Abstract
Protein misfolding in the endoplasmic reticulum (ER) contributes to the pathogenesis of many diseases. Although oxidative stress can disrupt protein folding, how protein misfolding and oxidative stress impact each other has not been explored. We have analyzed expression of coagulation factor VIII (FVIII), the protein deficient in hemophilia A, to elucidate the relationship between protein misfolding and oxidative stress. Newly synthesized FVIII misfolds in the ER lumen, activates the unfolded protein response (UPR), causes oxidative stress, and induces apoptosis in vitro and in vivo in mice. Strikingly, antioxidant treatment reduces UPR activation, oxidative stress, and apoptosis, and increases FVIII secretion in vitro and in vivo. The findings indicate that reactive oxygen species are a signal generated by misfolded protein in the ER that cause UPR activation and cell death. Genetic or chemical intervention to reduce reactive oxygen species improves protein folding and cell survival and may provide an avenue to treat and/or prevent diseases of protein misfolding.
Keywords: factor VIII, oxidative stress, unfolded protein response
Although endoplasmic reticulum (ER) stress and oxidative stress are closely linked events, the molecular pathways that couple these processes are poorly understood (1). Reactive oxygen species (ROS) originate during oxygen-using cellular metabolic processes, such as oxidative phosphorylation within mitochondria. The ER provides a unique oxidizing environment for protein folding and disulfide bond formation before transit to the Golgi compartment. During disulfide bond formation ROS are formed as a product of electron transport from thiol groups in proteins through protein disulfide isomerase (PDI) and ER oxidoreductase 1 (ERO1) to reduce molecular oxygen to form the oxidant hydrogen peroxide. It has been suggested that oxidation of cysteine residues during disulfide bond formation in the ER may significantly contribute to oxidative stress (2, 3). The unfolded protein response (UPR) is an adaptive signaling pathway designed to prevent the accumulation of misfolded proteins in the ER lumen. Studies also suggest the UPR is designed to minimize the stress of oxidative protein folding (2). The UPR is signaled through the protein kinases inositol-requiring protein 1α and PKR-related ER kinase and the activating transcription factor 6α (4, 5). Chronic unresolved accumulation of unfolded proteins in the ER leads to apoptosis. To elucidate the relationship between unfolded protein accumulation in the ER lumen, oxidative stress, and apoptosis, we have analyzed the secretion of coagulation factor VIII (FVIII), a large glycoprotein that is deficient in the X chromosome-linked bleeding disorder hemophilia A. As FVIII is prone to misfolding in the ER lumen, FVIII expression provides a unique approach to manipulate the ER stress response that does not rely on pharmacological intervention, where any connection between ER stress and ROS would be difficult to dissect.
FVIII is comprised of three domains in the order A1-A2-B-A3-C1-C2 (Fig. 1A). Although the liver produces FVIII, the particular cell type responsible for the majority of FVIII circulating in the plasma has yet to be definitively identified (6, 7). As FVIII is expressed at very low levels in vivo, the requirements for FVIII secretion have been characterized in cultured cells that express heterologous FVIII genes. These studies demonstrated that FVIII forms non-disulfide-bonded high molecular weight aggregates that are retained within the ER through interaction with the protein chaperones Ig-binding protein (BiP/GRP78), calnexin, and calreticulin (8–11). In addition, FVIII trafficking from ER to the Golgi complex is facilitated through interaction with the lectin LMAN1/MCFD2 complex (12, 13). As FVIII is susceptible to misfolding in the ER, its expression induces transcription of ER stress-response genes through the UPR (14). Here we show that unfolded FVIII accumulation in the ER lumen activates the UPR, causes oxidative stress, and induces apoptosis. Furthermore, antioxidants prevent ER stress-induced oxidative damage, activation of the UPR, and apoptosis, and improve FVIII secretion. The findings demonstrate an unprecedented link by which protein misfolding in the ER and ROS act in concert to activate the UPR and cause cell death. In addition, ROS can cause protein misfolding in the ER and prevent protein secretion.
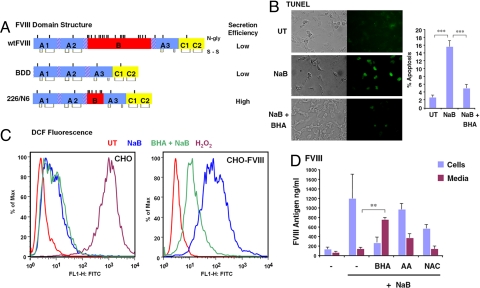
Fig. 1.
Induction of FVIII expression causes oxidative stress and apoptosis in CHO cells. (A) Schematic shows the domain structure of FVIII and deletion constructs used in these experiments. Positions of disulfide bonds and N-linked glycosylation sites are depicted. (B) wtFVIII-expressing CHO cells (CHO-FVIII) were treated with NaB (5 mM) for 24 h and then analyzed by TUNEL assay. TUNEL-positive cells were quantified from three separate experiments. (C) Control CHO cells and CHO-FVIII cells were treated with NaB for 24 h before staining with DCF for analysis by flow cytometry. Where indicated, cells were treated with NaB in the presence of BHA (10 μM). CHO cells were treated with H2O2 for 30 min before DCF staining as a positive control. (D) CHO-FVIII cells were treated with NaB in the presence or absence of BHA, ascorbic acid (500 μM; AA), or N-acetylcysteine (500 μM; NAC). Anti-oxidants were added 1 h before NaB treatment. After 24 h, conditioned medium and cells were harvested for analysis FVIII antigen. Data represent the mean of three independent experiments.
