Abstract
Categorical perception (CP) of color is the faster and more accurate discrimination of two colors from different categories than two colors from the same category, even when same- and different-category chromatic separations are equated. In adults, color CP is lateralized to the left hemisphere (LH), whereas in infants, it is lateralized to the right hemisphere (RH). There is evidence that the LH bias in color CP in adults is due to the influence of color terms in the LH. Here we show that the RH to LH switch in color CP occurs when the words that distinguish the relevant category boundary are learned. A colored target was shown in either the left- or right-visual field on either the same- or different-category background, with equal hue separation for both conditions. The time to initiate an eye movement toward the target from central fixation at target onset was recorded. Color naming and comprehension was assessed. Toddlers were faster at detecting targets on different- than same-category backgrounds and the extent of CP did not vary with level of color term knowledge. However, for toddlers who knew the relevant color terms, the category effect was found only for targets in the RVF (LH), whereas for toddlers learning the color terms, the category effect was found only for targets in the LVF (RH). The findings suggest that lateralization of color CP changes with color term acquisition, and provide evidence for the influence of language on the functional organization of the brain.
Keywords: visual field, color perception
The influence of color language on color perception and cognition has been debated for many decades (1, 2). One argument is that the color lexicon, in dividing the spectrum of color into discrete categories, changes perceptual differences among colors so that colors from the same linguistic category appear more similar than colors from different categories (3). There is converging support for this “Whorfian” hypothesis that language affects color perception. For example, categorical perception (CP) of color—faster or more accurate discrimination between two colors from different categories than two colors from the same category of an equivalent chromatic separation (4)—is only found in adult speakers if their color lexicon marks the categorical difference (3, 5, 6). Moreover, color CP is lateralized to the “language dominant” left hemisphere (LH) in adults (7–11) and LH CP is eliminated by verbal but not visual interference (7), both of which imply linguistic involvement in CP.
Despite the overwhelming evidence that color CP in adults depends on language, there is also evidence that color CP can be language independent. This comes from a series of developmental studies that find that infants as young as 4 months respond categorically to color on a range of tasks and across a range of color category boundaries (9, 12–16). However, this prelinguistic CP, in contrast to the LH-lateralized color CP in adults, appears to be lateralized to the right hemisphere (RH) (9). One interpretation of this finding is that two forms of color CP exist: a lexicalized form of CP that is predominantly LH based, and a nonlexicalized form of CP that is predominantly RH based. If this interpretation is correct, the RH to LH switch in color CP should occur when the words that distinguish the color category boundary are learned. Previous research with toddlers at the stage of color term acquisition indicates that category effects are found irrespective of color term knowledge (17). However, even though the extent of CP does not appear to change with color language learning, the lateralization of CP may. The current investigation tests this hypothesis by assessing lateralization of color CP in toddlers who are learning or who have already learned color terms.
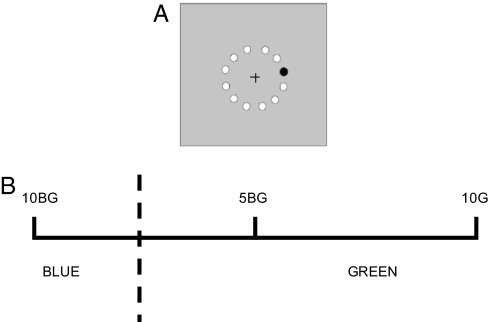
Toddlers between the ages of 2 and 5 years were tested by using a design and task identical to previous studies of lateralized CP (8, 9). A colored target appeared in the left or right visual field on a colored background that was either from the same color category (within-category) or from a different category (between-category) from the target (Fig. 1A). The hue separation for within- and between-category conditions was equated by using the Munsell color system and stimuli were from the blue-green region. Color CP is exhibited if targets are detected faster on between-category backgrounds than on within-category backgrounds. Lateralized color CP is exhibited if the category difference is greater for targets in one visual field than the other. In previous studies, both infants and adults show blue-green CP on this task. For adults the effect is stronger when targets appear in the RVF (LH) than the LVF (8, 9).* In contrast, for infants the effect is only found for targets in the LVF (RH; 9).†
Fig. 1.
