
We estimated the genetic correlations between the sexes using the variance due to the overall sire effects (Vsire) and the variance component of the interaction between sire and sex (VSIRE × SEX). We obtained these estimates by a single restricted maximum likelihood ANOVA with sire and dam nested within sire as random effects, sex as a fixed effect, sire by sex, and dam nested within sire by sex as factors (42, 43). We estimated the intersex genetic correlation as:
with variances computed as in Astles et al. (43).
We calculated standard errors for heritabilities as suggested by Lynch and Walsh (42) for unbalanced designs. Standard errors for intrasexual genetic correlations were calculated using the jackknife because there is considerable debate and no consensus over the best estimates for standard errors and statistical significance associated with genetic correlations (40, 42). There is no agreed upon or suggested solution for standard error associated with our estimates of intersexual correlations (42, 43). However, as is usual with studies of genetic correlations (42, 43), our goal here is to describe general patterns of covariance rather than to make point estimates.
Acknowledgments.
We thank C. J. Bird and C.M. House for assistance with experiments; B. H. Bleakley, J. D. Blount, J. Hunt, T. Moore, N. J. Royle, and T. Tregenza for comments on the manuscript; B. Brodie, T. Moore, and R. Preziosi for enlightening discussions; and 3 anonymous referees for extensive and helpful reviews. This project was funded by a grant from the Natural Environment Research Council (to A.J.M. and P.T.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Clutton-Brock TH. The Evolution of Parental Care. Princeton: Princeton Univ Press; 1991. [Google Scholar]
- 2.Reynolds JD, Goodwin NB, Freckleton RP. Evolutionary transitions in parental care and live bearing in vertebrates. Phil Trans R Soc London B. 2002;357:269–281. doi: 10.1098/rstb.2001.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itzkowitz M, Santangelo N, Richter M. Parental division of labour and the shift from minimal to maximal role specializations: an examination using a biparental fish. Anim Behav. 2001;61:1237–1245. [Google Scholar]
- 4.Emlen ST, Wrege PH. Division of labour in parental care behaviour of a sex-role-reversed shorebird, the wattled jacana. Anim Behav. 2004;68:847–855. [Google Scholar]
- 5.del Hoyo J, Elliot A, Sargatal J. Handbook of the Birds of the World. New World Vultures to Guinea Fowl. Vol 2. Barcelona: Lynx Edicions; 1994. [Google Scholar]
- 6.Houston AI, Székely T, McNamara JM. Conflict between parents over care. Trends Ecol Evol. 2005;20:33–38. doi: 10.1016/j.tree.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Queller DC. Why do females care more than males? Proc Roy Soc London B. 1997;264:1555–1557. [Google Scholar]
- 8.Kokko H, Jennions M. It takes two to tango. Trends Ecol Evol. 2003;18:103–104. [Google Scholar]
- 9.Fisher RA. The Genetical Theory of Natural Selection. 2nd Ed. New York: Dover Publications; 1958. [Google Scholar]
- 10.Lande R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution. 1980;34:292–305. doi: 10.1111/j.1558-5646.1980.tb04817.x. [DOI] [PubMed] [Google Scholar]
- 11.Lande R. In: Sexual Selection: Testing the Alternatives. Andersson MB, Bradbury JW, editors. New York: John Wiley & Sons; 1987. pp. 83–94. [Google Scholar]
- 12.Schluter D. Adaptive radiation along genetic lines of least resistance. Evolution. 1996;50:1766–1774. doi: 10.1111/j.1558-5646.1996.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 13.Freeman-Gallant CR, Rothstein MD. Apparent heritability of parental care in savannah sparrows. Auk. 1999;116:1132–1136. [Google Scholar]
- 14.MacColl AD, Hatchwell BJ. Heritability of parental effort in a passerine bird. Evolution. 2003;57:2191–2195. doi: 10.1111/j.0014-3820.2003.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 15.Smiseth PT, Dawson C, Varley E, Moore AJ. How do caring parents respond to mate loss? Differential response by males and females. Anim Behav. 2005;69:551–559. [Google Scholar]
- 16.Eggert AK, Müller JK. In: Social Behavior in Insects and Arachnids. Choe JC, Crespi BJ, editors. Cambridge, UK: Cambridge Univ Press; 1997. pp. 216–236. [Google Scholar]
- 17.Scott MP. The ecology and behaviour of burying beetles. Ann Rev Ent. 1998;43:595–618. doi: 10.1146/annurev.ento.43.1.595. [DOI] [PubMed] [Google Scholar]
- 18.Smiseth PT, Moore AJ. Behavioural dynamics between caring males and females in a beetle with facultative biparental care. Behav Ecol. 2004;15:612–628. [Google Scholar]