Results
wtFVIII Expression Induces Oxidative Stress and Apoptosis In Vitro.
To analyze the relationship between protein misfolding in the ER and oxidative stress, we analyzed Chinese hamster ovary (CHO)-H9 cells that were engineered for transcriptional induction of wild-type human FVIII (wtFVIII) in response to the addition of sodium butyrate (NaB) to the culture medium (14). In this system, NaB increases the synthesis of wtFVIII mRNA and protein, although the majority of the newly synthesized wtFVIII protein is not secreted from the cell, but rather aggregates in a complex with BiP within the ER lumen (10). Induction of wtFVIII synthesis in these cells causes distention of the ER lumen, a characteristic of ER stress (15), and transcriptional activation of UPR genes (14). TUNEL staining indicated that NaB treatment led to apoptosis in ≈16% of the cells that express wtFVIII (Fig. 1B). NaB did not induce apoptosis in CHO cells that did not express wtFVIII (data not shown). To determine whether accumulation of misfolded wtFVIII in the ER lumen can generate ROS, cells were stained with dichlorofluorescein (DCF), for which fluorescence requires production of the superoxide ion (16). Although DCF fluorescence did increase in control CHO cells upon treatment with NaB, DCF fluorescence dramatically increased more than 100 fold upon induction of wtFVIII expression in CHO-FVIII cells (Fig. 1C).
Antioxidants Prevent Oxidative Stress and Improve wtFVIII Secretion In Vitro.
To begin to address whether oxidative stress induced by wtFVIII expression interferes with wtFVIII secretion, we asked whether antioxidants can influence wtFVIII secretion. Butylated hydroxyanisole (BHA) is a lipid-soluble antioxidant that has been shown to suppress TNFα-induced cell death (17, 18). Addition of BHA to the medium of CHO-H9 cells at the time of NaB treatment significantly reduced apoptosis (Fig. 1B) and DCF fluorescence observed upon wtFVIII induction (Fig. 1C). BHA addition at 12 h after NaB treatment did not reduce the apoptosis observed at 24 h (data not shown). BHA treatment also decreased the intracellular accumulation of wtFVIII and increased secretion of wtFVIII into the medium by approximately four fold (Fig. 1D). The amount of FVIII antigen in the medium correlated with the amount of activity (data not shown), suggesting that the secreted FVIII was properly folded. Treatment with weaker antioxidants—N-acetylcysteine or ascorbic acid—had a lesser, more inconsistent effect on reducing DCF fluorescence (data not shown) and increasing wtFVIII secretion (Fig. 1D). These findings show that BHA treatment reduces oxidative stress and apoptosis in response to increased wtFVIII expression, and this correlates with increased secretion of functional wtFVIII. In addition, as the BHA-mediated increase in wtFVIII secretion into the medium corresponded with a decrease in intracellular antigen, the increase in wtFVIII production was likely a consequence of increased secretion and not increased cell survival.
To test whether oxidative stress was a unique feature observed upon wtFVIII expression, we analyzed CHO cells that were engineered for tetracycline-induced expression of the vitamin K-dependent clotting factor VII (FVII). Upon tetracycline induction of FVII, FVII accumulated within the ER, activated the UPR, and caused apoptosis [supporting information (SI) Fig. S1 A–C]. Under these conditions, there was a ≈30-fold increase in DCF fluorescence that was largely prevented by addition of BHA to medium (Fig. S1D). BHA treatment increased secretion of FVII by ≈2.5-fold (Fig. S1E). Therefore, induction of either wtFVIII or FVII, secreted proteins that are prone to misfolding in the ER lumen, generates ROS and induces the UPR and apoptosis. Furthermore, BHA treatment reduces ROS accumulation and apoptosis, and increases secretion of FVII and wtFVIII in cultured mammalian cells.
Compared with wtFVIII and B Domain Deletion, 226/N6 Is More Efficiently Secreted In Vivo.
We then asked whether FVIII accumulation in the ER induces ROS in vivo. For these studies, we compared the effects of expression of FVIII variants that exhibit different secretion efficiencies. Previous studies demonstrated that deletion of the heavily glycosylated B domain (residues 741-1647; B domain deletion [BDD]) produces a FVIII molecule that is inefficiently transported out of the ER, similar to wtFVIII (19). In contrast, a smaller BDD molecule (226/N6, which retains an additional 226 aa with 6 N-linked glycosylation sites from the B domain) is secreted ≈10-fold more efficiently than either wtFVIII or BDD, presumably because of increased engagement with the lectin chaperone machinery of the cell (Fig. 1A) (20). wtFVIII, BDD, and 226/N6 each contain eight disulfide bonds so that proper oxidative folding of each of these molecules requires the transfer of 16 electrons from cysteine residues within FVIII to molecular oxygen to form hydrogen peroxide. Therefore, comparison of the ROS production upon expression of these different forms of FVIII should provide insight into whether protein accumulation in the ER or de novo disulfide bond formation are significant factors that contribute to ROS production.