Characteristics of the stimulus display and stimuli. (A) Illustration of the display. Black circle shows target, white circles show other possible target locations. (B) Munsell codes of the stimuli; stimuli varied in hue at constant value and chroma. Hue separations were five Munsell hue units apart. The target was either in the same color category as the background (5BG and 10BG, both green) or in the adjacent category (5BG and 10G, green and blue). The dashed line indicates the category boundary.
In the first study that used the target detection task to test for lateralized color CP (8), adults indicated whether the target appeared on the left or the right, and reaction time was analyzed. Because infants are unable to respond in this way, the subsequent study that tested both infants and adults (9) used a different measure. Eye movements were recorded and, after central fixation, the time that elapsed between target onset and the initiation of eye movement toward the target was measured. Because participants were centrally fixated until the initiation of the eye-movement, the target was lateralized for the duration of the measure. Adults tested with the eye movement initiation time measure showed a LH color CP bias, as in previous reaction time studies, confirming the suitability of the measure for studying lateralization effects. In the current study, because toddlers are also unable to reliably indicate the spatial location of a briefly presented target, the eye movement initiation time measure was used again. To account for changes in chromatic sensitivity across the lifespan (19), stimuli had a smaller hue difference than those used for infants and a larger hue difference than those used for adults, but all other aspects of the task remained the same.
To assess whether lateralization of color CP varies with color term knowledge, toddlers were also tested on color naming and comprehension tasks. Color naming tasks assessed ability to name colors with the appropriate term, whereas color comprehension tasks assessed ability to identify colors that match a given term. If a child has systematic knowledge of a term then they should be accurate on both tasks (20). As CP was tested across the blue-green boundary, knowledge of the terms blue and green was assessed. If a color term is reliably known, then that term should be applied or responded to correctly for the best example (the focal stimulus) of the category and for other category exemplars. Therefore, color term knowledge was assessed for focal blue and green as well as the nonfocal blue and green stimuli used in the target detection task. Another criterion for knowing a color term is that it is used only for exemplars from that category and is not applied to other color categories (20). Such over-extensions are common in children just starting to use color terms (20, 21). Therefore, color term knowledge for the focals of the other basic chromatic categories (yellow, red, pink, orange, purple, and brown) was also assessed. A previous in-depth and systematic study of color term acquisition found that the toddlers tested acquired reliable knowledge of blue and green terms ≈37 months (20). Therefore, within the age range of 2–5 years, it was expected that there would be a group of toddlers who were learning these terms, and a group of toddlers who had mastered both of these terms. Toddlers were divided into two groups based on their naming and comprehension, and the pattern of lateralized color CP was compared for the two groups of toddlers.
Results
Naming and Comprehension.
Each of the naming and comprehension tasks provided a measure of the number of blue and green stimuli correctly named and identified. These four measures were entered as variables into a principal components analysis. The four measures loaded onto one component that explained 70.2% of the variance on the measures. Weights on this component were calculated for each toddler. Inspection of weights revealed two distinct groups of toddlers: those with negative or minimal weights (8 males and 11 females) and those with large positive weights (5 males and 13 females). Table 1 gives accuracy scores on each of the four measures and the accuracy scores for naming and identifying the non-blue and non-green focal stimuli for the two groups of toddlers.
Table 1.
Number of correct responses for the naming and comprehension measures (target detection stimuli/focal blue and green/other focals), for toddlers with large positive weights and negative or minimal weights, on a component identified by a principal components analysis that explained 70.2% of the variance in color naming and comprehension
| Measure | +ve weights | −ve weights |
|---|---|---|
| Naming target detection blue and green, max = 3 | 2.89 (0.32) | 0.58 (0.77) |
| Identifying target detection blue and green, max = 3 | 2.89 (0.32) | 1.47 (0.96) |
| Naming focal blue and green, max = 2 | 2.00 (0.00) | 0.84 (0.60) |
| Identifying focal blue and green, max = 2 | 2.00 (0.00) | 1.37 (0.68) |
| Other focal stimuli named, max = 6 | 5.72 (0.57) | 3.58 (1.46) |
| Other focal stimuli identified, max = 6 | 5.89 (0.47) | 2.95 (1.27) |
Standard deviations are given in parentheses.
