Abstract
The conditions leading to the induction of adaptive Foxp3+ regulatory T cells (T-regs) from peripheral T cells in vivo are incompletely understood. Here, we show that unresponsiveness of T cells to IL-6 by T cell-selective deletion of gp130 or immunization of wild-type mice with antigen in incomplete Freund's adjuvant (IFA), which fails to induce IL-6, promotes the conversion of peripheral CD4+ T cells into adaptive Foxp3+ T-regs. Thus, both T cell-conditional gp130 knockout (KO) mice immunized with MOG35-55 in complete Freund's adjuvant (CFA) and wild-type mice immunized with MOG35-55 in IFA develop overwhelming antigen-specific T-reg responses and are protected from experimental autoimmune encephalomyelitis (EAE). Depletion of T-regs restores T helper (Th)17 responses and clinical EAE in MOG/CFA-immunized T cell-conditional gp130 KO mice, but not in MOG/IFA-immunized wild-type mice. We conclude that in the absence of T-regs, IL-6 signaling is dispensable for the induction of Th17 cells, and alternative pathways exist to induce Th17 cells and EAE in the absence of IL-6 signaling. However, IL-6 signaling is dominant in inhibiting the conversion of conventional T cells into Foxp3+ T-regs in vivo, and in the absence of IL-6 signaling, no other cytokine can substitute in inhibiting T-reg conversion. These data identify IL-6 as an important target to modulate autoimmune responses and chronic inflammation.
Keywords: experimental autoimmune encephalomyelitis, multiple sclerosis, IL-21, tolerance, incomplete Freund's adjuvant
Foxp3+ regulatory T cells (T-regs) are critical for the maintenance of peripheral tolerance, and deletion of Foxp3+ T-regs results in multiorgan autoimmunity (1). Naturally occurring Foxp3+ T-regs are generated in the thymus (2) and are released into the peripheral immune compartment during early postnatal development. In the peripheral immune compartment, IL-2 is an essential growth factor for the proliferation of T-regs, whereas TGF-β is important for their maintenance (3). Apart from naturally occurring CD4+CD25+Foxp3+ T-regs, several subsets of T-regs have been described that are induced from naïve conventional T cells in the peripheral immune compartment under specific circumstances (for review, see ref. 4). However, under physiological conditions, it is believed that induced Foxp3+ T-regs are generated mainly in the gut and possibly in other immunological niches that contain high local concentrations of TGF-β and are colonized by specialized types of antigen-presenting cells (5, 6).
Recently, we have discovered a reciprocal developmental relationship between Foxp3+ T-regs and T helper (Th)17 cells because TGF-β triggers the expression of Foxp3 in naïve T cells, whereas IL-6 inhibits the TGF-β-driven expression of Foxp3, and TGF-β plus IL-6 together induce retinoid-related orphan receptor (ROR)-γt triggering the developmental program of Th17 cells (7). In the absence of IL-6, IL-21, which is a member of the IL-2 family of cytokines, can substitute for IL-6, and activation with TGF-β plus IL-21 might constitute an alternative pathway to induce Th17 cells (8). Together, these findings suggested that IL-6 and possibly IL-21 are switch factors between the induction of T-regs and Th17 cells. IL-6 was initially described as B cell-stimulatory factor (9) and as an important trigger of acute-phase responses. IL-6 uses a receptor complex consisting of the ligand-binding subunit IL-6Rα (CD126) and the signaling subunit gp130 (10). Whereas gp130 is ubiquitously expressed, the expression of IL-6Rα is restricted to hepatocytes, intestinal epithelial cells, endocrine glands, and leukocytes with the exception of naïve B cells (for review see ref. 11). Mice deficient in gp130 have been generated. However, in contrast to Il6−/− mice, homozygous loss of gp130 is perinatally lethal (12). In fact, gp130 is the receptor signaling subunit for at least 6 additional members of the IL-6 family of cytokines, including IL-11, oncostatin M, leukemia inhibitory factor, cardiotrophin-like cytokine, ciliary neurotrophic factor, and cardiotrophin-1. Furthermore, gp130 is able to trigger 2 major signaling pathways, i.e., the SHP-2/ERK pathway and the STAT3 pathway (for review, see ref. 11). Interestingly, decreased gp130-triggered SHP/ERK signaling and increased gp130-triggered STAT3 signaling result in autoimmunity (13).
Here, we investigated the role of IL-6 in the generation of an immune response to MOG35-55, by using genetically modified mice in which unresponsiveness to IL-6 is restricted to T cells. We found that IL-6 critically prevented the conversion of naïve CD4+ T cells into Foxp3+ T-regs in vivo, and conversely, vaccination protocols that did not induce large amounts of IL-6 resulted in an immune response dominated by Foxp3+ T-regs. Furthermore, we show that immunization with antigen emulsified in incomplete Freund's adjuvant promotes the de novo generation of Foxp3+ T-regs to an extent that is sufficient to confer antigen-specific tolerance. Hence, we illustrate that “absence of inflammatory signals” is consistent with absence of IL-6-induction, which places this cytokine at a nodal point in the shaping of an adaptive immune response.
Results
Responsiveness of T Cells to IL-6 Determines Susceptibility to Experimental Autoimmune Encephalomyelitis (EAE).
