Abstract
The extracellular glutamate concentration ([glu]o) rises during cerebral ischemia, reaching levels capable of inducing delayed neuronal death. The mechanisms underlying this glutamate accumulation remain controversial. We used N-methyl-d-aspartate receptors on CA3 pyramidal neurons as a real-time, on-site, glutamate sensor to identify the source of glutamate release in an in vitro model of ischemia. Using glutamate and l-trans-pyrrolidine-2,4-dicarboxylic acid (tPDC) as substrates and dl-threo-β-benzyloxyaspartate (TBOA) as an inhibitor of glutamate transporters, we demonstrate that energy deprivation decreases net glutamate uptake within 2–3 min and later promotes reverse glutamate transport. This process accounts for up to 50% of the glutamate accumulation during energy deprivation. Enhanced action potential-independent vesicular release also contributes to the increase in [glu]o, by ≈50%, but only once glutamate uptake is inhibited. These results indicate that a significant rise in [glu]o already occurs during the first minutes of energy deprivation and is the consequence of reduced uptake and increased vesicular and nonvesicular release of glutamate.
During brain ischemia, extracellular glutamate concentration ([glu]o) increases, reaching levels that activate the N-methyl-d-aspartate type of glutamate receptor (NMDAR), thereby causing neuronal death (1). A number of hypotheses have been advanced to explain this pathological increase in [glu]o (2, 3). Whereas some studies suggest that the source of accumulating glutamate is vesicular (synaptic) (4–6), others provide evidence for nonvesicular mechanisms (7–11).
Previous investigations usually have used biochemical (e.g., microdialysis) or radiolabeling techniques to measure excitatory amino acid concentrations at predefined time points (3, 7–9). These methods, however, provide low spatial and/or temporal resolution and offer little information on glutamate levels at the synaptic cleft, where the receptors mediating excitotoxicity are located. To detect changes in ambient glutamate levels during energy deprivation (ED) in real time, we have adopted an alternative approach in which the NMDARs of neurons undergoing ED are used as high-affinity glutamate sensors (12). This technique has allowed us to determine the relative contribution of vesicular and nonvesicular release to the increase in [glu]o during ED.
Materials and Methods
Organotypic Hippocampal Slice Preparation.
Experiments were performed on rat organotypic hippocampal slice cultures. Tissue slices of 400-μm thickness were prepared from 6-day-old rats and cultured by means of the roller-tube technique as described pre-viously (13).
Experimental Conditions.
After 12–25 days in vitro, cultures were transferred to a recording chamber mounted onto the stage of an upright microscope (Axioscop FS 2; Zeiss), and then they were superfused with external solution (137 mM Na+/2.7 mM K+/146.2 mM Cl−/2.8 mM Ca2+/0.5 mM Mg2+/11.6 mM HCO3−/0.4 mM H2PO4−/5.6 mM d-glucose) at 29–31°C, pH 7.4. To induce ED, glucose was replaced with 2 mM 2-deoxyglucose, and 5 mM sodium cyanide (NaCN) was added (14).
Electrophysiological Recordings.
Whole cell patch-clamp recordings were obtained from CA3 pyramidal cells (Axopatch 200B amplifier; Axon Instruments, Foster City, CA) with microelectrodes (2–5 MΩ) filled with 122.5 mM Cs-gluconate/10 mM Hepes/8 mM NaCl/10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA). Cells were considered acceptable if the holding current at +40 mV was <600 pA, with access resistances from 8 to 16 MΩ. Experiments to record miniature excitatory postsynaptic currents (mEPSCs) were performed in the presence of 20 μM d-2-amino-5-phosphonovaleric acid (dAPV). Changes in input conductance were assessed by applying 0.4-s voltage commands of ±10 mV. l-Glutamate, NMDA, and l-trans-pyrrolidine-2,4-dicarboxylic acid (tPDC) were applied locally by pressure-ejection as indicated (NeuroPhore; Medical System, Great Neck, NY). Cultures treated with tetanus toxin (100 ng/ml), as well as their respective controls, were incubated for 3 days in serum-free medium to avoid nonspecific binding with serum proteins (15). For monopolar stimulation, current pulses (20–100 μA for 100 μs) were applied with a tungsten electrode.
Drugs and Chemicals.
1,2,3,4-Tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide (NBQX), tPDC, and dAPV were purchased from Tocris Neuramin (Bristol, U.K.); BAPTA, picrotoxin, glutamate, and NMDA were purchased from Sigma; and tetrodotoxin was purchased from Latoxan (Valence, France). dl-threo-β-benzyloxyaspartate (TBOA) was a gift from K. Shimamoto (Suntory Institute for Bioorganic Research, Osaka), and L-644711 was a gift from Merck.
Data Analysis.
Signals were filtered at 2 kHz and digitally recorded on computer by using clampex 7 (Axon Instruments). Numerical data are expressed as mean ± SEM. Student's t test was used to compare means. P < 0.05 was considered significant.