As can be seen in Table 1, toddlers with positive weights were largely accurate at naming and identifying blue and green target detection stimuli (2 mistakes were made by 2 toddlers) and completely accurate in naming and identifying blue and green focal stimuli. These toddlers never used blue and green terms when asked to name other focal stimuli, and never pointed to blue and green focals inappropriately. These toddlers also had largely accurate naming and comprehension of focals for other color categories. Toddlers with negative or minimal weights made many mistakes when naming and identifying focal blue and green and target detection stimuli and made mistakes at naming and identifying other focal colors. On the basis of this analysis, the toddlers with positive weights were deemed to have acquired an accurate and appropriate understanding and use of the terms “blue” and “green,” not only for focal stimuli, but also for other exemplars of the two categories, and were therefore classified as “Namers.” Those toddlers with negative or minimal weights were deemed to be learning how to understand and apply the color terms “blue” and “green” appropriately, and were therefore classified as “Learners.” The mean age of the Learners was 32 months (SD = 7.5), and the mean age of the Namers was 46 months (SD = 3.8).
Target Detection.
Trials were excluded if the eye-movement signal was lost, (mean number of trials lost per toddler = 4.95, SD = 3.47), if multiple eye movements around the screen were made before the eye movement to the target (mean number of trials lost per toddler = 2.58, SD = 2.29), or if the target was not fixated at all (mean number of trials lost per toddler = 3.42, SD = 3.56). This left on average 21.1 trials per toddler (SD = 5.47), and all toddlers had at least 2 trials per condition. The time taken to initiate an eye movement to the target was calculated as the time from target onset (central fixation) up until the start of the eye movement to the target (as in 9). Table 2 gives the median initiation time for within- and between-category conditions for LVF and RVF targets, averaged for Learners and Namers.
Table 2.
Median initiation times (SD) for within- and between-category target detection of LVF or RVF targets, averaged for Learners and Namers
| LVF | RVF | ||
|---|---|---|---|
| Learners | Within-category | 625 (315) | 520 (150) |
| Between-category | 408 (97) | 467 (112) | |
| Namers | Within-category | 498 (187) | 502 (117) |
| Between-category | 454 (163) | 428 (79) |
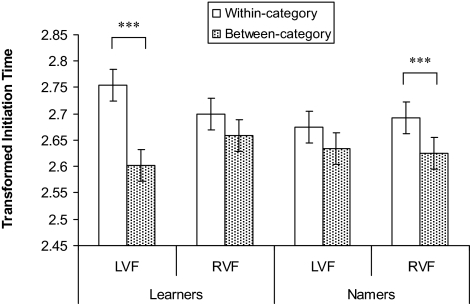
Data were positively skewed for all conditions (largest skew Z = 4.90 < 1.96; Shapiro-Wilk (37) = 0.73, P < 0.001), and therefore data were log transformed. Fig. 2 gives the transformed data for the median initiation time (ms) for each visual field, and for within- and between-category conditions. A three-way repeated measures ANOVA with category (within/between), visual field (left/right), and group (Learners/Namers) was conducted on the transformed time to initiate measure. Responses were faster to between-category targets (mean = 2.71, SD = 0.11) than to within-category targets (mean = 2.63, SD = 0.94), [F (1, 35) = 14.43, MSE = .015, P < 0.005, ηp2 = .29]. There was no effect of visual field or group (largest F = .61, smallest P = 0.44), and no significant two-way interactions (largest F = 1.16, smallest P = 0.29). The three-way interaction was significant, [F (1, 35) = 5.11, MSE = .009, P < 0.05, ηp2 = .13], and reflected a significant category effect for the Learners in the LVF [t (18) = 3.77, P < 0.005] but not the RVF [t (18) = 1.20, P = 0.25], and for the Namers in the RVF [t (17) = 3.29, P < 0.005] but not the LVF, [t (17) = 0.98, P = 0.34].