- 19.Lock JE, Smiseth PT, Moore AJ. Selection, inheritance and the evolution of parent-offspring interactions. Am Nat. 2004;164:13–24. doi: 10.1086/421444. [DOI] [PubMed] [Google Scholar]
- 20.Eggert AK, Reinking M, Müller JK. Parental care improves offspring survival and growth in burying beetles. Anim Behav. 1998;55:97–107. doi: 10.1006/anbe.1997.0588. [DOI] [PubMed] [Google Scholar]
- 21.Kölliker M, Brinkhof MWG, Heeb P, Fitze PS, Richner H. The quantitative genetic basis of offspring solicitation and parental response in a passerine bird with biparental care. Proc R Soc London B. 2000;267:2127–2132. doi: 10.1098/rspb.2000.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agrawal AF, Brodie ED, 3rd, Brown J. Parent-offspring coadaptation and the dual genetic control of maternal care. Science. 2001;292:1710–1712. doi: 10.1126/science.1059910. [DOI] [PubMed] [Google Scholar]
- 23.Hunt J, Simmons LW. The genetics of maternal care: Direct and indirect genetic effects on phenotype in the dung beetle Onthophagus taurus. Proc Natl Acad Sci USA. 2002;99:6828–6832. doi: 10.1073/pnas.092676199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hager R, Johnstone RA. The genetic basis of family conflict resolution in mice. Nature. 2003;421:533–535. doi: 10.1038/nature01239. [DOI] [PubMed] [Google Scholar]
- 25.Curley JP, Barton S, Surani A, Keverne EB. Coadaptation in mother and infant regulated by a paternally expressed imprinted gene. Proc R Soc London B. 2004;271:1303–1309. doi: 10.1098/rspb.2004.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maestripieri D. Genetic aspects of mother-offspring conflict in rhesus macaques. Behav Ecol Sociobiol. 2004;55:381–387. [Google Scholar]
- 27.Merilä J, Sheldon BC, Kruuk LEB. Explaining stasis: Microevolutionary studies in natural populations. Genetica. 2001;112–113:119–222. [PubMed] [Google Scholar]
- 28.Kölliker M, Richner H. Parent-offspring conflict and the genetics of offspring solicitation and parental response. Anim Behav. 2001;62:395–407. [Google Scholar]
- 29.Nakagawa S, Gillespie DOS, Hatchwell BJ, Burke T. Predictable males and unpredictable females: Sex difference in repeatability of parental care in a wild bird population. J Evol Biol. 2007;20:1674–1681. doi: 10.1111/j.1420-9101.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- 30.Thomas GH, Székely T, Reynolds JD. Sexual conflict and the evolution of breeding systems in shorebirds. Adv Study Behav. 2007;37:270–342. [Google Scholar]
- 31.Cheverud JM, Moore AJ. In: Quantitative Genetics Studies of Behavioral Evolution. Boake CRB, editor. Chicago: Univ Chicago Press; 1994. pp. 67–100. [Google Scholar]
- 32.Wolf JB, Brodie ED., III The coadaptation of parental and offspring characters. Evolution. 1998;52:299–308. doi: 10.1111/j.1558-5646.1998.tb01632.x. [DOI] [PubMed] [Google Scholar]
- 33.Wolf JB, Brodie ED, 3rd, Moore AJ. Interacting phenotypes and the evolutionary process. II. selection resulting from social interactions. Am Nat. 1999;153:254–266. doi: 10.1086/303168. [DOI] [PubMed] [Google Scholar]
- 34.Kölliker M, Brodie ED, 3rd, Moore AJ. The coadaptation of parental supply and offspring demand. Am Nat. 2005;166:506–516. doi: 10.1086/491687. [DOI] [PubMed] [Google Scholar]
- 35.Beeler AE, Rauter CM, Moore AJ. Pheromonally-mediated mate attraction by males of the burying beetle Nicrophorus orbicollis: Alternative calling tactics conditional on both intrinsic and extrinsic factors. Behav Ecol. 1999;10:578–584. [Google Scholar]
- 36.Müller JK, Eggert AK, Sakuluk SK. Carcass maintenance and biparental care in burying beetles: Are males redundant? Ecol Ent. 1998;23:195–200. [Google Scholar]
- 37.Eggert AK. Alternative male mate-finding tactics in burying beetles. Behav Ecol. 1992;3:243–254. [Google Scholar]
- 38.Müller JK, Braunisch V, Hwang W, Eggert AK. Alternative tactics and individual reproductive success in natural associations of the burying beetle, Nicrophorus vespilloides. Behav Ecol. 2006;18:196–203. [Google Scholar]
- 39.Smiseth PT, Darwell CT, Moore AJ. Partial begging: An empirical model for the early evolution of offspring signaling. Proc R Soc London B. 2003;270:1773–1777. doi: 10.1098/rspb.2003.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roff DA. Introduction to Computer-Intensive Methods of Data Analysis. Cambridge, UK: Cambridge Univ Press; 2006. [Google Scholar]
- 41.Houle D. Comparing evolvability and variability of quantitative traits. Genetics. 1992;130:195–204. doi: 10.1093/genetics/130.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- 43.Astles PA, Moore AJ, Preziosi RF. A comparison of methods to estimate cross-environment genetic correlations. J Evol Biol. 2006;19:114–122. doi: 10.1111/j.1420-9101.2005.00997.x. [DOI] [PubMed] [Google Scholar]