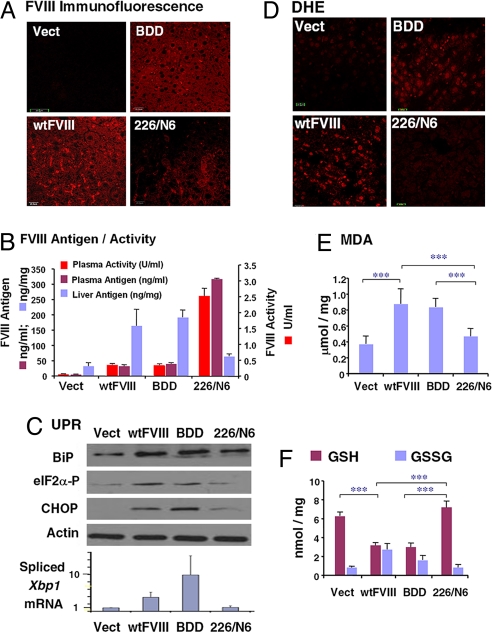
The effect of FVIII expression in hepatocytes in vivo was studied by hydrodynamic delivery of FVIII DNA expression vectors into the tail veins of mice. Under these conditions, FVIII is expressed in the majority of hepatocytes (Fig. 2A). Injection of these vectors did not cause significant liver damage or necrosis. However, the levels of serum alanine aminotransferase and aspartate aminotransferase were slightly elevated in wtFVIII- and BDD- injected mice after 24 h (Fig. S2C). Injection of either wtFVIII or BDD vector DNA produced detectable amounts of FVIII activity and antigen in the plasma after 24 h (Fig. 2B). In contrast, injection of vector encoding 226/N6 produced approximately seven- to eightfold greater levels of both FVIII antigen and FVIII activity in the plasma compared with wtFVIII or BDD (Fig. 2B). For all constructs, the amount of functional activity in the plasma correlated with levels of FVIII antigen, suggesting that the majority of the secreted antigen was functional. In addition, analysis of FVIII antigen in liver extracts demonstrated that 226/N6 accumulated to a lesser extent than either wtFVIII or BDD (Fig. 2B), consistent with the improved secretion efficiency (20). These findings show that hydrodynamic tail vein injection provides a reliable method to express FVIII in the majority of the hepatocytes and that 226/N6 is more efficiently secreted from the liver into the plasma than either wtFVIII or BDD.
Fig. 2.
FVIII expression induces ER stress, oxidative stress, and apoptosis upon in vivo expression in liver. DNA expression vectors were delivered by tail-vein injection into WT C57BL/6 mice. After 24 h, blood and liver tissues were isolated for analysis. (A) Liver tissue sections were analyzed for immunolocalization of FVIII antigen. (B) FVIII antigen in plasma samples and liver extracts was measured by ELISA. FVIII activity in plasma samples was measured using the COAMATIC assay kit. For activity measurements, the background of murine FVIII activity was subtracted (0.35 U/ml). (C) Western blot analysis of liver tissue for detection of BiP, phospho-eIF2α, and CHOP. Densitometry indicated that BiP was increased three fold and eIF2α-P was increased two fold in mice injected with wtFVIII and BDD compared with 226/N6. Spliced Xbp1 mRNA in liver tissue was measured by real-time RT-PCR. (D) Fresh frozen liver sections were prepared and stained with 2 μM dihydroethidine hydrochloride for 30 min at 37 °C. Sections were analyzed by fluorescence microscopy. (E) Malondialdehyde was measured in liver homogenates. (F) Liver lysates were analyzed for GSH and GSH and oxidized glutathione content. Data represent the mean and SD from three different animals in B, E, and F.
wtFVIII and BDD Cause Oxidative Stress, Activate the UPR, and Induce Apoptosis In Vivo.
We then characterized whether accumulation of FVIII in the ER causes UPR activation. Western blot analysis demonstrated increased expression of BiP, a sentinel marker of UPR activation, and C/EBP-homologous protein CHOP/GADD153 in liver extracts from mice injected with vector encoding wtFVIII or BDD, compared with mice that received 226/N6 vector or empty vector (Fig. 2C). In addition, phosphorylation of the α subunit of eukaryotic translation initiation factor 2 (eIF2α), a marker for PKR-related ER kinase activation, and splicing of Xbp1 mRNA, a marker for inositol-requiring protein 1α activation (Fig. 2C), were increased upon injection of either wtFVIII or BDD compared with 226/N6 or empty vector. Therefore, whereas expression of either wtFVIII or BDD activated the UPR, expression of 226/N6 did not significantly activate the UPR.