We and others have shown that IL-6-deficient mice are resistant to EAE (7, 8, 14). In the absence of IL-6, Th17 responses are impaired whereas T-reg responses are dominant, suggesting that IL-6 is a critical factor that shifts the immune response from a T-reg response toward a pathogenic Th17 response (8). However, IL-6 has also been shown to induce the expression of vascular cell adhesion molecule (VCAM) on endothelial cells (14). Because the interaction between the integrin very-late antigen 4 (VLA-4) on T cells and VCAM is crucial for the transmigration of encephalitogenic T cells across the blood brain barrier (15), the failure of IL-6-deficient mice to up-regulate VCAM has also been proposed to be responsible for their resistance to EAE.
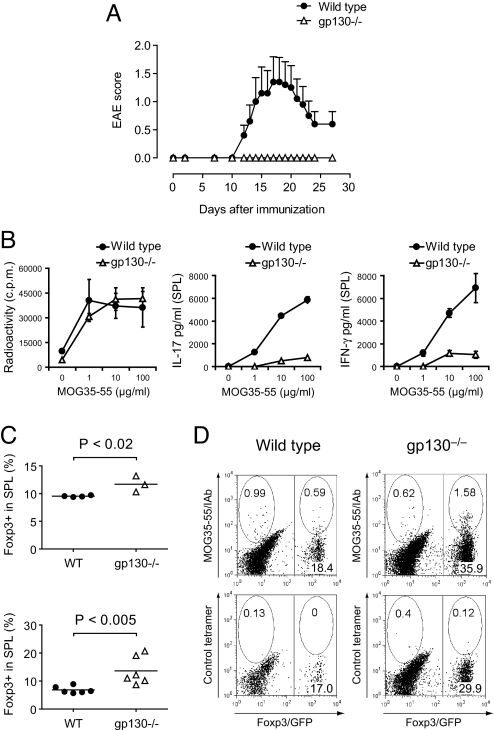
Here, we sought to discriminate these 2 effects by investigating genetically modified mice (henceforth called gp130−/− mice) that were created by crossing CD4-Cre+/+ mice with gp130flox/flox mice. As a consequence, responsiveness to IL-6 is selectively eliminated in T cells, whereas other cell types including endothelial cells are not affected. Additionally, we crossed gp130−/− mice with Foxp3gfp.KI mice (7, 16) so that we could track Foxp3+ T-regs based on the expression of green fluorescent protein (GFP). We immunized wild-type or gp130−/− mice with MOG35-55/complete Freund's adjuvant (CFA). Whereas wild-type animals developed EAE, gp130−/− mice were, like Il6−/− mice, completely resistant to EAE (Fig. 1A). This suggested that the absence of IL-6 signaling in T cells and not in other cellular targets of IL-6 was responsible for the resistance to the disease. We wondered whether gp130−/− mice, analogously to IL-6-deficient animals, had a reduced Th17 response. When we tested the recall response after immunization with MOG35-55/CFA, the antigen-specific production of IL-17 and IFN-γ was significantly reduced in gp130−/− mice (Fig. 1B). However, we detected an enhanced T-reg response in gp130−/− mice ex vivo upon immunization with MOG35-55/CFA (Fig. 1C Upper), and in contrast to wild-type mice, the fraction of Foxp3+ T-regs in gp130−/− CD4+ T cells increased even further after in vitro stimulation of MOG35-55-sensitized splenocytes (Fig. 1C Lower), suggesting that the lack of IL-6 signaling in T cells is sufficient to skew the immune response toward the expansion of Foxp3+ T-regs at the expense of Th17 cells. When tracking antigen-specific Foxp3+ T-regs by staining with MOG35-55/IAb tetramers, we found that the fraction of MOG35-55/IAb-reactive Foxp3+ T-regs in the compartment of activated CD4+ T cells was also increased in gp130−/− mice compared with their wild-type counterparts (Fig. 1D). Thus, lack of responsiveness to IL-6 seems to promote the generation (or expansion) of antigen-specific Foxp3+ T-regs despite the presence of an inflammatory milieu in vivo.
Fig. 1.
Unresponsiveness of T cells to IL-6 confers resistance to EAE caused by lack of Th17 cells and an increased T-reg response. (A) Wild-type or T cell-conditional gp130−/− mice on the Foxp3gfp.KI background were immunized with MOG35-55/CFA plus pertussis toxin and followed for signs of EAE (mean clinical score ± SEM, n = 10). (B) On day 10 after immunization, splenocytes were isolated and restimulated with MOG35-55 in vitro. The proliferative response was measured by [3H]thymidine incorporation, and the cytokine production in 48-h culture supernatants was determined by ELISA. Mean of triplicate cultures is shown. (C) Frequency of Foxp3+ T-regs in the splenic CD4+ T cell compartments of MOG35-55/CFA-immunized wild-type and gp130−/− mice as determined by the expression of Foxp3/GFP ex vivo (Upper) and after in vitro restimulation with MOG35-55 (Lower). (D) MOG35-55/IAb tetramer staining in MOG35-55-stimulated splenocytes from in vivo-sensitized wild-type and gp130−/− mice. The splenocytes were isolated on day 11 after immunization with MOG35-55/CFA followed by restimulation in vitro for 4 days. The gate was set on blasting CD4+ T cells. Representative cytograms are shown.
