Adiponectin, an adipocyte-derived hormone, is a versatile player involved in the regulation of energy homeostasis, cardiovascular disease, and cancer (1). Numerous studies highlighting the beneficial aspects of circulating adiponectin complexes have raised the question as to which regulatory mechanisms govern its synthesis and secretion from adipocytes. Our current understanding of the production of adiponectin still has many gaps. Recent studies have identified critical players that control various aspects of the synthesis and secretion of adiponectin (2–4). In this issue of PNAS, a new player, DsbA-L, has been added to the list of existing chaperones involved in the maturation of adiponectin, such as BiP, ERp44, Ero1-Lα, and GGA1, all of which work in tandem to ensure correct formation of the tertiary and quaternary structure of adiponectin (5).
Adiponectin shows at least 3 complexes in circulation: trimer, hexamer, and high-molecular-weight forms (HMW) of 18–36 monomers (6). The 3 forms of adiponectin display distinct biochemical characteristics and exert nonoverlapping biological functions (7–9). Biochemical analysis of purified complexes and in vivo studies suggest that different forms of adiponectin do not interconvert after secretion. These results further underline the importance of the adipocyte, not only as the major producer cell type governing the total levels of adiponectin in circulation, but also as the key regulator of the relative complex distribution of the different forms present in plasma (6).
Oligomerization and Secretion of Adiponectin
BiP, an ER resident chaperone, interacts with adiponectin and regulates the early folding and posttranslational modifications (P.E.S., unpublished data) (Fig. 1). The hydrophobic interactions leading to the formation of the globular domains are sufficient to initialize trimerization of adiponectin (10). The only exposed cysteine in adiponectin, Cys-39, can form a covalent bond between 2 monomers. This intratrimeric disulfide bond is not essential for trimer formation but may contribute to the stability (6) and could secure an energetically charged (“spring-loaded”) conformation that, on reduction, translates into a conformational change in the globular domain. ERp44, an ER/Golgi resident chaperone, binds adiponectin through Cys-39 (2). This interaction retains adiponectin intracellularly before further oxidative folding with the assistance of Ero1-Lα and protein disulfide bond isomerase (PDI).
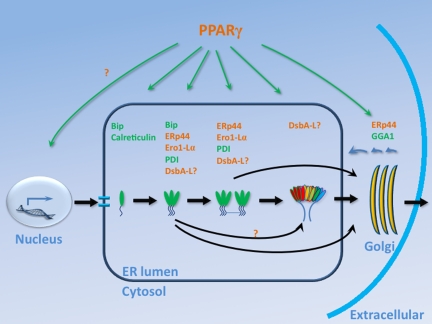
Fig. 1.
The synthesis and secretion of adiponectin. Adiponectin has a collagenous tail and a globular head that fold with the help of BiP. The head domains of 3 monomers interact with each other and form trimeric adiponectin. The intratrimeric disulfide bond formation is stimulated by ER chaperones, ERp44, Ero1-Lα, PDI, and potentially DsbA-L. An intertrimeric disulfide bond connects 2 trimers to form hexameric adiponectin. Either the trimer or the hexamer of adiponectin are the building block for HMW of 18–36 monomers. Movement through the Golgi may involve GGA1. PPARγ has been shown to stimulate the expression of various chaperones and thus enhance the folding and secretion of adiponectin. In the secretory pathway, postulated PPARγ targets are shown in green, whereas confirmed PPARγ targets are shown in red. DsbA-L may play roles in multiple steps of adiponectin secretion.
In the trimeric adiponectin, the free cysteine from the third monomer can pair up with the free cysteine from another trimer, and connect them covalently to form a hexamer. Either hexameric or trimeric adiponectin complexes may be the building blocks for the HMW form of 18–36 monomers. All 3 complexes travel to Golgi for further modification and eventually are released from the cell with the help of GGA1 (Golgi localizing γ-adaptin ear homology domain ARF-binding protein) (4). Adiponectin monomers, in contrast, are predicted to be thermodynamically unstable and can indeed never be found either in plasma or intracellularly.
DsbA-L and Adiponectin
In this issue of PNAS, Liu and colleagues identified DsbA-L as a new chaperone interacting with adiponectin and participating in HMW formation (5). DsbA-L, originally termed glutathione transferase kappa, was initially isolated from the mitochondrial fraction of rat liver (11). Recently, Ladner et al. (12) solved the structure of GST-kappa that reveals a surprising overall difference compared with the canonical GST transferases. A structural comparison reveals that GST-kappa is in fact more closely related to DsbA, the primary catalyst of disulfide bond formation in Escherichia coli (12). Based on the close structural similarities to DsbA, GST-kappa was renamed as “DsbA like protein” (DsbA-L).
Liu et al. (5) demonstrated that overexpression of DsbA-L increases the intracellular levels and the secretion of adiponectin, in particular, the HMW form, indicating that DsbA-L is an integral component of adiponectin complex formation and secretion in adipocytes. In 3T3-L1 adipocytes, they found that incubation with the PPARγ agonist rosiglitazone increases the secretion and HMW formation of adiponectin and this is accompanied by a concomitant up-regulation of DsbA-L. However, TNF-α suppresses adiponectin release and concurrently down-regulates DsbA-L. These correlations were further strengthened in rodent models on high-fat diets and overweight/obese subjects that revealed significant correlations between DsbA-L levels in adipocytes and adiponectin levels in plasma.