We next explored whether the accumulation of FVIII in the ER lumen can generate ROS, leading to oxidative damage. Oxidative stress was monitored by using dihydroethidine staining of liver sections and by direct analysis of lipid peroxidation (malondialdehyde) in liver extracts (Fig. 2 D and E). Mice that received either wtFVIII or BDD vectors exhibited increased levels of ROS and lipid peroxidation. The increased ROS was also associated with depletion of intracellular glutathione (GSH) and a corresponding increase in oxidized glutathione (Fig. 2F). In contrast, the increase in oxidation products was not observed in mice that received 226/N6 vector or empty vector.
Because chronic or severe UPR activation can initiate apoptosis, we studied whether expression of poorly secreted FVIII causes apoptosis in the liver. TUNEL staining indicated a large percentage of apoptotic cells in mice injected with wtFVIII or BDD vectors, but not in mice injected with 226/N6 vector or empty vector (Fig. 3A and Fig. S2D). The results from TUNEL were also consistent with analysis of hematoxylin and eosin-stained liver sections (Fig. S2 A and B).
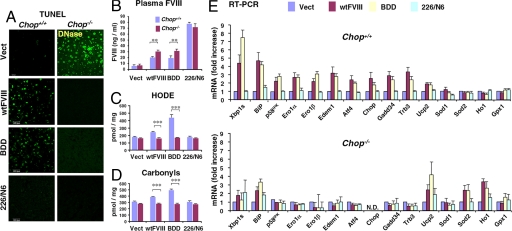
Fig. 3.
Chop deletion attenuates the UPR, apoptosis, and oxidative damage upon wtFVIII and BDD expression. Chop+/+ and Chop−/− mice were injected with empty vector or vector containing wtFVIII, BDD, or 226/N6. Liver tissue and plasma samples were isolated after 24 h for analysis. (A) Representative images from TUNEL staining of liver sections are shown. (B) Blood was collected by retro-orbital bleed and analyzed by anti-human FVIII ELISA (n = 3). (C and D) Protein oxidation (i.e., carbonyls) and lipid peroxidation (i.e., HODEs) in liver extracts were measured as described in SI Materials and Methods. (E) Total RNA was isolated from livers of Chop+/+ and Chop−/− mice injected with empty vector, wtFVIII, BDD, or 226/N6 and analyzed by quantitative real-time RT-PCR using specific primers (Table S1). Values represent the mean of three mice injected with each DNA vector. The values were normalized to 18S rRNA and expressed as induction in fold relative to empty vector.
Chop Deletion Protects from Oxidative Stress and Apoptosis upon wtFVIII and BDD Expression.
As the ER stress-induced apoptotic cell death pathway is, at least in part, mediated through CHOP (21, 22), and CHOP has been implicated in ROS production (23–25), we asked whether CHOP is required for apoptosis and ROS production in response to FVIII expression. Compared with Chop+/+ mice injected with wtFVIII or BDD vectors, apoptosis was significantly reduced in livers from Chop−/− mice that were injected with these vectors (Fig. 3A and Fig. S2D). The plasma levels of wtFVIII and BDD expressed in Chop−/− mice were modestly but significantly increased compared with those in Chop+/+ mice (Fig. 3B). In contrast, the plasma levels of 226/N6 were not significantly affected by Chop deletion. Whereas expression of wtFVIII or BDD significantly increased lipid peroxidation (hydroxyoctadecadienoic acid [HODE]) and protein oxidation (i.e., carbonyls)—sensitive and quantitative markers of ROS production—in the livers of Chop+/+ mice, these oxidation products were not increased in Chop−/− mice (Fig. 3 C and D). In addition, in contrast to Chop+/+ mice, GSH depletion was not observed in Chop−/− mice that received wtFVIII or BDD vector (Fig. S3). From these findings we conclude that both oxidative stress and the apoptotic response to wtFVIII or BDD expression requires CHOP.
To provide insight into the mechanism by which Chop deletion protects hepatocytes from apoptosis upon wtFVIII or BDD expression, we analyzed gene expression by real-time RT-PCR. In Chop+/+ mice, expression of wtFVIII or BDD, but not 226/N6, induced expression of UPR adaptive functions including BiP, p58IPK, the ER degradation-enhancing mannosidase-like protein EDEM1 (Edem1), spliced Xbp1 mRNA, and Atf4 (Fig. 3E). The expression levels of proapoptotic genes downstream of CHOP, Gadd34, and Trb3, were also induced upon wtFVIII or BDD expression. In contrast, the induction of these genes was significantly attenuated in Chop-null mice that were injected with the wtFVIII or BDD vectors (Fig. 3E). In addition, whereas the expression of ER oxidases 1α and 1β (Ero1α/β promote oxidation in the ER) was increased in Chop+/+ mice injected with wtFVIII or BDD vectors, the expression of these genes was not induced in Chop−/− mice. Western blot analysis of liver extracts from Chop−/− mice demonstrated that Chop deletion also attenuated UPR activation at the protein level (Fig. S4). The gene expression analysis also indicated the expression levels of some genes encoding an antioxidant response (uncoupling protein 2 [Ucp2] and superoxide dismutase 2 [Sod2]) were elevated in the Chop−/− mice that were injected with wtFVIII or BDD vectors (Fig. 3E). These results demonstrate that expression of either wtFVIII or BDD, but not 226/N6, induce the UPR, apoptosis, and oxidative stress in a manner that requires CHOP.