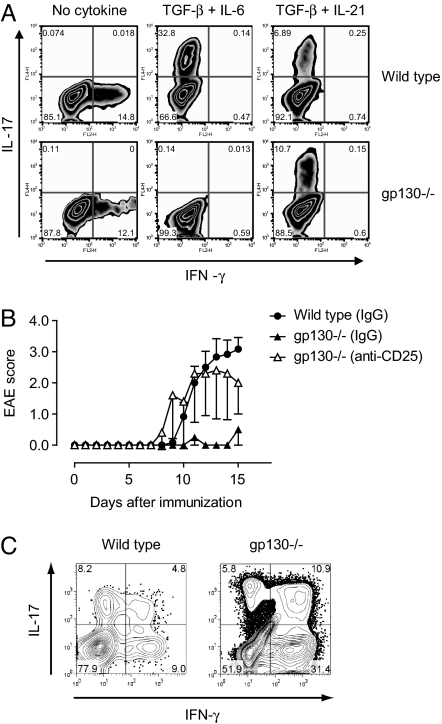
Both IL-6 and IL-21 are, together with TGF-β, capable of inducing Th17 cells. Therefore, we wanted to know whether the induction of Th17 cells would occur in the complete absence of IL-6 signaling in T cells. Naïve CD4+ T cells from wild-type and gp130−/− mice were purified by flow cytometry and differentiated in vitro in the presence of TGF-β plus IL-6 or TGF-β plus IL-21. Whereas wild-type cells responded to both cytokine mixtures by becoming Th17 cells, the induction of Th17 cells was abrogated in gp130−/− T cells in response to TGF-β plus IL-6 (Fig. 2A). However, TGF-β plus IL-21 induced Th17 cells as efficiently in gp130−/− T cells as in wild-type T cells (Fig. 2A), suggesting that the combination of TGF-β plus IL-21 is operational independently of IL-6-mediated signal transduction. To test this hypothesis in vivo, we treated gp130−/− mice with a control antibody or depleted them of CD4+CD25+Foxp3+ T-regs by means of a monoclonal antibody against CD25. This system allowed us to investigate the induction of pathogenic T cell populations in vivo in the absence of an exaggerated T-reg response that confounds the induction of effector T cell populations. Control antibody-treated gp130−/− mice were resistant to EAE; however, T-reg-depleted gp130−/− mice developed EAE with kinetics and severity similar to wild-type control animals (Fig. 2B). Despite the absence of IL-6 signaling, T-reg-depleted gp130−/− mice not only developed EAE, but also mounted a Th17 response both in the peripheral immune compartment and the CNS (Fig. 2C), suggesting that IL-6 signaling is dispensable for the induction of pathogenic Th17 responses in vivo, at least under conditions of reduced T-reg levels.
Fig. 2.
The combination of TGF-β plus IL-21 induces Th17 cells in gp130−/− mice. (A) Naïve T cells were purified from wild-type or gp130−/− mice and differentiated in vitro with either TGF-β plus IL-6 or TGF-β plus IL-21. The frequency of IL-17- and IFN-γ-positive T cells was determined by intracellular cytokine staining. (B) gp130−/− mice were either treated with control IgG (n = 3) or depleted of T-regs by treatment with a monoclonal antibody to CD25 (PC61, 2 × 0.5 mg) (8) 5 and 3 days before immunization with MOG/CFA (n = 5). As further control group, T-reg-competent wild-type mice were included (n = 6). The mean EAE score of each group is shown. Data represent 1 of 3 independent experiments. (C) At the peak of disease, mononuclear cells were isolated from the CNS of wild-type animals and T-reg-depleted gp130−/− mice followed by stimulation with PMA/ionomycin and intracellular cytokine staining for IL-17 and IFN-γ. The numbers in the quadrants of the cytograms indicate percentages of cytokine-positive cells in the CNS-derived CD4+ T cell compartment. One representative experiment is shown. Because gp130−/− mice that were not depleted of T-regs did not develop EAE, the T cellular infiltrate into the CNS of these mice was insufficient to perform intracellular cytokine staining.
Immunization with Incomplete Freund's Adjuvant (IFA) Fails to Induce IL-6 and Th17 Cells but Induces Antigen-Specific T-Regs.