Nomenclature.
When referring to transporters, “uptake” signifies unidirectional influx, and “release” signifies unidirectional efflux of glutamate. “Net uptake” describes the net transport of glutamate across the membrane, that is, uptake-release.
Results
Changes in [glu]o were detected by monitoring the current flow through NMDARs on CA3 pyramidal cells in hippocampal slice cultures (12, 16). For recording NMDAR-mediated responses, cells were held at +40 mV in the presence of NBQX (25 μM), picrotoxin (300 μM), and tetrodotoxin (0.5 μM).
Extracellular Glutamate Rapidly Activates NMDARs During ED.
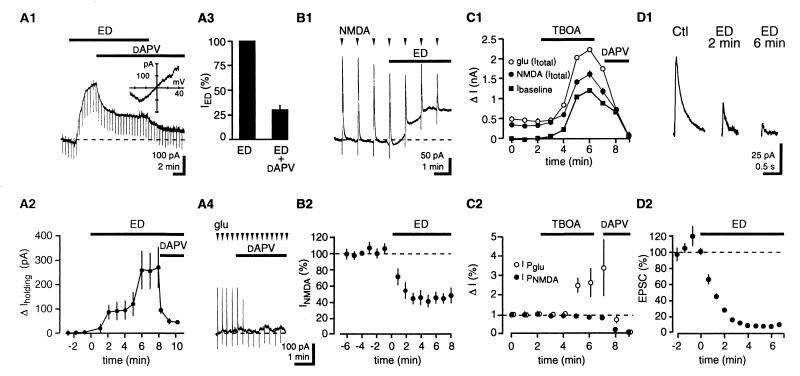
ED induced an outward current in CA3 pyramidal cells that reached an amplitude of 218 ± 64 pA after 7 min of ED. This response was reduced by 70 ± 5% with application of the NMDAR antagonist dAPV (100 μM; n = 17; P < 0.05). dAPV did not induce an inward current under control (Ctl) conditions (5 ± 9 pA; n = 7; P < 0.05) (Fig. 1A).
Figure 1.
NMDARs are activated by [glu]o during ED. (A1) Single trace depicting that ED-induced outward current in CA3 pyramidal cells is decreased by dAPV. Downward deflections represent responses to −10 mV voltage steps. (Inset) The current–voltage relationship of dAPV-sensitive current displays a negative slope at hyperpolarized potentials, characteristic of NMDAR currents. (A2) Pooled data; n = 17. (A3) dAPV reduces the current to 30 ± 5% of its initial value. (A4) Under Ctl conditions, dAPV blocks outward currents induced by glutamate pulses (400 μM in pipette; 150 ms) without affecting baseline holding current. (B1) Single trace depicting that responses to NMDA pulses decrease during ED. (B2) Pooled data; n = 14. (C1) An increase in ambient [glu]o is not sufficient to occlude NMDAR-mediated responses. Time course of the effect of TBOA on baseline holding current and NMDAR-mediated responses to NMPA and glutamate puffs. (C2) Time course of the effect of TBOA on NMDA and glutamate puffs after subtraction of baseline holding current. (D1) Depression of NMDAR-mediated EPSCs. (D2) Time-course graph. Stimulus frequency, 0.1 Hz; bin size, six stimuli; n = 4. dAPV concentration is 100 μM in this and in subsequent figures.
The dAPV-insensitive current displayed a linear current–voltage (I–V) relationship with a reversal potential close to 0 mV, and may reflect inhibition of Na+K+ ATPase (17), as application of the specific antagonist ouabain (5 μM) under Ctl conditions generated a current with a similar I–V relationship (n =3; data not shown).
The observed NMDAR activation may result from an increase in [glu]o or reflect an enhancement in NMDAR sensitivity to [glu]o caused by ED. When brief pulses of NMDA (500 μM in pipette; 80- to 200-ms duration) were applied close to the recorded neuron, NMDA responses decreased to 45 ± 10% of Ctl responses after 4 min of ED (Ctl, 599 ± 30 pA; n =14; P < 0.05) (Fig. 1B), indicating a decrease in sensitivity of NMDARs during ED. To rule out the possibility that the decrease of the NMDA transient was caused by occlusion with ED-mediated NMDAR activation, the effects of alternating pulses of glutamate (400 μM in pipette 1; 80- to 200-ms duration) and NMDA (500 μM in pipette 2) were compared during control conditions and in the presence of TBOA, a nontransportable competitive inhibitor of glutamate transporters (12, 18). As illustrated in Fig. 1C1, extracellular glutamate accumulation induced by TBOA that produced an outward current similar in amplitude to the one observed during ED failed to reduce the amplitude of responses to pressure-applied NMDA (n = 7). In fact, responses to pressure-applied NMDA remained constant, indicating that the receptors were not saturated. In addition, consistent with the block of glutamate uptake, responses to pressure application of glutamate were potentiated and greatly exceeded responses to pressure-applied NMDA (Fig. 1C2) (12).