Fig. 2.
Between-category targets are detected faster than within-category, yet only when targets appear in the LVF for those learning blue and green color terms (Learners), and only when targets appear in the RVF for those who have learned blue and green color terms (Namers). The dependent measure is the log transformation of initiation time (ms), and error bars are within-subjects 95% confidence intervals calculated by using the error term from the three-way interaction (22). ***, indicates significant difference at P < 0.005.
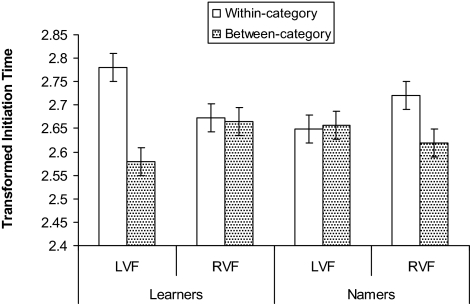
As the Learners were on average younger than the Namers, the pattern of lateralization of category effects for the two groups may be due to age rather than color term knowledge. However, when age was added as a covariate to the above ANOVA the pattern and significance of the three-way interaction was preserved, [F (1, 34) = 11.73, MSE = .008, P < 0.005, ηp2 = .26], and it can be seen in Fig. 3 that even when the variation in age is statistically accounted for, the pattern of lateralization of category effects for the two groups remains.
Fig. 3.
When age was added as a covariate to the analysis, the pattern of lateralization of CP for the Namers and Learners remained. As in Fig. 2, there is a category effect only for targets in the LVF for Learners and only for targets in the RVF for Namers. The dependent measure is the estimated means of the log transformation of initiation time (ms), estimated for when variance due to age is accounted for. Error bars are within-subjects 95% confidence intervals calculated by using the error term from the three-way interaction (22).
General Discussion.
Toddlers were faster at detecting targets on between-category than within-category backgrounds, and the extent of the blue-green CP was not different for toddlers who were learning the terms for blue and green compared with toddlers who had learned these terms. This replicates a previous study that found that the extent of color CP in toddlers was not affected by color term acquisition (17). The current study found that what did vary with color naming was the lateralization of the category effect. For toddlers with incomplete knowledge of terms blue and green, CP was found only for targets in the LVF (RH). For toddlers who could use blue and green terms with a high degree of accuracy, reliability, and appropriateness, CP was found only for targets in the RVF (LH). We therefore find the same pattern of lateralization for toddlers still learning blue and green terms as for prelinguistic infants (RH CP), and the same pattern of lateralization for toddlers with blue and green color term knowledge as for adults (LH CP).
Consideration needs to be given as to whether the apparent RH to LH “switch” in lateralization of color CP that occurs between the ages of two and five is attributable to color term learning or whether other factors may explain this pattern of results. Those who had learned the terms for blue and green were generally (although not always) older than those who were still learning the terms. However, when the variation due to age was statistically controlled, the pattern of lateralization of CP for the two groups of toddlers remained the same. Additionally, there is at present no clear language-independent account for why color CP should be RH lateralized in the younger toddlers but LH lateralized in the older toddlers.‡ However, further studies can be conducted to confirm that the changes in lateralization of color CP found in the current study are due to color term knowledge rather than age related structural differences in the brain. For example, the age of color term acquisition is variable across cultures (25), so with cross-cultural investigation it would be possible to hold age constant while comparing two groups of toddlers with different levels of color term knowledge. It is of course also important to see whether the changes in lateralization of color CP around the time of color term acquisition extend to other color category boundaries.
The findings of the current study are consistent with the hypothesis that there is a form of lexicalized color CP that is lateralized to the LH after color term acquisition and a form of nonlexicalized color CP that is lateralized to the RH before color term acquisition. If further support for this hypothesis is provided then there are several issues that need to be clarified. First, what categories are prelinguistically available in the RH? How do these categories compare extensionally to linguistic color categories, and is their extension governed by similar forces? Recent work suggests that linguistic color category systems across languages near-optimally partition the irregular surface of perceptual color space, in that they tend to maximize similarity within categories, and minimize it across categories (26, 27). An appealing but unexplored possibility is that prelinguistic categories in the RH are shaped by similar forces.