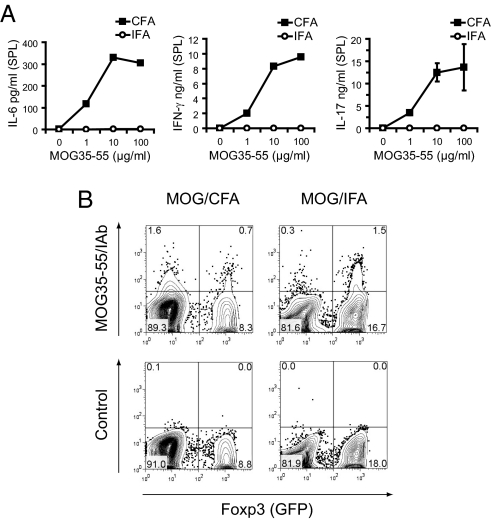
Emulsion of protein and peptide antigens in IFA has commonly been described to result in a Th2 type of response (17). We postulated that exposure of wild-type mice to antigen emulsified in IFA would prevent IL-6 production and could therefore lead to the generation of Foxp3+ T-regs. Indeed, in contrast to immunization with MOG35-55/CFA, IL-6 was not induced when C57BL/6 mice were immunized with MOG35-55/IFA (Fig. 3A). Furthermore, immunization with MOG35-55/IFA, in contrast to immunization with MOG35-55/CFA, did not trigger antigen-specific production of IFN-γ and IL-17 (Fig. 3A). This finding confirmed and extended earlier results showing that in the absence of Mycobacterium tuberculosis extract as adjuvant, immunization of wild-type mice failed to induce productive Th1 responses (17). We did not observe a measurable induction of IL-4 or IL-10 (data not shown), suggesting that there was no strong skewing toward a Th2 type of response. We then tracked antigen-specific T cells in the Foxp3/GFP− (effector T cell, T-eff) and the Foxp3/GFP+ (T-reg) compartment in Foxp3gfp.KI mice by using MOG35-55/IAb tetramer staining. Interestingly, we found that in contrast to immunization with MOG35-55/CFA (Fig. 3B), immunization with MOG35-55/IFA led to the preferential expansion of MOG-specific Foxp3+ T-regs and only insufficiently supported the priming/expansion of antigen-specific T-eff cells (Fig. 3B).
Fig. 3.
Immunization with MOG35-55 in IFA results in the generation/expansion of MOG35-55 specific Foxp3+ T-regs and does not support the induction of Th17 cells. Wild-type Foxp3gfp.KI mice were immunized with MOG35-55 emulsified in CFA vs. IFA. After 10 days, splenocytes were isolated and stimulated with MOG35-55. Supernatants were analyzed for the indicated cytokines by cytometric bead array (A), and the fractions of MOG35-55-specific CD4+ T cells in the Foxp3− and Foxp3+ compartments were determined by tetramer staining (B).
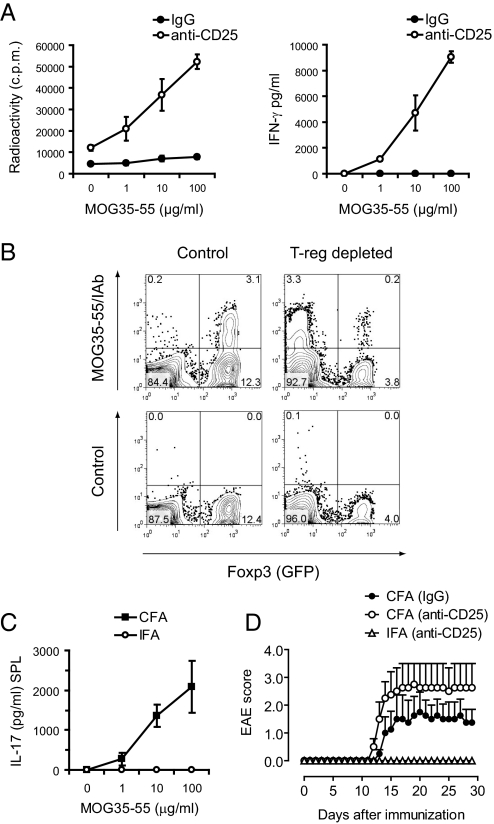
Next, we wanted to differentiate whether the lack of priming/expansion of IFN-γ and IL-17 producing CD4+ T cells upon immunization with IFA was caused by an overwhelming T-reg response. Thus, similarly to the strategy in gp130−/− mice, naturally occurring CD4+CD25+ T-regs were depleted by using an anti-CD25 antibody, and the depleted mice were then immunized with IFA. Surprisingly, the number of IFN-γ-producing Th1 cells was greatly increased in T-reg-depleted compared with nondepleted mice immunized with MOG35-55/IFA (Fig. 4 A and B). However, antigen-specific Th17 cells were not generated in T-reg-depleted MOG/IFA-immunized animals (Fig. 4C), and MOG/IFA immunization failed to induce EAE despite the depletion of T-regs (Fig. 4D). It has recently been reported that adjuvant-free induction of IFN-γ in vivo appears to be innocuous and potentially even protective in autoimmune diseases (18). Taken together, in contrast to T-reg-depleted gp130−/− mice that developed a Th17 response and became susceptible to EAE upon immunization with MOG/CFA, immunization with MOG/IFA failed to induce Th17 cells and EAE in T-reg-depleted wild-type animals. Together, these data suggest that immunization with CFA leads to activation of alternative signaling pathways in T cells that allow for the generation of Th17 cells independently of IL-6/IL-6R signaling, whereas immunization with IFA fails to do so.
Fig. 4.
Immunization with MOG/IFA fails to induce Th17 cells in vivo. (A) Foxp3gfp.KI mice were treated with control IgG or depleted of CD4+CD25+ T-regs by i.p. administration of a monoclonal antibody to CD25 followed by immunization with MOG/IFA. Splenic recall cultures were tested for antigen-specific proliferation and cytokine production by ELISA. Mean ± SD of triplicate cultures is shown. (B) The fraction of MOG35-55 specific T cells in the Foxp3− and Foxp3+ compartments of MOG/IFA-immunized control and T-reg-depleted Foxp3gfp.KI mice was measured by tetramer staining. (C) Wild-type Foxp3gfp.KI mice were depleted of T-regs and immunized with MOG/CFA or MOG/IFA. After 10 days, the antigen-specific IL-17 response was tested in splenocytes by ELISA. Mean ± SD of triplicate cultures is shown. (D) In a parallel experiment, T-reg-depleted and MOG/CFA (n = 4, control) vs. MOG/IFA (n = 7) immunized mice were monitored for EAE. Mean clinical score ± SEM is shown.