The dAPV-sensitive current was not significantly different when ED was induced in the presence of N2 instead of NaCN (N2 + 2-deoxyglucose, 212 ± 76 pA after 7 min; n = 3; P > 0.05; data not shown). Similar dAPV-sensitive currents during ED also were observed in CA1 pyramidal cells (CA1, 193 ± 44 pA; CA3, 153 ± 45 pA after 7 min of ED; n =3; P > 0.05; data not shown).
To test the effect of ED on action potential (AP)-dependent vesicular release, we evoked NMDAR-mediated EPSCs by stimulating neurons in the CA3 region with an extracellular monopolar electrode (20–100 μA for 100 μs) in the absence of tetrodotoxin. During ED, NMDAR EPSCs were depressed to 12 ± 4% of Ctl values after 3 min (Ctl, 163 ± 31 pA; n =4; P < 0.05) (Fig. 1D). Because NMDAR sensitivity is depressed to 50% of Ctl during ED, this result indicates that evoked glutamate release decreases to ≈24% of Ctl values (12%/50%) during ED. Therefore, in agreement with previous data (see refs. 2 and 3 for reviews), these experiments exclude AP-dependent release as a major source of glutamate during ED.
Vesicular Release of Glutamate.
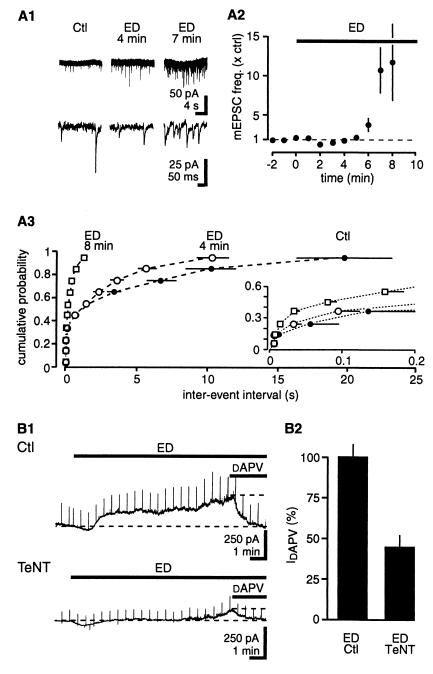
An elevation in [glu]o may be caused either by an increase in release or by a decrease in uptake. We first examined whether AP-independent vesicular release underlies glutamate accumulation by measuring α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated mEPSCs at −60 mV. The frequency of mEPSCs progressively increased throughout ED, reaching 10.3 ± 3.1 times Ctl values after 7 min (Ctl, 2.9 ± 1.6 Hz; n = 5; P < 0.05) (Fig. 2A). mEPSC amplitude was not affected (90 ± 6% of Ctl after 8 min of ED; P > 0.05; data not shown). To determine whether increased spontaneous vesicular release contributes to the increased [glu]o during ED, slice cultures were incubated with tetanus toxin (TeNT) to inhibit vesicular fusion (19, 20). In TeNT-treated slice cultures, no mEPSCs were observed during ED (data not shown), and the NMDAR-mediated current after 9 min of ED was reduced to 44 ± 7% of the value obtained in interleaved control cultures (Ctl, 283 ± 23 pA; n = 9; P < 0.05) (Fig. 2B), suggesting that approximately half of the [glu]o is of vesicular origin.
Figure 2.
ED increases AP-independent vesicular release of glutamate. (A1) Sample traces of AMPA receptor-mediated miniature EPSCs at −60 mV, in the presence of dAPV and picrotoxin at two different time resolutions. (A2) Time-course plot of the increase in mEPSC frequency during ED. (A3) Cumulative plot for interevent intervals. (Inset) Expanded time scale; n = 5. (B1) Inhibition of vesicular release with TeNT limits the increase in [glu]o during ED. (B2) Summary histogram of normalized values of the dAPV-sensitive current after 9 min of ED for Ctl and TeNT-treated cultures; n = 9.
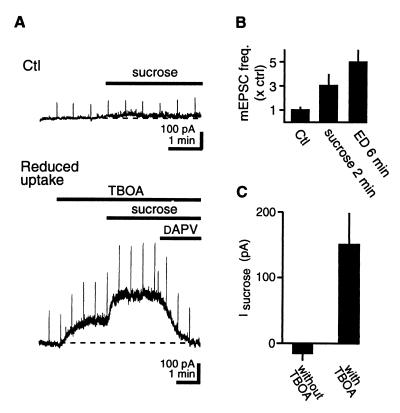
To test directly whether enhanced vesicular release can lead to detectable increases in [glu]o, we artificially stimulated vesicular release with extracellular sucrose (21). Although sucrose (500 μM for 2 min) increased the frequency of mEPSCs to the same extent as did 6 min of ED (sucrose, 3.1 ± 0.9 × Ctl; ED, 5.0 ± 1.1 × Ctl; n = 5; P > 0.05), this did not induce an NMDAR-mediated current (sucrose, −15 ± 11; n = 6) (Fig. 3). However, when glutamate uptake was inhibited with TBOA (250 μM), application of sucrose immediately increased [glu]o (152 ± 48 pA; n = 6) (Fig. 3). In slice cultures treated with TeNT, sucrose did not increase [glu]o in the presence of TBOA (0 ± 23 pA; n = 5; data not shown). These results show that an increase in the frequency of AP-independent vesicular release raises [glu]o only if glutamate uptake is reduced. We therefore examined whether net glutamate uptake is reduced during ED.