Antioxidant Treatment Attenuates UPR Activation and Apoptosis upon wtFVIII and BDD Expression.
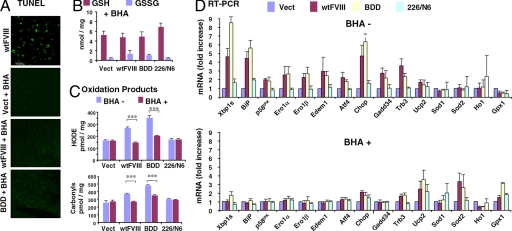
To test the requirement for oxidative stress for the UPR and apoptotic response, we analyzed the effect of antioxidant treatment. WT mice were fed chow supplemented with BHA for 4 days before DNA delivery. BHA feeding dramatically reduced apoptosis (Fig. 4A), reduced glutathione depletion (Fig. 4B), and reduced the accumulation of oxidized proteins and lipids (Fig. 4C) upon expression of wtFVIII or BDD. BHA feeding had no effect on these parameters in mice that received the 226/N6 vector or empty vector. mRNA expression analysis demonstrated that expression of wtFVIII or BDD induced the UPR, as previously observed in Chop+/+ mice (Fig. 3E vs. Fig. 4D). In contrast, BHA feeding attenuated UPR activation of genes encoding adaptive, as well as apoptotic, functions, upon delivery of wtFVIII or BDD vectors (Fig. 4D). Western blot analysis indicated that BHA feeding also suppressed UPR activation at the protein level (Fig. S4). Interestingly, BHA feeding also increased expression of the antioxidative stress response genes Ucp2 and Sod2 in a manner similar to the effect of Chop deletion, suggesting that antioxidant treatment and Chop deletion may act through a common mechanism to improve hepatocyte function.
Fig. 4.
BHA feeding suppresses oxidative stress and apoptosis and improves wtFVIII and BDD secretion in vivo. WT (A-C and E) or hemophilia A Fviii−/− (D) mice were fed with normal chow or chow supplemented with BHA for 4 days and then DNA expression vectors were injected into the tail vein. After 24 h, plasma and liver samples were harvested: (A) TUNEL, (B) glutathione, (C) HODEs and carbonyls, and (D) real-time RT-PCR. Expression values were normalized to 18S rRNA and the fold induction is expressed relative to empty vector. B-D depict three independent mice.
Antioxidant Treatment Improves wtFVIII and BDD Secretion In Vivo.
Significantly, analysis of FVIII antigen demonstrated that BHA feeding reduced intracellular accumulation of wtFVIII and BDD in the liver, and this correlated with increased secretion into the plasma, by eight fold and three fold, respectively (Fig. 5A). BHA increased wtFVIII and BDD secretion in WT mice (data not shown), as well as hemophilia A Fviii−/− mice (Fig. 5A). The increase in FVIII antigen was proportional to the increase in FVIII activity (data not shown), suggesting that the secreted FVIII was folded properly. In contrast, BHA feeding did not have a significant effect on secretion of the well secreted 226/N6 molecule. In addition, injection of mice with the superoxide dismutase mimetic Mn (iii)tetrakis(4-benzoic acid) porphyrin (MnTBAP) also improved BDD secretion and attenuated both UPR induction and apoptosis (Fig. S5 A-C). We also extended these observations to another model of gene delivery by using helper-dependent adenovirus delivery (26). Upon adenovirus delivery, BHA feeding improved the secretion of BDD (Fig. S6).
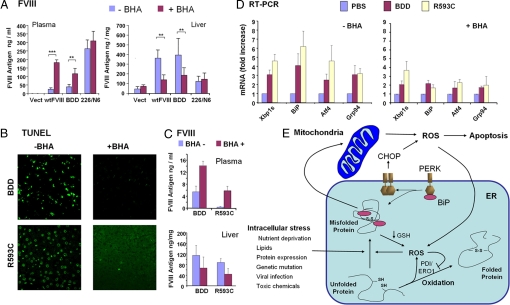
Fig. 5.
BHA feeding improves secretion of wtFVIII and BDD in vivo. Hemophilia A Fviii−/− mice were fed with normal chow or chow supplemented with BHA for 4 days before gene delivery. (A) FVIII antigen in plasma and liver samples was measured at 24 h after DNA injection of the indicated vectors. (B–D) DNA vectors encoding FVIII-BDD or R593C-BDD (R593C) (27) were injected into tail vein. After 24 h, plasma and liver samples were harvested for TUNEL (B), FVIII antigen in plasma and liver (C), and real-time RT-PCR (D). A, C, and D depict three independent mice. (E) Protein misfolding and oxidative stress create a vicious cycle leading to ER stress and cell death. ROS are generated by exposure to multiple stresses and also as a byproduct of mitochondrial respiration. Protein misfolding may cause ROS through changes in oxidative phosphorylation as a consequence of energy depletion or Ca2+ release from the ER. In addition, GSH can be consumed to reduce improperly paired disulfide bonds within misfolded proteins. ROS production can interfere with protein folding by inactivating PDI/ERO1 thiol-disulfide exchange reactions and/or by causing aberrant disulfide bond formation. ER stress activates CHOP expression that may lead to ROS production through induction of Ero1 or Gadd34. In this model, ROS can cause protein misfolding, UPR activation, and CHOP induction.
