Neuronal networks in the brain oscillate in various frequency bands, and such oscillations can be detected by observing local field potential or EEG activity (1). Oscillations in the gamma frequency band (30–80 Hz) have drawn special attention because of their link to a variety of cognitive processes including sensory binding (2), attention selection (3), and memory (4, 5). Of particular physiological interest are questions of how gamma oscillations are generated and synchronized across brain regions. The study by Middleton et al. in this issue of PNAS (6) provides novel insights into these questions: it identifies 2 subcircuits within the entorhinal cortex (EC) that are capable of generating gamma oscillations at different frequencies. Moreover, it presents data implying that these generators recruit different neuronal pathways in their communication with the hippocampus. Their study points out for the first time the specialized role of inhibitory interneuron types in generating oscillatory patterns at different frequencies. Given that both the hippocampo–EC system and gamma oscillations are linked to memory processing, the temporal interactions between the EC and the hippocampus at these distinct gamma frequencies could be involved in different aspects of mnemonic processes.
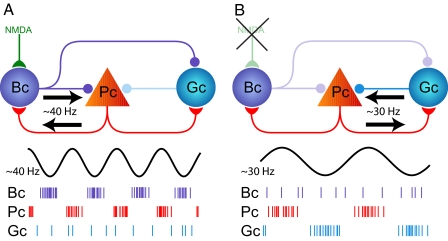
The report by Middleton et al. (6) examines the mechanism behind how gamma oscillations are generated in the EC with an emphasis on the effect of the NMDA receptor activation: a key glutamatergic receptor for synaptic plasticity and memory formation. Using an in vitro preparation of the EC, they demonstrate that gamma oscillations slow down when NMDA receptors (NMDARs) are blocked by ketamine (an NMDAR antagonist). They then show that NMDA-dependent (fast, ≈40 Hz) and NMDA-independent (slow, ≈30 Hz) gamma rhythms are generated by functionally distinct subcircuits (Fig. 1). Both subcircuits consist of EC excitatory principal cells and inhibitory interneurons whose reciprocal interactions lead to oscillations. However, Middleton et al. find that these subcircuits include 2 distinct types of interneurons. The interneurons participating in the first subcircuit are the basket cells whose cell bodies are located in the layer II of the EC. NMDAR activation leads to a prominent tonic excitation of these basket cells that interact with principal cells to generate ≈40-Hz gamma rhythm (Fig. 1A). These basket cells, however, have another function: they suppress the activity of interneurons in the second subcircuit. The cell bodies of these latter interneurons are located in layer III of the EC and are called “goblet” cells, newly-discovered by the Middleton et al. study (6). In the absence of NMDAR-mediated excitation in the EC, the decreased spiking activity of the basket interneurons reduces the inhibition they exert on goblet cells. The release of goblet cells from inhibition allows them to participate in the reciprocal interaction with principal cells (Fig. 1B), leading to the generation of the second gamma rhythm at a lower frequency (≈30 Hz). Based on the results of the experiments, a biophysical model was created that confirmed that these 2 types of gamma oscillations can be generated by using the microcircuit architecture described above. Finally, the Middleton et al. study (6) shows that hippocampal regions in vitro have a selective preference for slow or fast gamma oscillations: whereas the CA1 region preferentially oscillates with ≈40-Hz oscillations, the CA3 region prefers slower ≈30-Hz oscillations. Hence NMDA-dependent EC gamma oscillations may preferentially synchronize with the CA1 region, whereas NMDA-independent EC gamma oscillations may recruit coherent oscillations with the CA3 region.
Fig. 1.
Schematic illustration of the gamma-generating EC microcircuit described in Middleton et al.'s study (6). The circuit is composed of 2 subcircuits: the principal cell (Pc)–basket cell (Bc) and the principal cell–goblet cell (Gc) loops. These 2 subcircuits generate mutually exclusive ≈40- and ≈30-Hz gamma oscillations. (Upper) Illustration of the microcircuit. (Lower) Raster plots illustrate the firing of the different cell types during gamma oscillations. (A) Fast (≈40 Hz) gamma oscillations are generated through the recurrent interactions between basket cells and principal cells. Such interaction requires NMDAR-mediated depolarization of basket cells. Basket cells inhibit goblet cells, preventing the activation of the pyramidal cell–goblet cell loop. (B) Slow (≈30 Hz) gamma oscillations are generated similarly by goblet cells and principal cells. In the absence of NMDAR-mediated excitation, the spiking activity of basket cells is reduced, releasing goblet cells to interact with principal cells.