Lack of Responsiveness to IL-6 Promotes the Conversion of Conventional CD4+ T Cells into Foxp3+ T-Regs in Vivo.
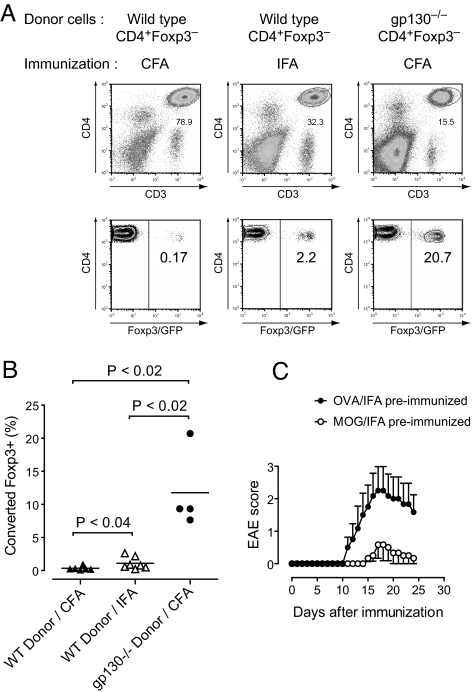
Alternative signaling pathways such as IL-21/IL-21R signaling can substitute for IL-6/IL-6R signaling in inducing Th17 cells. However, IL-6/IL-6R signaling appears to be dominant in inhibiting the de novo induction of Foxp3+ T-regs in vivo. To confirm this hypothesis, CD4+Foxp3− T cells derived from Foxp3gfp.KI mice were adoptively transferred into Rag1-deficient recipients followed by immunization with either MOG35-55/CFA or MOG35-55/IFA. After 20 days, CD3+CD4+ T cells were recovered from the spleens of host mice and tested for the expression of Foxp3. As reported before (16), immunization with CFA blocked the de novo generation of Foxp3+ T-regs (Fig. 5A). In contrast, immunization with IFA consistently induced a small but significant fraction of Foxp3+ T-regs (Fig. 5 A and B). Most importantly, when CD4+Foxp3− T cells from gp130−/− mice (crossed to Foxp3gfp.KI) were transferred, significant conversion of Foxp3− T cells into Foxp3+ T-regs was observed even after immunization with CFA (Fig. 5 A and B).
Fig. 5.
IL-6 inhibits the conversion of conventional Foxp3− T cells into Foxp3+ T-regs and blocks the generation of antigen-specific T-regs in vivo. (A) CD4+Foxp3− T cells from naïve wild-type or gp130−/− mice on the Foxp3gfp.KI background were purified by flow cytometry and transferred into Rag1-deficient recipients followed by immunization with MOG/CFA or MOG/IFA as indicated. On day 20 after immunization, splenocytes were isolated, and the CD4+ T cell compartment was assessed for expression of Foxp3. (B) Fraction of converted Foxp3+ T cells in the transferred wild-type or gp130-deficient T cell populations after immunization with MOG35-55/CFA or MOG35-55/IFA as indicated. Statistical analysis was performed by using Student's t test. (C) Immunization with MOG35-55/IFA induces antigen-specific tolerance. Wild-type mice were either preimmunized with OVA323–339/IFA (control) or MOG35-55/IFA. After 7 days, both groups were rechallenged with MOG35-55/CFA plus pertussis toxin. Mean clinical score ± SEM (n = 6 in each group).
Collectively, these data illustrate that IL-6 signaling in CD4+ T cells blocks the conversion of Foxp3− into Foxp3+ T-regs. Furthermore, in the absence of IL-6 induction (immunization with MOG35-55/IFA) or in the absence of IL-6 signaling in CD4+Foxp3− T cells (transfer of gp130−/− T cells), de novo generation of T-regs does occur in vivo. In gp130−/− T cells, the conversion of Foxp3− T cells into Foxp3+ T-regs cannot be suppressed even upon immunization with MOG/CFA, suggesting that no alternative signaling pathway can substitute for IL-6/IL-6R signaling in inhibiting T-reg conversion.
MOG35-55/IFA Induces Antigen-Specific Tolerance.
In contrast to immunization with antigen/CFA where antigen-specific Foxp3+ T-regs are exclusively recruited from preexisting (and expanding) naturally occurring T-regs, immunization with antigen/IFA leads to the establishment of a profound T-reg response that is fueled by conversion of Foxp3− into Foxp3+ T-regs. To explore whether the induction of antigen-specific T-regs by immunization with MOG35-55/IFA could be exploited in a clinical setting, we compared disease development and clinical course of EAE in mice that were “tolerized” with an irrelevant peptide (OVA323-339) emulsified in IFA or with MOG35-55/IFA followed by immunization with MOG35-55/CFA. Wild-type mice that did not receive a MOG35-55/IFA injection before immunization with MOG35-55/CFA developed regular EAE with paralysis of the hind limbs (OVA/IFA-preimmunized group, Fig. 5C). In contrast, most of the animals that were administered MOG35-55/IFA 1 week before rechallenge with MOG35-55/CFA were protected from EAE (Fig. 5C). In addition to the markedly reduced incidence, those animals in the MOG/IFA-tolerized group that nevertheless developed disease had a delayed onset of EAE and a milder disease course resulting in a significantly decreased disease burden. These results further support the idea that MOG35-55/IFA is a potent means to induce antigen-specific tolerance that relies on the de novo induction of antigen-specific Foxp3+ T-regs.