Second, does the apparent RH to LH “switch” in lateralization of color CP, seen when color words are learned, indicate a permanent loss of color CP in the RH? Or does color CP persist in the RH, albeit normally suppressed by language, to reappear when lexical color codes are not activated? Examples of RH color CP in adults (8, 11), may seem to indicate that RH CP persists in some circumstances after color term acquisition. However, RH CP of color in adults is weaker than LH CP (8), and becomes stronger with long reaction times (11), suggesting an origin not in the RH itself but rather in cross-callosal transfer from the LH. Importantly, there is LH but not RH CP in two callosotomy patients whose corpus callosum had been surgically severed (7, 28). The suppression hypothesis predicts that these patients would exhibit RH CP, because the severing of the corpus callosum would remove the possibility of LH linguistic suppression. Therefore, the lack of RH CP in callosotomy patients supports the hypothesis that there is a permanent loss of RH CP after color term acquisition. Further research is needed to verify whether the onset of language leads to loss, or mere suppression, of color CP in the RH—and if it is loss, as currently appears, by what mechanism that loss occurs.
Nonetheless, the present findings clarify the debate over the contribution of language to color CP. It appears that there is a prelinguistic RH substrate for categorical responding to color, that is replaced or suppressed by mechanisms of language when color terms are learned. Consequently, the research also has implications for our understanding of the effect of language acquisition on the brain. The influence of language learning on the functional organization of the brain has been demonstrated in studies that have found changes in lateralization of language-related brain activity that appear to be related to language proficiency in infants and toddlers (29). Here, we suggest that knowing a word (in this case color words) may also change the lateralization of the relevant perceptual categories. Considering the pervasive nature of categorization from infancy onwards (30), there is ample scope to see whether these effects generalize to CP and categorization in other domains. Such research will have implications for our understanding of the nature of categorization across development and the interaction between language and cognition (31, 32).
Materials and Methods
Participants.
Forty-nine toddlers (2–5 years) took part in the study. Of these, 12 were not included in the final study due either to excessive head movement preventing accurate calibration (6 toddlers) or to insufficient trials completed for one or more of the conditions (6 toddlers). The mean age of the final sample was 39.08 months (SD = 9.12); there were 24 females and 13 males. None of the toddlers had a family history of color vision deficiency.
Apparatus and Experimental Set Up.
The apparatus and experimental set up was identical to that in Franklin et al. (9). Stimuli were displayed on a Sony Trinitron CRT monitor (model GDM-F520). Toddlers sat 50 cm away and at eye level to the monitor in a dark room. An ASL 504 pan/tilt eye-tracking camera, recording at 50 Hz, tracked eye movements at 0.5° accuracy. A video of what the participant was shown, with the eye movement output superimposed, was recorded. This video was digitized by using an analogue to digital video converter (Canopus ADVC-300) and digital video was analyzed by using i-Movie 2.1.2 software.
Stimuli.
As shown in the top half of Fig. 1B, the stimuli for the target detection task varied only in Munsell hue, with Munsell value and chroma kept constant (value = 6, chroma = 8). Adjacent stimuli were separated by 5 Munsell hue units and straddled the blue-green boundary (7.5BG)§. Two stimuli were green (10G and 5BG) and the third was blue (10BG): see Table 3 for the CIE, 1931, Y,x,y chromaticity coordinates of these Munsell codes when emulated on the monitor. The chromaticity coordinates were verified with a Cambridge Research Systems ColorCal colorimeter.
Table 3.