Figure 3.
Increased vesicular release is not sufficient to account for increased [glu]o. (A) (Top) Increasing mEPSC frequency with sucrose under Ctl conditions induces negligible outward current. (Bottom) After blockade of glutamate uptake with TBOA, sucrose induces a large NMDAR-mediated outward current (different cell from above). (B) The frequency of mEPSCs is not significantly different after 2 min treatment with sucrose or 6 min of ED. Values are normalized to mEPSC frequency during Ctl; n = 8. (C) Pooled data for the amplitude of NMDAR-mediated currents induced by sucrose alone and by sucrose in presence of TBOA; n = 6.
Impaired Net Glutamate Uptake During ED.
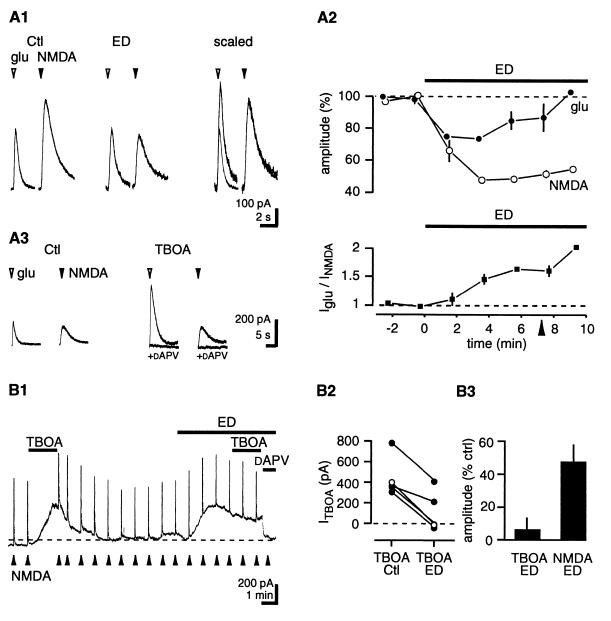
The extracellular concentration of glutamate after brief local application depends on transporter function, whereas the concentration of NMDA after a similar application does not, because NMDA is not transported (12, 22). We therefore measured the relative changes in glutamate versus NMDA responses to monitor transporter function during ED. Alternating pulses of glutamate (400 μM in pipette 1; 80 to 200 ms) and NMDA (500 μM in pipette 2) were applied close to the patched neuron. In agreement with the results in Fig. 1B, NMDA responses were depressed to about half those of Ctl after 7 min of ED (NMDA, 48 ± 8%). In contrast, glutamate responses only decreased to 79 ± 5% of Ctl (n = 8; P < 0.05) (Fig. 4A). Both responses were abolished by dAPV (data not shown). The glutamate/NMDA response ratio increased throughout ED and doubled after 9 min (2.0 ± 0.8 × preED), which could be mimicked by inhibiting uptake with TBOA (250 μM) (glutamate/NMDA, 6.9 ± 2.4 × preTBOA; n = 7) (Fig. 4A3). These results indicate that ED rapidly reduces net glutamate uptake capacity and suggest that failure of transporters of the EAAT family contributes to the increase in [glu]o.
Figure 4.
ED reduces net glutamate uptake. (A1) Brief alternating applications of glutamate and NMDA were used to evaluate the net effect of glutamate transporter function on glutamate responses. In the sample traces, both responses decrease in amplitude, but the NMDA response is decreased to a greater extent than is the glutamate response. (Right) Both traces were scaled to the NMDA response amplitude under Ctl conditions. (A2) Pooled data. (Upper) Glutamate responses decrease less than NMDA responses do. (Lower) The glutamate/NMDA response ratio reveals the time course of the rundown in glutamate transporter function. Applications were alternated every 15 s. Bin size, four applications; n = 8. The arrowhead indicates the time when sample traces in A1 were recorded. (A3) Inhibition of glutamate transporters with TBOA (250 μM) increases the glutamate/NMDA response ratio. Note that NMDA responses are unaffected. Traces show averages of three consecutive responses. (B1) TBOA increases [glu]o under Ctl conditions, but its effect is occluded during ED. In the sample trace, upward deflections are responses to 200-ms NMDA applications. (B2) Illustration of data from individual cells. The open circles denote the cell in A1; the closed circles denote the other four cells tested. (B3) Pooled values; n = 5. Note that TBOA responses are depressed by a factor of 8 as compared with NMDA responses.