Discussion
In this work, we investigated the role of IL-6 in the lineage decision of antigen-specific CD4+ T cells during an autoimmune response in vivo. We found that unresponsiveness to IL-6 restricted to T cells is sufficient to mount a massive T-reg response in vivo that prevents the induction of Th1 and Th17 effector cells and results in complete resistance to EAE. However, the failure to induce Th17 cells in gp130−/− mice is not caused by an intrinsic inability of gp130−/− CD4+ T cells to become Th17 cells because the combination of TGF-β plus IL-21 induced the expression of IL-17 in naive gp130−/− CD4+ T cells. Also, T-reg-depleted gp130−/− mice were able to mount a pathogenic Th17 response upon immunization with MOG/CFA in vivo. Thus, alternative pathways exist to induce Th17 cells in the absence of IL-6 signaling. However, IL-6 has a dual role because it also suppresses the induction of Foxp3. Here, IL-6/IL-6R signaling in CD4+ T cells constitutes a dominant pathway because in the absence of IL-6R signaling but in the presence of an intact IL-21/IL-21R system, the induction of Foxp3 was still not suppressed, and the mice developed an overwhelming T-reg response even if CFA was used as an adjuvant. Consistent with these findings, we show that immunization of wild-type mice with autoantigen in IFA fails to induce IL-6 and promotes the development of antigen-specific T-regs instead of antigen-specific effector T cells. This immunization protocol can be used to induce antigen-specific tolerance protecting from EAE.
IL-6 is a potent factor to switch immune responses from the induction of Foxp3+ T-regs to pathogenic Th17 cells in vivo. There is accumulating molecular evidence that a single naïve T cell can develop into both a functional T-reg cell and an IL-17-producing T cell (19). TGF-β is necessary to induce the expression of both Foxp3, the master transcription factor of T-regs, and ROR-γt, the essential transcription factor of Th17 cells (20). Although necessary for the expression of both Foxp3 and ROR-γt, TGF-β enhances the function of Foxp3 but inhibits the function of ROR-γt (20). Only when additional signaling of “proinflammatory” cytokines such as IL-6 or IL-21 is operational, the TGF-β-mediated functional inhibition of ROR-γt is released, and Th17 cells are induced. Here, we show that after T-reg depletion, the development of Th17 cells is possible in the absence of IL-6 signaling, suggesting that other factors can compensate for IL-6 effects in inducing Th17 cells. It has recently been shown that STAT3, ROR-γt, and ROR-α are required to induce IL-17 in T cells (21–23). Although IL-6 and IL-21 use totally unrelated receptors, both recruit STAT3 as downstream signaling molecule (24). Thus, IL-21R signaling can bypass defects in IL-6R signaling and induce Th17 cells. STAT3 is also necessary and might even be sufficient to inhibit Foxp3 because STAT3-deficient T cells show excessive induction of Foxp3 when activated in the presence of TGF-β plus IL-6 (25 and data not shown). However, in the case of a deficient IL-6R system, the induction of Foxp3 cannot be suppressed either, and Foxp3+ T-regs are massively induced, suggesting that activation of STAT3 by other factors such as IL-21 is qualitatively or quantitatively insufficient to compensate for IL-6 in the inhibition of Foxp3 induction and the generation of functional T-regs in vivo. We conclude that IL-6/IL-6R (gp130)/STAT3 signaling has a dominant function in the suppression of Foxp3 in vivo. This idea is supported by the fact that under conditions of high availability of IL-6, IL-21R KO mice do not exhibit enhanced induction of T-regs and are susceptible to EAE (26).
Collectively, these data illustrate why IL-6 is pivotal in dictating the balance between induced T-regs and Th17 cells in vivo and show that the de novo generation of Foxp3+ T-regs actually occurs in the secondary lymphoid compartment in the absence of IL-6. Blockade of IL-6 signaling seems to be a promising strategy to control autoimmune responses, and a recent report confirmed that preventive administration of a monoclonal antibody to IL-6R that is already successfully used in juvenile idiopathic rheumatoid arthritis abrogates the buildup of inflammation in EAE caused by a decreased Th17 response (27). Interestingly, immunization with MOG/IFA provides an antigenic stimulus but fails to induce IL-6. IFA has long been known to induce “unresponsiveness” of T cells. However, the potential underlying mechanisms were poorly defined. On one hand, passive mechanisms such as anergy induction and deletion of autoreactive T cells were discussed (28). However, active mechanisms like immune deviation toward a Th2 type of response (17) and induction of regulatory T cells (29, 30) were reported. Active mechanisms of tolerance induction by immunization with antigen/IFA were supported by the possibility of transferring protection from the development of autoimmune disease to naïve host animals by adoptive transfer of T cells from IFA-immunized donor animals (30). We revisited this issue by using a unique combination of tools including Foxp3gfp.KI reporter mice and a MOG35-55/IAb tetramer to track well-defined Foxp3+ regulatory T cells. Our data are consistent with early observations by Swanborg and colleagues (29, 30) who described the induction of “suppressor cells” in the peripheral immune compartment of MBP/IFA-immunized rats and in a later report suggested that these suppressor cells might use TGF-β to keep potentially autoreactive encephalitogenic T cells in check. It is likely that the suppressor cells described by Swanborg and colleagues are identical to antigen-specific Foxp3+ T-regs that are overwhelmingly induced by immunization with antigen/IFA. In the present work, we also define that the mechanism by which IFA induces T-regs is conversion of Foxp3− into Foxp3+ T cells. We demonstrate that lack of responsiveness to IL-6 in T cells or the failure to induce IL-6 is necessary and sufficient to promote this conversion. This sheds light on the mechanism of how conversion of Foxp3− T cells into Foxp3+ T-regs might take place in vivo and explains why this phenomenon can be observed “under noninflammatory” conditions (31).