As antioxidant treatment improved secretion of wtFVIII and BDD, we asked whether BHA feeding could increase the secretion of a FVIII molecule having a missense mutation Arg593Cys (R593C) that causes protein misfolding and retention within the ER (27). This common mutation has been identified in patients with mild hemophilia A characterized by reductions in both FVIII antigen and activity. BHA feeding attenuated apoptosis and UPR gene induction observed upon expression of R593C-BDD (Fig. 5 B and D). BHA feeding preferentially increased the secretion of the folding-defective R593C-BDD mutant compared with BDD (Fig. 5C). The findings show that reduction in ROS can increase secretion of proteins prone to misfolding in an animal model in vivo.
Discussion
Our findings show that accumulation of unfolded protein in the ER lumen is sufficient to produce ROS and that both ROS and unfolded protein are required in concert to activate the UPR and apoptosis. The findings suggest that unfolded protein in the ER lumen signals ROS production as a second messenger to activate the UPR and induce apoptosis (Fig. 5E). Although it is not presently known how protein misfolding in the ER may generate ROS, several possible mechanisms exist. First, misfolded proteins bind protein chaperones, such as BiP, that consume ATP that may stimulate mitochondrial oxidative phosphorylation to produce ROS as a byproduct. Second, ROS may be produced as a consequence of disulfide bond formation in the ER during the transfer of electrons from thiol groups in folding substrates through PDI and ERO1 to molecular oxygen to produce hydrogen peroxide (2, 3, 28). As all FVIII molecules we analyzed harbor the same number of disulfide bonds, the increased ROS production in response to wtFVIII or BDD expression, but not upon 226/N6 expression, is not likely a direct consequence of de novo disulfide bond formation. Alternatively, GSH may be consumed during reduction of unstable and/or improper disulfide bonds in misfolded proteins (29). Consistent with this hypothesis, GSH levels were depleted in response to wtFVIII and BDD expression, but not in response to 226/N6 expression. Finally, protein misfolding in the ER lumen can cause Ca2+ leak from the ER (30) and uptake into the mitochondria to disrupt the electron transport chain. Although further studies are required to elucidate how protein misfolding in the ER lumen produces ROS, our studies demonstrate that accumulation of misfolded protein in the ER lumen is sufficient to initiate a self-perpetuating vicious cycle of ER stress and oxidative stress that, when unresolved, leads to cell death.
Previous studies of the mutant Z allele of α-1 antitrypsin and of prion protein have suggested there is an association between abnormal protein folding in the ER and oxidative stress (31, 32). In addition, cells compromised in ER function, such as defective UPR or ER-associated protein degradation, are susceptible to ROS production (2, 33). Our findings extend these observations by showing that protein misfolding in the ER lumen can initiate ROS production. In addition, ROS are an essential component in the events leading to protein misfolding in the ER and ER stress-induced apoptosis. ROS could exacerbate protein misfolding in the ER lumen by oxidizing amino acids in folding proteins or modifying chaperone and/or ERAD functions, thereby amplifying UPR signaling. The ability for the antioxidative stress response to limit ROS accumulation and protein misfolding may be especially important for function and survival of cells that have a high protein-folding load and/or are susceptible to oxidative stress, such as B lymphocytes or pancreatic β-cells, or in cells that are exposed to a variety of environmental insults (34, 35) (Fig. 5E).
The ER stress-induced apoptotic cell death pathway is, at least in part, mediated through CHOP (21, 23), as Chop−/− cells and mice are protected from ER stress-induced apoptosis (21, 22, 25, 36). Although the precise mechanism by which CHOP mediates apoptosis is unknown, CHOP activates the transcription of numerous cell death genes including Gadd34, Ero1, Bim, and Trb3. Of note, Gadd34 encodes a subunit of protein phosphatase 1 that directs dephosphorylation of eIF2α to promote protein synthesis and oxidation of the ER under conditions of ER stress (37, 38). In addition, increased expression of ERO1 causes hyperoxidation of the ER in yeast (28). We have shown that Chop deletion reduces oxidative stress, attenuates UPR induction, and prevents apoptosis in hepatocytes during conditions of unfolded protein accumulation in the ER lumen. Intriguingly, antioxidant treatment also reduced UPR induction (including CHOP), reduced oxidative stress, and reduced apoptosis. Moreover, the ER stress-induced alterations in gene expression observed upon Chop deletion mirrored the changes in gene expression caused by antioxidant treatment. The findings suggest that antioxidants and Chop deletion may improve ER function through a common mechanism.