Transporter function was further assessed by comparing the effects of TBOA under Ctl versus ED conditions. We previously showed that inhibiting glutamate uptake with TBOA under Ctl conditions causes an increase in [glu]o (12). If net glutamate uptake is reduced during ED, it should occlude that action of TBOA. In five experiments, TBOA (250 μM) was applied under Ctl conditions and again during ED. The NMDAR-mediated current induced by TBOA during ED was reduced to 6 ± 7.5% of that of preED (preED, 500 ± 89 pA; n = 5; P < 0.05) (Fig. 4B). This reduction cannot be attributed to desensitization or saturation of NMDARs, because when compared with Ctl conditions, the depression of TBOA responses during ED was 8.0 ± 2.1 times greater than the depression of responses to pulses of NMDA in the same cell (n = 5; P < 0.05). These results indicate that net uptake by glutamate transporters is decreased during ED.
Glutamate Efflux Increases During ED.
After prolonged ischemia, extracellular K+ and intracellular Na+ concentrations rise, altering the electrochemical gradients driving glutamate uptake. Under these conditions, glutamate flux through the transporters is predicted to reverse, increasing glutamate release and consequently reducing net glutamate uptake (10, 22, 23). We therefore examined whether the reduction in net uptake during ED described above could reflect reverse glutamate transport.
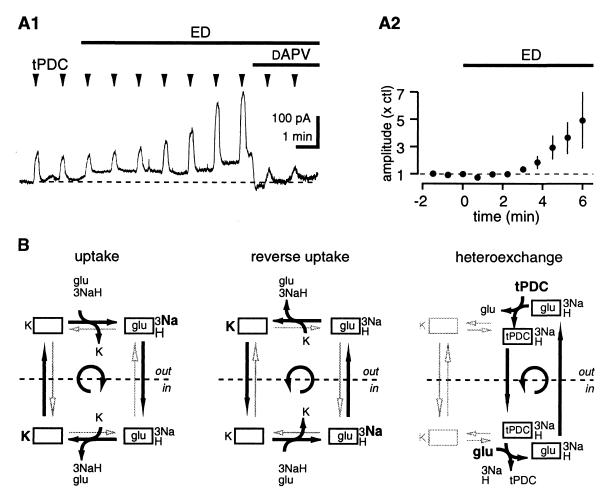
Net glutamate release (negative net glutamate uptake) by transporters is seen under two conditions,: (i) reverse transport and (ii) heteroexchange, where a transporter substrate such as tPDC (24) is exchanged for intracellular glutamate (25, 26). The glutamate release step is common to both processes (23, 25) (Fig. 5B). Therefore, if the conditions prevailing during ED facilitate reverse glutamate transport, this process will also be associated with an increase in the release of glutamate by heteroexchange. In the following experiment, heteroexchange was thus used as a tool to monitor reverse glutamate transport. When pulses of the transportable uptake inhibitor tPDC (500 μM in pipette; 100 to 500 ms) were applied close to a patched neuron, we observed fast transient NMDAR-mediated currents. In contrast, long (≈3 s) pulse applications of the nontransportable inhibitor TBOA at a high concentration (1 mM) were necessary to induce detectable currents (12) (n = 8; data not shown). Given that tPDC increased [glu]o at rates 18 times greater than those of TBOA (TBOA mean rising slope, 44 ± 7 pA/s; tPDC mean rising slope, 804 ± 166 pA/s; n = 8; data not shown), and that tPDC and TBOA have similar affinities for transporters (18, 25), this finding suggests that tPDC evoked glutamate release by heteroexchange. When ED was induced, the amplitude of tPDC-evoked currents progressively increased, reaching 4.9 ± 2.1 times the Ctl value after 6 min, (n = 5; P < 0.05) (Fig. 5A).
Figure 5.
ED promotes reverse glutamate transport. (A) Glutamate heteroexchange increases during ED. Sample trace showing that brief (300 ms) tPDC applications induce progressively more release of glutamate during ED. (A2) Pooled data; n = 7. (B) Glutamate efflux can occur during reverse transport or during heteroexchange. Pulses of tPDC will shift the transporter into the “glu/3Na+/H+ inside” state. The glutamate binding site is then returned to the outside through transport of either K+ or glutamate. During ED, the latter pathway (common to heteroexchange and reverse transport) is favored. See Results for details. This figure is adapted from refs. 22 and 24.
This increase in NMDAR activation may reflect decreased clearance of tPDC-evoked glutamate release rather than increased heteroexchange. If this were the case, however, the greater tPDC response could not exceed the potentiation of the Iglu/INMDA current ratio (Fig. 4A2), because the increase in this ratio reflects a decrease in net uptake during ED. The potentiation of the tPDC response was about 3 times greater than the Iglu/INMDA ratio (490% vs. 160% after 6 min ED), suggesting that the increase in tPDC responses reflects a genuine increase in evoked glutamate release during ED. Thus, conditions promoting net glutamate release through the transporters progressively develop within a few minutes of ED.