In conclusion, these findings have an important impact on the attempt to generate adaptive antigen-specific Foxp3+ T-regs and skew immune responses for therapeutic applications in vivo. Indeed, as soon as IL-6 production or signaling is blocked, immunogenic vaccination protocols are likely to be converted into tolerizing regimens in that exposure to antigen in the absence of IL-6 promotes the induction of antigen-specific Foxp3+ T-regs.
Materials and Methods
Animals.
Foxp3gfp KI mice were generated as described (7, 16). CD4-Cre+/+ mice and gp130flox/flox mice were provided by W. Müller (Faculty of Life Sciences, University of Manchester, UK) (32) and bred onto the Foxp3gfp.KI background. Because CD4-Cre deletes in all T cells when they are at the double-positive stage in thymic development, CD4-Cre+/+ × gp130flox/flox mice lack gp130 in all T cells. All animals were on pure C57BL/6 background. Animals were kept in a conventional, pathogen-free facility at the Harvard Institutes of Medicine (Boston, MA), and all experiments were carried out in accordance with the guidelines prescribed by the standing committee of animals at Harvard Medical School, Boston.
Induction of EAE and Adoptive Transfer Experiments.
EAE was induced by s.c. immunization of mice into the flanks with 100 μL of an emulsion of 100 μg of MOG35-55 peptide (MEVGWYRSPFSRVVHLYRNGK) and 250 μg of M. tuberculosis extract H37 Ra (Difco) in adjuvant oil (CFA). In addition, the animals received 200 ng of pertussis toxin (List Biological Laboratories) i.p. on days 0 and 2. Where indicated, MOG35-55 was emulsified in IFA (without M. tuberculosis extract). Mice immunized with MOG35-55/IFA did not receive pertussis toxin. Clinical signs of EAE were assessed according to the following score: 0, no signs of disease; 1, loss of tone in the tail; 2, hind limb paresis; 3, hind limb paralysis; 4, tetraplegia; 5, moribund.
In the adoptive transfer experiments, recipient Rag1-deficient animals received 2 × 106 flow cytometrically sorted CD4+Foxp3− T cells from naïve wild-type or gp130−/− donor mice i.p. in 0.5 mL of sterile PBS. The host mice were checked for proper reconstitution of CD4 T cells in the peripheral blood on day 10 after transfer and immunized s.c. with MOG/CFA plus pertussis toxin vs. MOG/IFA on day 20 after transfer. Three weeks later, splenocytes were isolated and tested for the expression of Foxp3/GFP by flow cytometry.
T Cell Proliferation and Differentiation.
Cells were cultured in DMEM/10% FCS supplemented with 5 × 10−5 M 2-mercaptoethanol, 1 mM sodium pyruvate, nonessential amino acids, l-glutamine, and 100 units of penicillin and 100 μg of streptomycin per ml. In antigen-specific recall assays, 2.5 × 106/ml splenocytes or draining lymph node cells were cultured in round-bottom wells for 72 h with the indicated concentration of MOG35-55 without the addition of IL-2. During the last 16 h, cells were pulsed with 1 μCi of [3H]thymidine (PerkinElmer) followed by harvesting on glass fiber filters and analysis of [3H]thymidine incorporation in a β-counter (1450 Microbeta, Trilux, PerkinElmer).
For in vitro T cell differentiation, CD4+ cells from naïve splenocytes and lymph node cells were isolated by using anti-CD4+ beads (Miltenyi) and further purified by flow cytometry into CD4+CD62LhighFoxp3/GFP− T cells. T cells were stimulated for 3 days with plate-bound antibody to CD3 (145-2C11, 4 μg/ml) plus soluble antibody to CD28 (PV-1, 2 μg/mL) or by soluble anti-CD3 (2 μg/mL) in the presence of irradiated syngeneic splenocytes as antigen-presenting cells. Where indicated, the medium was supplemented with recombinant cytokines (R&D Systems): human TGF-β1 (3 ng/mL), mouse IL-6 (30 ng/mL), and mouse IL-21 (100 ng/mL).
Cytokine Production.
Culture supernatants were collected after 48 h, and cytokine concentrations were determined by ELISA or by cytometric bead array (BD Biosciences) according to the manufacturer's instructions.