CIE (1931), Y,x,y chromaticity coordinates of the target detection stimuli
| Stimulus | Y | x | y |
|---|---|---|---|
| 10G6/8 | 19.47 | 0.242 | 0.368 |
| 5BG6/8 | 19.47 | 0.224 | 0.331 |
| 10BG6/8 | 19.47 | 0.212 | 0.295 |
| Gray | 19.47 | 0.326 | 0.335 |
White point of monitor as measured on screen: Y = 64.80 cd/m2, x = 0.326, y = 0.335. The stimuli emulated a reflectance of 30.05.
The target detection stimuli were also used for the naming and comprehension tasks, as well as a set of stimuli that were the best examples (focals) of the 8 chromatic categories (33) within the constraints of gamut of the monitor. See Table 4 for CIE, 1931, Y,x,y chromaticity coordinates.
Table 4.
CIE (1931), Y,x,y chromaticity coordinates of the focal stimuli
| Stimulus name | Y | x | y |
|---|---|---|---|
| Red | 9.86 | 0.556 | 0.309 |
| Yellow | 48.54 | 0.464 | 0.435 |
| Green | 16.24 | 0.294 | 0.575 |
| Blue | 9.86 | 0.202 | 0.228 |
| Pink | 48.54 | 0.374 | 0.325 |
| Purple | 5.38 | 0.277 | 0.171 |
| Orange | 35.37 | 0.525 | 0.417 |
| Brown | 5.38 | 0.497 | 0.391 |
Target Detection Design and Procedure.
The design and procedure of the target detection task were identical to those of (9). Adjacent stimuli in Fig. 1B formed within- and between-category pairs. One stimulus in a pair appeared as the target (diameter = 3 cm, visual angle of 3.5°), with the other stimulus as the background (40 × 30 cm), with this allocation reversed on half of the trials. There were 12 possible locations for the target that were arranged radially around a central fixation point to the left or to the right (see Fig. 1A). The location of the target in these 12 positions was randomized with the constraint that the target appeared equally on the left and on the right for within- and between-category conditions. There were 32 trials in total, with 16 trials each for within- and between-category conditions, and trials were presented in a randomized order. Each trial began once participants were centrally fixated (as indicated by eye-tracking output) on a looming and contracting black and white central attention getter. A gray screen (see Table 3 for chromaticity coordinates) was then presented for 250 ms, followed by presentation of the target and background for four seconds. Before the task commenced, the eye-tracker was calibrated for the participant by using a 2-point calibration procedure (9, 16).
Naming and Comprehension Design and Procedure.
After the target detection task, naming and comprehension were assessed for the target detection stimuli and the focals. Comprehension tasks were made into a game about pointing to the cartoon rabbit that was holding the balloon of a certain color. Stimuli within a set (target detection/focal) were presented simultaneously as oval balloon shapes in a random location on a gray background and all “balloons” were held by cartoon rabbits (see Table 1 for Y,x,y chromaticity coordinates). Toddlers were asked “which rabbit is holding the X balloon?” where X indicates a given color term. For the target detection stimuli there were two trials (X = blue or green), and on each trial children were also asked, “Are there any other rabbits holding the X balloon?” For the focal stimuli there were eight trials (X = each of the focals). Naming tasks were made into a game about naming the color of a cartoon rabbit's balloon. Stimuli within a set (target detection/focal) were presented simultaneously as oval balloon shapes in a random location on a gray background. A cartoon rabbit appeared to be holding one of the “balloons” and toddlers were asked “what color is the rabbit's balloon?” On each trial the rabbit held a different balloon, and the location of the balloons changed across trials. This led to three naming trials for the target detection stimuli, and eight for the focal stimuli. Naming and comprehension were always assessed for the target detection stimuli first, and comprehension was always assessed before naming.
Acknowledgments.
The authors thank the toddlers and their parents. We also thank Laura Bevis and Justine Cornforth for assistance with testing and recruitment and Nigel Woodger for technical support. Support from U.S. National Science Foundation grants 0418283 and 0418404 is also acknowledged.
Footnotes
The authors declare no conflict of interest.
On this task and others, a relatively weak LVF advantage for between-category discriminations in adults does sometimes occur but only in experiments where there are relatively long response times. This pattern suggests that cross-callosal transfer and/or scanning may be the cause of the apparent CP effect in the LVF, and consequently, that RVF/LH CP may be the only real phenomenon in normal adults (18).