Discussion
Using NMDARs on CA3 pyramidal cells as a real-time, on-site sensor to monitor [glu]o, we found that enhanced vesicular release, in combination with an acute decrease in net glutamate uptake, produces an increase in [glu]o during ED. These findings indicate that transporter function is perturbed within a few minutes of ED. At this time, the massive ionic redistribution that accompanies anoxic depolarization, recorded as a sudden increase of several nanoamperes in the holding current (data not shown), has not yet occurred (27).
NMDAR Activation and Desensitization During ED.
NMDAR responses were reported to be potentiated after ischemia (28). In this paper, we show that during ED, neuronal responses to puffs of NMDA decrease by ≈50%. This desensitization seems to be specific for NMDARs, as the amplitude of AMPA receptor-mediated mEPSCs was not changed during ED and may be attributable to a decrease in energy-dependent receptor phosphorylation (29), dephosphorylation (30, 31), or increases in extracellular H+, Zn2+, or K+ concentrations during ischemia (32–35).
Vesicular Versus Nonvesicular Release.
The functional consequences of the initial increase in AP-independent vesicular release during ED remain speculative (2, 6, 36, 37). Our experiments with TeNT indicate that this type of vesicular release is responsible for ≈50% of extracellular glutamate accumulation at the onset of ED. Importantly, by dissociating vesicular release from transporter uptake with sucrose and TBOA, we show that AP-independent vesicular release contributes to the increase in [glu]o only when glutamate uptake is impaired. With prolonged ED, the proportion of glutamate released by vesicular means, however, is likely to decrease rapidly with progressive ATP depletion (38).
Reverse glutamate transport also contributes to the increase in [glu]o during ED, because application of TBOA, which blocks (i.e., stops the cycling of) transporters, produced an inward current in three of five cells (26 ± 16 pA) (Fig. 4B2), indicating that a source of glutamate was blocked and that net release was occurring (i.e., in these cells, glutamate release by transporters was greater than was uptake). Other sources of glutamate, such as arachidonic acid-sensitive release (39) and swelling-activated release (9) are unlikely to contribute to the TeNT-resistant rise in [glu]o. Indeed, arachidonic acid-sensitive glutamate release is blocked by TeNT (39), and L-644711 (1 mM), a specific antagonist of volume-sensitive Cl− channels (9), did not reduce NMDAR activation after 7 min of ED (n = 5; data not shown).
Mechanism of Net Uptake Decrease.
Two independent observations indicate that net glutamate uptake is decreased during ED by using two independent approaches. First, the glutamate/NMDA response ratio was increased. This result is independent of the molecular or pharmacological identification of the transporters, and reflects the net function of all glutamate uptake systems in the hippocampal slice culture. Second, the increase in [glu]o caused by the nontransportable inhibitor TBOA was occluded, demonstrating the involvement of the EAAT family of transporters.
Net uptake can be reduced as a consequence of decreased uptake or increased release of glutamate by transporters. The latter possibility was examined by using tPDC as a substrate to induce glutamate release by heteroexchange. Using heteroexchange to monitor reverse transport is justified, because the rate-limiting step in the transport reaction is thought to be common to both heteroexchange and reverse transport (23, 25). We observed an increase in tPDC heteroexchange during ED, reflecting increased glutamate release. This increase could result from a rise in intracellular Na+ or glutamate concentrations during ED, both of which increase heteroexchange and reverse transport (40). In parallel, factors released during ischemia are known to inhibit glutamate transporters (41–44), which could also contribute to the decrease in net uptake.
What Is the Concentration of Extracellular Glutamate Attained During ED?
The interstitial glutamate concentration achieved during ED can be estimated by comparing the desensitization-corrected, dAPV-sensitive increase in membrane conductance caused by ED versus a saturating concentration of NMDA at 0 mV (800 μM) (45–49) as measured with brief voltage steps (400 ms; +5 mV). By using this protocol, the increase in conductance after 5 min of ED was ≈34% of the increase caused by 800 μM NMDA (ΔED 5 min-Ctl, +227 ± 25%; ΔNMDA-Ctl, +690 ± 100%; n = 7; data not shown), which, based on concentration-response curves for glutamate-induced NMDAR activation (EC50 = 1.1 μM; Hill coefficient = 1.4), corresponds to a glutamate concentration of 650–700 nM (46, 47, 50). After 9 min of ED, this value reaches 900 nM (ΔED 9 min-Ctl, +307 ± 80%; n = 6). Thus, after about 10 min of ED, but before anoxic depolarization, [glu]o in the vicinity of NMDARs is roughly 1 μM, or 500 times the minimal value transporters can maintain (2 nM) (51). This glutamate concentration, which approximately corresponds to the EC50 of NMDARs, kills 50% of cerebral cortex neurons in culture when uptake is inhibited (52).