Oxidative stress has been implicated in many diseases associated with protein misfolding, such as protein aggregation diseases including prion disease, Alzheimer disease, and Parkinson disease, as well as diabetes, metabolic disease, inflammation, and atherosclerosis (39–44). There is evidence that supports the idea that antioxidants and diet modification can alleviate oxidative stress and prove beneficial in these diverse disease states (18, 45, 46). In particular, antioxidants can reduce insulin resistance in animal models (47, 48). Recent studies suggest that protein misfolding in the ER may also lead to insulin resistance (49). In support of this idea, chemical chaperones 4-phenyl butyric acid and tauroursodeoxycholic acid, which are thought to improve protein folding, increased insulin sensitivity (49). It is possible that these chaperones may act to improve ER protein folding through their antioxidant properties.
Traditional therapy for hemophilia A involves protein replacement with plasma-derived and, more recently, recombinant-derived FVIII. However, this costly approach is hampered by development of anti-FVIII inhibitory antibodies, limited supply, potential for pathogen transmission, and poor access to the venous circulation. FVIII gene transfer offers one potential solution to these problems. As the B domain is not required for functional FVIII activity, most gene therapy strategies use B domain-deleted FVIII, similar to the BDD we have described here. Unfortunately, to date, clinical studies using retroviral-mediated and adenoviral-mediated delivery of FVIII have not produced therapeutic levels of FVIII in the plasma (50, 51). A limited study with adenovirus suggests hemophilia gene therapy may be limited by inflammatory responses associated with administration of recombinant adenovirus (50). The ROS produced as a consequence of FVIII misfolding may exacerbate inflammatory responses, as well as stimulate production of anti-FVIII antibodies. We have shown that BHA feeding prevents apoptosis, suppresses ER stress, and increases the secretion of FVIII delivered by adenovirus, as well as a folding-defective functional FVIII mutant R593C that is known to cause hemophilia A (27). Therefore, antioxidants may provide a useful adjuvant to improve FVIII production in patients who receive gene therapy or who have mutations that disrupt FVIII folding. These findings should also encourage the evaluation of antioxidant treatment to improve folding of different substrates and in different diseases associated with protein misfolding.
Methods
Mice.
Male C57BL/6 mice were purchased from Jackson Laboratory. Control and Fviii−/− (exon 16 deletion) mice in a C57BL/6 background at 6 to 8 weeks were housed under pathogen-free conditions at the University of Michigan Laboratory Animal Medicine facility. Chop+/+ and Chop−/− mice (21) were kindly provided by David Ron (New York, NY) and bred into a C57BL/6 background. The University Committee on the Use and Care of Animals approved animal protocols.
Hydrodynamic Tail Vein Injections.
The expression vectors for wtFVIII, BDD, and 226/N6 were previously described (20). Vectors for FVIII-BDD and R593C-BDD were kindly provided by J. Voorberg (Amsterdam, The Netherlands) (27). Plasmid DNA samples (100 μg) were diluted in 2.5 ml lactated Ringer buffer and infused over 10 sec into the tail vein. Retro-orbital blood collection was performed at 24 h after injection for measure of FVIII activity and antigen in the plasma (20).
Supplementary Material
Acknowledgments.
We thank Dr. D.T. Rutkowski and D. Ginsburg for critical reading of this paper. This work was supported by National Institutes of Health Grants DK042394, HL052173, and HL057346 and Human Frontier Science Program Grant RGP31/2005. R.J.K. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809677105/DCSupplemental.
References
- 1.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 2.Harding HP, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 3.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 5.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 6.Do H, Healey JF, Waller EK, Lollar P. Expression of factor VIII by murine liver sinusoidal endothelial cells. J Biol Chem. 1999;274:19587–19592. doi: 10.1074/jbc.274.28.19587. [DOI] [PubMed] [Google Scholar]
- 7.Hollestelle MJ, et al. Tissue distribution of factor VIII gene expression in vivo-a closer look. Thromb Haemost. 2001;86:855–861. [PubMed] [Google Scholar]
- 8.Dorner AJ, Bole DG, Kaufman RJ. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987;105:2665–2674. doi: 10.1083/jcb.105.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swaroop M, Moussalli M, Pipe SW, Kaufman RJ. Mutagenesis of a potential immunoglobulin-binding protein-binding site enhances secretion of coagulation factor VIII. J Biol Chem. 1997;272:24121–24124. doi: 10.1074/jbc.272.39.24121. [DOI] [PubMed] [Google Scholar]
- 10.Tagliavacca L, Wang Q, Kaufman RJ. ATP-dependent dissociation of non-disulfide-linked aggregates of coagulation factor VIII is a rate-limiting step for secretion. Biochemistry. 2000;39:1973–1981. doi: 10.1021/bi991896r. [DOI] [PubMed] [Google Scholar]
- 11.Pipe SW, Morris JA, Shah J, Kaufman RJ. Differential interaction of coagulation factor VIII and factor V with protein chaperones calnexin and calreticulin. J Biol Chem. 1998;273:8537–8544. doi: 10.1074/jbc.273.14.8537. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, et al. Bleeding due to disruption of a cargo-specific ER-to-Golgi transport complex. Nat Genet. 2003;34:220–225. doi: 10.1038/ng1153. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Kaufman RJ, Ginsburg D. LMAN1 and MCFD2 form a cargo receptor complex and interact with coagulation factor VIII in the early secretory pathway. J Biol Chem. 2005;280:25881–25886. doi: 10.1074/jbc.M502160200. [DOI] [PubMed] [Google Scholar]