In a lateralized CP study the visual field variable provides a control against possible stimulus bias. Thus, even if the chosen color space artificially reduces the cross boundary distance relative to the within distance (or vice versa), the differences between the two visual fields with respect to within versus between judgments remain valid.
The amount of myelination of the corpus callosum increases into early adulthood (23). Additionally, in posterior associative cortex there is greater RH than LH blood flow at one year and greater LH than RH blood flow after three years, possibly related to the emergence of visuo-spatial abilities (RH) and language abilities (LH) around those ages (24). However, there is currently no clear link between these changes in the brain and the changes in lateralization of color CP found here.
Munsell is a standardized color metric based on extensive psychophysical judgments and is commonly used in adult color category studies (4). The between- and within-category pairs were equated in this metric, as well as in the CIE (1976, L*u*v*) perceptual color metric (≈18ΔE).
References
- 1.Bornstein MH. Hue categorization and color naming: Physics to sensation to perception. In: Pitchford NJ, Biggam CP, editors. Progress in Colour Studies Volume II. Psychological Aspects. Amsterdam/Philadelphia: John Benjamins; 2006. pp. 35–68. [Google Scholar]
- 2.Bornstein MH. Hue categorization and color naming: Cognition to language to culture. In: MacLaury RM, Paramei GV, Dedrick D, editors. Anthropology of Colour: Interdisciplinary Multilevel Modeling. Amsterdam/Philadelphia: John Benjamins; 2008. pp. 3–27. [Google Scholar]
- 3.Kay P, Kempton W. What is the Sapir-Whorf hypothesis? Am Anthropol. 1984;86:65–79. [Google Scholar]
- 4.Bornstein MH, Korda NO. Discrimination and matching within and between hues measured by reaction times: Some implications for Categorical Perception and levels of processing. Psychol Res. 1984;46:207–222. doi: 10.1007/BF00308884. [DOI] [PubMed] [Google Scholar]
- 5.Roberson R, Davies IRL, Davidoff J. Color categories are not universal: Replications and new evidence from a stone-age culture. J Exp Psychol Gen. 2000;129:369–398. doi: 10.1037//0096-3445.129.3.369. [DOI] [PubMed] [Google Scholar]
- 6.Winawer J, et al. Russian blues reveal effects of language on color discrimination. Proc Natl Acad Sci USA. 2007;104:7780–7785. doi: 10.1073/pnas.0701644104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert AL, Regier T, Kay P, Ivry RB. Whorf hypothesis is supported in the right visual field not the left. Proc Natl Acad Sci USA. 2006;103:489–494. doi: 10.1073/pnas.0509868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drivonikou GV, et al. Further evidence that Whorfian effects are stronger in the right visual field than the left. Proc Natl Acad Sci USA. 2007;104:1097–1102. doi: 10.1073/pnas.0610132104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franklin A, et al. Categorical Perception of color is lateralized to the right hemisphere in infants, but to the left hemisphere in adults. Proc Natl Acad Sci USA. 2008;105:3221–3225. doi: 10.1073/pnas.0712286105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert AL. Berkeley: Univ of California; 2007. Lateralized effects of language on perception. PhD dissertation. [Google Scholar]
- 11.Roberson D, Pak HS, Hanley JR. Categorical Perception of colour in the left and right visual field is verbally mediated: Evidence from Korean. Cogn. 2007;107:752–762. doi: 10.1016/j.cognition.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Bornstein MH, Kessen N, Weiskopf S. Color vision and hue categorization in young infants. J Exp Hum Percept Perform. 1976;1:115–129. doi: 10.1037//0096-1523.2.1.115. [DOI] [PubMed] [Google Scholar]