In conclusion, our results indicate that [glu]o increases during the early phase of ED because of increased vesicular release, decreased uptake, and increased reverse transport. Thus, net uptake is affected even without a massive breakdown in ionic gradients, suggesting that chronic low-level energy deficits could also compromise glutamate transport and lead to the pathological stimulation of glutamate receptors in various neurodegenerative diseases (53).
Acknowledgments
We thank Dr. Keiko Shimamoto for the gift of TBOA; C. Heuss and A. Lüthi for useful discussions and for reading the manuscript; and R. Dürr, L. Heeb, R. Kägi, H. Kasper, and L. Rietschin for technical assistance. This work was supported by the Swiss National Science Foundation (Grant 31–45547.95) and the Prof. Dr. Max Cloëtta Foundation.
Abbreviations
- [glu]o
extracellular glutamate concentration
- ED
energy deprivation
- NMDA
N-methyl-d-aspartate
- NMDAR
NMDA receptor
- dAPV
d-2-amino-5-phosphonovaleric acid
- Ctl
control
- mEPSC
miniature excitatory postsynaptic current
- TBOA
dl-threo-β-benzyloxyaspartate
- tPDC
l-trans-pyrrolidine-2,4-dicarboxylic acid
- AP
action potential
- TeNT
tetanus toxin
Noted Added in Proof
While this manuscript was under review, similar findings were reported from a study by Rossi et al. (54).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Choi D W, Rothman S M. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 2.Martin R L, Lloyd H G, Cowan A I. Trends Neurosci. 1994;17:251–257. doi: 10.1016/0166-2236(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 3.Obrenovitch T P, Urenjak J. Prog Neurobiol. 1997;51:39–87. doi: 10.1016/s0301-0082(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 4.Drejer J, Benveniste H, Diemer N H, Schousboe A. J Neurochem. 1985;45:145–151. doi: 10.1111/j.1471-4159.1985.tb05486.x. [DOI] [PubMed] [Google Scholar]
- 5.Bosley T M, Woodhams P L, Gordon R D, Balazs R. J Neurochem. 1983;40:189–201. doi: 10.1111/j.1471-4159.1983.tb12670.x. [DOI] [PubMed] [Google Scholar]
- 6.Hershkowitz N, Katchman A N, Veregge S. J Neurophysiol. 1993;69:432–441. doi: 10.1152/jn.1993.69.2.432. [DOI] [PubMed] [Google Scholar]
- 7.Madl J E, Burgesser K. J Neurosci. 1993;13:4429–4444. doi: 10.1523/JNEUROSCI.13-10-04429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roettger V, Lipton P. Neuroscience. 1996;75:677–685. doi: 10.1016/0306-4522(96)00314-4. [DOI] [PubMed] [Google Scholar]
- 9.Kimelberg H K, Goderie S K, Higman S, Pang S, Waniewski R A. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szatkowski M, Barbour B, Attwell D. Nature (London) 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- 11.Pocock J M, Nicholls D G. J Neurochem. 1998;70:806–813. doi: 10.1046/j.1471-4159.1998.70020806.x. [DOI] [PubMed] [Google Scholar]
- 12.Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gähwiler B H, Gerber U. Proc Natl Acad Sci USA. 1999;96:8733–8738. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gähwiler B H, McKinney R A, Debanne D, Robertson R T. In: Organotypic Slice Cultures of Neuronal Tissue. Banker G, Goslin K, editors. Cambridge, MA: MIT Press; 1998. pp. 461–498. [Google Scholar]
- 14.Dubinsky J M, Rothman S M. J Neurosci. 1991;11:2545–2551. doi: 10.1523/JNEUROSCI.11-08-02545.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergey G K, Bugalke H, Nelson P G. J Neurophysiol. 1987;57:121–131. doi: 10.1152/jn.1987.57.1.121. [DOI] [PubMed] [Google Scholar]
- 16.Sah P, Hestrin S, Nicoll R A. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara N, Higashi H, Shimoji K, Yoshimura M. J Physiol (London) 1987;384:131–151. doi: 10.1113/jphysiol.1987.sp016447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 19.Niemann H, Blasi J, Jahn R. Trends Cell Biol. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 20.Capogna M, McKinney R A, O'Connor V, Gähwiler B H, Thompson S M. J Neurosci. 1997;17:7190–7202. doi: 10.1523/JNEUROSCI.17-19-07190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fatt P, Katz B. J Physiol (London) 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 22.Sarantis M, Attwell D. Brain Res. 1990;516:322–325. doi: 10.1016/0006-8993(90)90935-5. [DOI] [PubMed] [Google Scholar]