MHC Class II IAb Construct and Generation of Soluble MHC Class II Molecules and IAb Multimeric Complexes.
Generation of the cDNA constructs encoding the IAb α- and β-chains of the MOG35-55/IAb monomer and staining with MOG35-55/IAb tetramers have been described (16, 33). Briefly, MOG35-55-stimulated primary spleen or lymph node cells were incubated with IAb tetramers (30 μg/mL) in DMEM supplemented with 2% FCS (pH 8.0) at room temperature for 2.5 h. The percentage of tetramer+ cells was determined in the CD4 gate of live (7-AAD−) cells. To control for unspecific binding, IAs control tetramers were used (33). Stained cells were analyzed on a FACSCalibur machine (BD Biosciences), and data analysis was performed by using FlowJo software (Tree Star, version 6.3.3).
Intracellular Cytokine Staining.
For intracellular cytokine staining, cells were stimulated with phorbol 12-myristate 13-acetate (PMA) (50 ng/mL; Sigma), ionomycin (1 μg/mL; Sigma), and monensin (GolgiStop 1 μL/mL; BD Biosciences) at 37 °C/10% CO2 for 4 h. After staining of surface markers (CD4), cells were fixed, permeabilized, and stained for intracellular cytokines by using Cytofix/Cytoperm and Perm/Wash buffer and antibodies to mouse IL-17 and IFN-γ (BD Biosciences) according to the manufacturer's instructions.
Acknowledgments.
This work was supported by National Institutes of Health Grants R01AI073542-01 (to M.O.) and 1R01NS045937-01, 2R01NS35685-06-, 2R37NS30843-11, 1R01A144880-03, 2P01A139671-07, 1P01NS38037-04, and 1R01NS046414 (to V.K.K); National Multiple Sclerosis Society Grants RG-2571-D-9 (to V.K.K.) and RG-3882-A-1 (to M.O.); and by the Juvenile Diabetes Research Foundation Center for Immunological Tolerance at Harvard Medical School. T.K. is the recipient of Heisenberg fellowship KO 2964/2-1 from the Deutsche Forschungsgemeinschaft. M.M. is supported by Deutsche Forschungsgemeinschaft Grant MI 1221/1-1. V.K.K. is the recipient of the Javits Neuroscience Investigator Award from the National Institutes of Health. A.A. and V.A.D. are supported by a postdoctoral fellowship from the National Multiple Sclerosis Society. A.W. is supported by the FP6 Marie Curie Research Training Network Grant MRTN-CT-2004-005632 (IMDEMI) and Deutsche Forschungsgemeinschaft Grants SFB490 and SFB/TR52.
Footnotes
The authors declare no conflict of interest.
References
- 1.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 5.Kamanaka M, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 8.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirano T, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 10.Taga T, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 11.Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida K, et al. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atsumi T, et al. A point mutation of Tyr-759 in interleukin 6 family cytokine receptor subunit gp130 causes autoimmune arthritis. J Exp Med. 2002;196:979–990. doi: 10.1084/jem.20020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eugster HP, Frei K, Kopf M, Lassmann H, Fontana A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur J Immunol. 1998;28:2178–2187. doi: 10.1002/(SICI)1521-4141(199807)28:07<2178::AID-IMMU2178>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of α4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korn T, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heeger PS, et al. Revisiting tolerance induced by autoantigen in incomplete Freund's adjuvant. J Immunol. 2000;164:5771–5781. doi: 10.4049/jimmunol.164.11.5771. [DOI] [PubMed] [Google Scholar]
- 18.Jain R, et al. Innocuous IFNγ induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med. 2008;205:207–218. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou L, et al. TGF-β-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing ROR-γt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-β and induction of the nuclear receptor ROR-γt. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov II, et al. The orphan nuclear receptor ROR-γt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR-α and ROR-γ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spolski R, Leonard WJ. Interleukin-21: Basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 25.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7097–7101. doi: 10.4049/jimmunol.180.11.7097. [DOI] [PubMed] [Google Scholar]
- 27.Serada S, et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:9041–9046. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marusic S, Tonegawa S. Tolerance induction and autoimmune encephalomyelitis amelioration after administration of myelin basic protein-derived peptide. J Exp Med. 1997;186:507–515. doi: 10.1084/jem.186.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conant SB, Swanborg RH. Autoreactive T cells persist in rats protected against experimental autoimmune encephalomyelitis and can be activated through stimulation of innate immunity. J Immunol. 2004;172:5322–5328. doi: 10.4049/jimmunol.172.9.5322. [DOI] [PubMed] [Google Scholar]
- 30.Swierkosz JE, Swanborg RH. Immunoregulation of experimental allergic encephalomyelitis: Conditions for induction of suppressor cells and analysis of mechanism. J Immunol. 1977;119:1501–1506. [PubMed] [Google Scholar]
- 31.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naïve T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betz UA, et al. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic, and pulmonary defects. J Exp Med. 1998;188:1955–1965. doi: 10.1084/jem.188.10.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy J, et al. Detection of autoreactive myelin proteolipid protein 139–151-specific T cells by using MHC II (IAs) tetramers. J Immunol. 2003;170:870–877. doi: 10.4049/jimmunol.170.2.870. [DOI] [PubMed] [Google Scholar]