- 13.Catherwood D, Crassini B, Freiberg K. The nature of infant memory for hue. Brit J of Dev Psychol. 1987;5:385–394. [Google Scholar]
- 14.Catherwood D, Crassini B, Freiberg K. The course of infant memory for hue. Aust J of Psychol. 1990;42:277–285. [Google Scholar]
- 15.Franklin A, Davies IRL. New evidence for infant color categories. Br J Dev Psychol. 2004;22:349–377. [Google Scholar]
- 16.Franklin A, Pilling M, Davies IRL. The nature of infant color categorization: Evidence from eye movements on a target detection task. J Exp Child Psychol. 2005;91:227–248. doi: 10.1016/j.jecp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Franklin A, Clifford A, Williamson E, Davies IRL. Color term knowledge does not affect Categorical Perception of color in toddlers. J Exp Child Psychol. 2005;90:114–141. doi: 10.1016/j.jecp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Kay P, Regier T, Gilbert AL, Ivry RB. Lateralized Whorf: Language influences perceptual decision in the right visual field. In: Minett JW, Wang WS-Y, editors. Language, Evolution, and the Brain. Hong Kong: The City University of Hong Kong Press; 2008. [Google Scholar]
- 19.Knoblauch K, Vital-Durand F, Barbur JL. Variation of chromatic sensitivity across the life span. Vision Res. 2000;41:23–36. doi: 10.1016/s0042-6989(00)00205-4. [DOI] [PubMed] [Google Scholar]
- 20.Pitchford NJ, Mullen KT. Is the acquisition of basic-colour terms in young children constrained? Perception. 2002;31:1349–1370. doi: 10.1068/p3405. [DOI] [PubMed] [Google Scholar]
- 21.Cruse DA. A note on the learning of colour names. J of Child Lang. 1977;4:305–311. [Google Scholar]
- 22.Loftus GR, Masson MEJ. Using confidence intervals in within-subjects designs. Psychol Bull Rev. 1994;1:479–490. [Google Scholar]
- 23.Giedd JN, et al. Development of the human corpus callosum during childhood and adolescence: A longitudinal MRI study. Prog Neuro-Psychopharm Biolog Psychiat. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 24.Chiron C, et al. The right hemisphere is dominant in human infants. Brain. 1997;120:1057–1065. doi: 10.1093/brain/120.6.1057. [DOI] [PubMed] [Google Scholar]
- 25.Bornstein MH. On the development of color naming in young children: Data and theory. Brain Lang. 1985;26:72–93. doi: 10.1016/0093-934x(85)90029-x. [DOI] [PubMed] [Google Scholar]
- 26.Regier T, Kay P, Khetarpal N. Color naming reflects optimal partitions of color space. Proc Natl Acad Sci USA. 2007;104:1436–1441. doi: 10.1073/pnas.0610341104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jameson K, D'Andrade R. In: Color Categories in Thought and Language. Hardin CL, Maffi L, editors. Cambridge, UK: Cambridge Univ Press; 1997. pp. 295–319. [Google Scholar]
- 28.Gilbert AL, Regier T, Kay P, Ivry RB. Support for lateralization of the Whorf effect beyond the realm of color discrimination. Brain and Language. 2008 doi: 10.1016/j.bandl.2007.06.001. in press. [DOI] [PubMed] [Google Scholar]
- 29.Mills DL, Conboy B, Paton C. Do changes in brain organization reflect shifts in symbolic functioning? In: Namy L, editor. Symbol Use and Symbolic Representation. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. pp. 123–153. [Google Scholar]
- 30.Mareschal D, Quinn PC. Categorization in infancy. Tren Cog Sci. 2001;5:443–450. doi: 10.1016/s1364-6613(00)01752-6. [DOI] [PubMed] [Google Scholar]
- 31.Bowerman M, Levinson C. Language Acquisition and Conceptual Development. Cambridge, UK: Cambridge Univ Press; 2001. [Google Scholar]
- 32.Mandler JM. Thought before language. Tren Cog Sci. 2004;8:508–513. doi: 10.1016/j.tics.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Heider ER. Universals in color naming and memory. J Exp Psychol. 1972;93:10–20. doi: 10.1037/h0032606. [DOI] [PubMed] [Google Scholar]