DsbA-L, a Molecular Chaperone or a Protein-Folding Catalyst?
PDI has 2 distinct activities, one as molecular chaperone and the other as protein-folding catalyst. PDI contains 2 CXXC (C, cysteine; X, any amino acid) active sites necessary for disulfide bond formation and isomerization. However, these sites are not essential for the molecular chaperone activity when PDI assists the refolding of client proteins that do not have a need for disulfide bond formation and isomerization (13).
Based on its structural similarities, DsbA-L is categorized as a member of the PDI family. Unlike most members of the PDI family, DsbA-L does not contain CXXC sites; this suggests that DsbA-L may not function specifically as a protein-folding catalyst. However, DsbA-L harbors a conserved S16XXS motif (S, serine; X, any amino acids). Ladner et al. (12) recently demonstrated that Ser-16 is the key residue that directly interacts with the thiol group present in glutathione during glutathione transferring. Interestingly, Liu et al. (5) further identified the Ser-16 residue in DsbA-L as essential for its role on adiponectin oligomerization and secretion. The interaction between DsbA-L and adiponectin may induce a conformational change in adiponectin and thus favors a disulfide bond exchange reaction with PDI, emphasizing the role of DsbA-L as a molecular chaperone for the assembly and secretion of adiponectin.
Overexpression of DsbA-L increases the intracellular levels and the secretion of adiponectin.
Transcriptional vs. Posttranslational Roles of PPARγ Activation for Adiponectin Secretion
Both rodent and human studies reveal that treatment with PPARγ agonists increases the circulating levels of adiponectin in vivo. However, the underlying mechanisms remain controversial. Some studies report that adiponectin mRNA levels are increased after PPARγ agonist treatment but others do not (14, 15). It is likely that PPARγ may exert its primary effect on adiponectin secretion by providing a more productive folding environment (2, 5) through transcriptional up-regulation of critical ER chaperones, including ERp44, Ero1-Lα and DsbA-L, which may thus lead to a stimulation of assembly and release of the HMW form.
The elegant studies performed by Liu and colleagues convincingly demonstrate the role of DsbA-L in adiponectin multimerization (5). These observations further stimulate an array of additional questions. Previous studies have shown that most intracellular DsbA-L is localized in peroxisomes and mitochondria. A colocalization experiment should identify whether a significant portion of DsbA-L is associated with adiponectin in the secretory pathway. Chaperones of the PDI family typically contain substrate binding sites that show low specificity for any given molecule and are ideally geared for the recognition of multiple substrates. On mapping the interacting domain of DsbA-L for the binding of adiponectin, more client molecules may be identified by using this binding pocket of DsbA-L as bait. These proteins may be subject to similar regulation as adiponectin.
The discovery of additional chaperones that participate in the process of adiponectin synthesis and maturation highlights these components as promising drug targets that have the potential to enhance endogenous adiponectin release from adipocytes. Given the extremely high levels of adiponectin mRNA present in adipocytes at any given time, optimizing the posttranslational events within the secretory pathway should be the most attractive approach toward a pharmacological intervention leading to the beneficial effects that are evident with increased total and HMW forms of adiponectin in plasma.
ACKNOWLEDGMENTS.
This work was supported by National Institutes of Health Grants R01-DK55758, , R01-CA112023, and JDRF 1-2008-16 (to P.E.S.).
Footnotes
The authors declare no conflict of interest.
See companion article on page 18302.
References
- 1.Kadowaki T, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang ZV, et al. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol. 2007;27:4698–4707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie L, et al. Intracellular trafficking and secretion of adiponectin is dependent on GGA-coated vesicles. J Biol Chem. 2006;281:7253–7259. doi: 10.1074/jbc.M511313200. [DOI] [PubMed] [Google Scholar]
- 5.Liu M, et al. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc Natl Acad Sci USA. 2008;165:18302–18307. doi: 10.1073/pnas.0806341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pajvani UB, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 7.Schraw T, et al. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008;149:2270–2282. doi: 10.1210/en.2007-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamauchi T, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, et al. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. 2006;281:16391–16400. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 11.Harris JM, Meyer DJ, Coles B, Ketterer B. A novel glutathione transferase (13–13) isolated from the matrix of rat liver mitochondria having structural similarity to class theta enzymes. Biochem J. 1991;278(Pt 1):137–141. doi: 10.1042/bj2780137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladner JE, et al. Parallel evolutionary pathways for glutathione transferases: Structure and mechanism of the mitochondrial class kappa enzyme rGSTK1–1. Biochemistry. 2004;43:352–361. doi: 10.1021/bi035832z. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Zhou Y, Wang C. Both the isomerase and chaperone activities of protein disulfide isomerase are required for the reactivation of reduced and denatured acidic phospholipase A2. EMBO J. 1997;16:651–658. doi: 10.1093/emboj/16.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasouli N, et al. Increased plasma adiponectin in response to pioglitazone does not result from increased gene expression. Am J Physiol. 2006;290:E42–E46. doi: 10.1152/ajpendo.00240.2005. [DOI] [PubMed] [Google Scholar]
- 15.Sharabi Y, et al. Effect of PPAR-gamma agonist on adiponectin levels in the metabolic syndrome: Lessons from the high fructose fed rat model. Am J Hypertens. 2007;20:206–210. doi: 10.1016/j.amjhyper.2006.08.002. [DOI] [PubMed] [Google Scholar]