- 23.Otis T S, Jahr C E. J Neurosci. 1998;18:7099–7110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bridges R J, Stanley M S, Anderson M W, Cotman C W, Chamberlin A R. J Med Chem. 1991;34:717–725. doi: 10.1021/jm00106a037. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths R, Dunlop J, Gorman A, Senior J, Grieve A. Biochem Pharmacol. 1994;47:267–274. doi: 10.1016/0006-2952(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 26.Volterra A, Bezzi P, Rizzini B L, Trotti D, Ullensvang K, Danbolt N C, Racagni G. Eur J Neurosci. 1996;8:2019–2028. doi: 10.1111/j.1460-9568.1996.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 27.Hansen A J. Physiol Rev. 1985;65:101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- 28.Hammond C, Crepel V, Gozlan H, Ben-Ari Y. Trends Neurosci. 1994;17:497–503. doi: 10.1016/0166-2236(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y T, Salter M W. Nature (London) 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 30.Lobner D, Lipton P. J Neurosci. 1993;13:4861–4871. doi: 10.1523/JNEUROSCI.13-11-04861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braunton J L, Wong V, Wang W, Salter M W, Roder J, Liu M, Wang Y T. Neuroscience. 1998;82:161–170. doi: 10.1016/s0306-4522(97)00286-8. [DOI] [PubMed] [Google Scholar]
- 32.Traynelis S F, Cull-Candy S G. Nature (London) 1990;345:347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- 33.Rassendren F A, Lory P, Pin J P, Nargeot J. Neuron. 1990;4:733–740. doi: 10.1016/0896-6273(90)90199-p. [DOI] [PubMed] [Google Scholar]
- 34.Ozawa S, Iino M, Tsuzuki K. J Neurophysiol. 1990;64:1361–1367. doi: 10.1152/jn.1990.64.5.1361. [DOI] [PubMed] [Google Scholar]
- 35.Choi D W, Koh J Y. Annu Rev Neurosci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 36.Katchman A N, Hershkowitz N. Neurosci Lett. 1994;168:217–220. doi: 10.1016/0304-3940(94)90454-5. [DOI] [PubMed] [Google Scholar]
- 37.Katchman A N, Hershkowitz N. J Neurophysiol. 1993;70:1–7. doi: 10.1152/jn.1993.70.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Prieto J, Sihra T S, Nicholls D G. J Neurochem. 1987;49:58–64. doi: 10.1111/j.1471-4159.1987.tb03394.x. [DOI] [PubMed] [Google Scholar]
- 39.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini B L, Pozzan T, Volterra A. Nature (London) 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 40.Kanner B I, Bendahan A. Biochemistry. 1982;21:6327–6330. doi: 10.1021/bi00267a044. [DOI] [PubMed] [Google Scholar]
- 41.Billups B, Attwell D. Nature (London) 1996;379:171–174. doi: 10.1038/379171a0. [DOI] [PubMed] [Google Scholar]
- 42.Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. J Neurosci. 1994;14:2924–2932. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spiridon M, Kamm D, Billups B, Mobbs P, Attwell D. J Physiol (London) 1998;506:363–376. doi: 10.1111/j.1469-7793.1998.363bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gegelashvili G, Schousboe A. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- 45.Verdoorn T A, Kleckner N W, Dingledine R. Mol Pharmacol. 1989;35:360–368. [PubMed] [Google Scholar]
- 46.Sather W, Dieudonne S, MacDonald J F, Ascher P. J Physiol (London) 1992;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakanishi N, Axel R, Shneider N A. Proc Natl Acad Sci USA. 1992;89:8552–8556. doi: 10.1073/pnas.89.18.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellomo M, Giuffrida R, Palmeri A, Sapienza S. Arch Ital Biol. 1998;136:215–223. [PubMed] [Google Scholar]
- 49.Beckstrom H, Julsrud L, Haugeto O, Dewar D, Graham D I, Lehre K F, Storm-Mathisen J, Danbolt N C. J Neurosci Res. 1999;55:218–229. doi: 10.1002/(SICI)1097-4547(19990115)55:2<218::AID-JNR9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 50.Beckman M L, Quick M W. J Membr Biol. 1998;164:1–10. doi: 10.1007/s002329900388. [DOI] [PubMed] [Google Scholar]
- 51.Billups B, Rossi D, Oshima T, Warr O, Takahashi M, Sarantis M, Szatkowski M, Attwell D. Prog Brain Res. 1998;116:45–57. doi: 10.1016/s0079-6123(08)60429-x. [DOI] [PubMed] [Google Scholar]
- 52.Frandsen A, Schousboe A. Int J Dev Neurosci. 1990;8:209–216. doi: 10.1016/0736-5748(90)90013-r. [DOI] [PubMed] [Google Scholar]
- 53.Beal M F, Hyman B T, Koroshetz W. Trends Neurosci. 1993;16:125–131. doi: 10.1016/0166-2236(93)90117-5. [DOI] [PubMed] [Google Scholar]
- 54.Rossi D J, Oshima T, Attwell D. Nature (London) 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]