The surprising finding in the Middleton et al. study (6) is that 2 gamma patterns are generated locally by 2 different types of interneurons. Previous work in vivo has demonstrated that hippocampal GABAergic interneuron types exhibit differentiable firing patterns in relation to network oscillations, indicating that they have specialized roles in controlling various oscillations in the brain (7). Similarly, previous work in vitro has demonstrated that both interneuron—interneuron and pyramidal cell–interneuron interactions can generate the gamma rhythm in the hippocampus, a result predicted by modeling work (8–12). The important finding of Middleton et al.'s study is the demonstration that certain interneurons have a specialized role in generating an oscillation at a certain frequency band. Moreover, their work also highlights that interneuron–interneuron interactions could regulate which rhythm generator subcircuit is activated at a given time. Because gamma patterns described in slice studies have been shown to share the same features as the ones present in vivo (12, 13), Middleton et al.'s study lays the foundation for future work in behaving animals.
In behaving animals, previous work has identified 2 distinct gamma-generating circuits in the hippocampus–EC system: one in the CA3–CA1 regions generating gamma oscillations locally, and another one in dentate gyrus driven by the EC (13). It is quite likely that the mechanisms by which gamma oscillatory patterns are generated in vivo are more complex than have been described so far. More detailed analysis will require simultaneous recordings in different subfields of the hippocampus and the EC to examine the details of gamma synchronization across these regions. Moreover, further investigation is needed to test whether gamma subbands (e.g., ≈30 and ≈40 Hz) exhibit differences in network synchronization and whether 2 distinct gamma patterns occur during in vivo physiological conditions within the EC.
What could be the functional significance of the 2 distinct EC gamma rhythms? Learning and memory are known to emerge from complex interactions among distributed brain networks. As the interface between the hippocampus and the neocortex, the EC occupies a critical gateway for the processing and transfer of information related to episodic and spatial memories. In this context Middleton et al.'s study (6) proposes that NMDAR activation could control the nature of the temporal interaction between the EC and the hippocampus by switching from one gamma pattern to another. Thus, NMDAR activation may govern the proportion by which each pathway is recruited to process information during, for example, the rapid acquisition of new information versus the retrieval of previously learned ones. Indeed, there are both human and animal studies suggesting that gamma oscillations in the hippocampus have such a role in memory processing, particularly in short-term memory (4, 5, 14). Interestingly, gamma oscillations are often nested within slower theta-band (4–12 Hz) oscillations (15). Thus, it has been proposed that memory sequences can be encoded by the firing patterns of cells in successive gamma cycles nested within each theta cycle (16). Recently, it was revealed in the hippocampus of behaving animals that gamma-related firing during theta oscillations can support the formation of such a temporal code (17). However, 2 distinct populations of hippocampal principal cells were differentiated in the CA1 region, representing movement trajectories and places, which each support different types of population codes within a theta cycle. Although the coding of movement trajectories may require the CA3 region (18), location representations could originate directly from the EC (19). Thus, the dual gamma generators described in the EC by Middleton et al. (6) and their differential communication with the CA3 and CA1 regions could support the 2 forms of coding observed.
Hippocampal regions in vitro have a selective preference for slow or fast gamma oscillations.
Acknowledgments.
We thank B. Micklem for preparing Fig. 1.
Footnotes
The authors declare no conflict of interest.
See companion article on page 18572.
References
- 1.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 2.Engel AK, Fries P, Singer W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 3.Fries P, Neuenschwander S, Engel AK, Goebel R, Singer W. Rapid feature selective neuronal synchronization through correlated latency shifting. Nat Neurosci. 2001;4:194–200. doi: 10.1038/84032. [DOI] [PubMed] [Google Scholar]
- 4.Howard MW, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs EC, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Middleton S, et al. NMDA receptor-dependent switching between different gamma rhythm-generating microcircuits in entorhinal cortex. Proc Natl Acad Sci USA. 2008;105:18572–18577. doi: 10.1073/pnas.0809302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 9.Traub RD, Whittington MA, Stanford IM, Jefferys JG. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- 10.Traub RD, et al. A model of gamma-frequency network oscillations induced in the rat CA3 region by carbachol in vitro. Eur J Neurosci. 2000;12:4093–4106. doi: 10.1046/j.1460-9568.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 11.Borgers C, Epstein S, Kopell NJ. Background gamma rhythmicity and attention in cortical local circuits: A computational study. Proc Natl Acad Sci USA. 2005;102:7002–7007. doi: 10.1073/pnas.0502366102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oren I, Mann EO, Paulsen O, Hajos N. Synaptic currents in anatomically identified CA3 neurons during hippocampal gamma oscillations in vitro. J Neurosci. 2006;26:9923–9934. doi: 10.1523/JNEUROSCI.1580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci USA. 2007;104:14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bragin A, et al. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 17.Senior TJ, Huxter JR, Allen K, O'Neill J, Csicsvari J. Gamma oscillatory firing reveals distinct populations of pyramidal cells in the CA1 region of the hippocampus. J Neurosci. 2008;28:2274–2286. doi: 10.1523/JNEUROSCI.4669-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL. Organization of hippocampal cell assemblies based on theta phase precession. Hippocampus. 2006;16:785–794. doi: 10.1002/hipo.20202. [DOI] [PubMed] [Google Scholar]
- 19.Brun VH, et al. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]