- 14.Dorner AJ, Wasley LC, Kaufman RJ. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J Biol Chem. 1989;264:20602–20607. [PubMed] [Google Scholar]
- 15.Rutkowski DT, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan TC, et al. Measurement of superoxide release in the phagovacuoles of immune complex-stimulated human neutrophils. J Immunol Methods. 1990;130:223–233. doi: 10.1016/0022-1759(90)90052-w. [DOI] [PubMed] [Google Scholar]
- 17.Vercammen D, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamata H, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 19.Pittman DD, et al. Biochemical, immunological, and in vivo functional characterization of B-domain-deleted factor VIII. Blood. 1993;81:2925–2935. [PubMed] [Google Scholar]
- 20.Miao HZ, et al. Bioengineering of coagulation factor VIII for improved secretion. Blood. 2004;103:3412–3419. doi: 10.1182/blood-2003-10-3591. [DOI] [PubMed] [Google Scholar]
- 21.Zinszner H, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oyadomari S, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCullough KD, et al. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marciniak SJ, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song B, et al. Genetic deletion of C/EBP homologous protein CHOP reduces oxidative stress, improves beta cell function, and prevents diabetes. J Clin Invest. 2008;10:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerullo V, et al. Correction of murine hemophilia A and immunological differences of factor VIII variants delivered by helper-dependent adenoviral vectors. Mol Ther. 2007;15:2080–2087. doi: 10.1038/sj.mt.6300308. [DOI] [PubMed] [Google Scholar]
- 27.Roelse JC, et al. Intracellular accumulation of factor VIII induced by missense mutations Arg593->Cys and Asn618->Ser explains cross-reacting material-reduced haemophilia A. Br J Haematol. 2000;108:241–246. doi: 10.1046/j.1365-2141.2000.01834.x. [DOI] [PubMed] [Google Scholar]
- 28.Sevier CS, et al. Modulation of cellular disulfide-bond formation and the ER redox environment by feedback regulation of Ero1. Cell. 2007;129:333–344. doi: 10.1016/j.cell.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Cuozzo JW, Kaiser CA. Competition between glutathione and protein thiols for disulphide-bond formation. Nat Cell Biol. 1999;1:130–135. doi: 10.1038/11047. [DOI] [PubMed] [Google Scholar]
- 30.Deniaud A, et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 31.Milhavet O, et al. Prion infection impairs the cellular response to oxidative stress. Proc Natl Acad Sci USA. 2000;97:13937–13942. doi: 10.1073/pnas.250289197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teckman JH, et al. Mitochondrial autophagy and injury in the liver in alpha 1-antitrypsin deficiency. Am J Physiol Gastrointest Liver Physiol. 2004;286:G851–862. doi: 10.1152/ajpgi.00175.2003. [DOI] [PubMed] [Google Scholar]
- 33.Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell. 2004;15:767–776. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 34.Scheuner D, Kaufman RJ. The Unfolded Protein Response: A Pathway That Links Insulin Demand with {beta}-Cell Failure and Diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masciarelli S, Sitia R. Building and operating an antibody factory: redox control during B to plasma cell terminal differentiation. Biochim Biophys Acta. 2008;1783:578–588. doi: 10.1016/j.bbamcr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Silva RM, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem. 2005;95:974–986. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23:1292–1303. doi: 10.1128/MCB.23.4.1292-1303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merad-Boudia M, et al. Mitochondrial impairment as an early event in the process of apoptosis induced by glutathione depletion in neuronal cells: relevance to Parkinson's disease. Biochem Pharmacol. 1998;56:645–655. doi: 10.1016/s0006-2952(97)00647-3. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Lipton SA. Molecular mechanisms of nitrosative stress-mediated protein misfolding in neurodegenerative diseases. Cell Mol Life Sci. 2007;64:1609–1620. doi: 10.1007/s00018-007-6525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uehara T, et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 42.Nathan C. Epidemic inflammation: pondering obesity. Mol Med. 2008;14:485–492. doi: 10.2119/2008-00038.Nathan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams KJ, Tabas I. Atherosclerosis and inflammation. Science. 2002;297:521–522. doi: 10.1126/science.297.5581.521. [DOI] [PubMed] [Google Scholar]
- 44.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kajimoto Y, Kaneto H. Role of oxidative stress in pancreatic beta-cell dysfunction. Ann N Y Acad Sci. 2004;1011:168–176. doi: 10.1007/978-3-662-41088-2_17. [DOI] [PubMed] [Google Scholar]
- 46.Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: a case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med. 2006;41:177–184. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 47.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 48.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.High KA. Update on progress and hurdles in novel genetic therapies for hemophilia. Hematology Am Soc Hematol Educ Program. 2007;2007:466–472. doi: 10.1182/asheducation-2007.1.466. [DOI] [PubMed] [Google Scholar]
- 51.Pierce GF, Lillicrap D, Pipe SW, Vandendriessche T. Gene therapy, bioengineered clotting factors and novel technologies for hemophilia treatment. J Thromb Haemost. 2007;5:901–906. doi: 10.1111/j.1538-7836.2007.02410.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.