Abstract
Motor learning and neuro-adaptations to drugs of abuse rely upon neuronal signaling in the striatum. Cyclin-dependent kinase 5 (Cdk5) regulates striatal dopamine neurotransmission and behavioral responses to cocaine. Although the role for Cdk5 in neurodegeneration in the cortex and hippocampus and in hippocampal-dependent learning has been demonstrated, its dysregulation in the striatum has not been examined. Here we show that strong activation of striatal NMDA receptors produced p25, the truncated form of the Cdk5 co-activator p35. Furthermore, inducible overexpression of p25 in the striatum prevented locomotor sensitization to cocaine and attenuated motor coordination and learning. This corresponded with reduced dendritic spine density, increased neuro-inflammation, altered dopamine signaling, and shifted Cdk5 specificity with regard to physiological and aberrant substrates, but no apparent loss of striatal neurons. Thus, dysregulation of Cdk5 dramatically affects striatal-dependent brain function and may be relevant to non-neurodegenerative disorders involving dopamine neurotransmission.
Keywords: p25, spines, addiction, dopamine, neurodegeneration
Neuronal signal transduction mediates the regulation of synaptic strength that underlies learning and memory. Dysregulation of synaptic signaling may result in neuropsychiatric and neurological disorders. Striatal medium spiny neurons (MSNs) receive midbrain dopaminergic and cortical glutamatergic input and mediate reward-based and motor learning. Furthermore, numerous psychiatric and neurodegenerative illnesses target striatal function. Study of the effects of dysregulation of signaling mechanisms that integrate striatal glutamate and dopamine neurotransmission may provide an understanding of the etiology of such disorders.
Cdk5 regulates dopamine neurotransmission (1, 2) and striatal neuron excitability (1, 3). Cocaine targets dopamine neurotransmission by elevating synaptic dopamine levels. Cdk5 modulates the effects of cocaine, including its ability to induce neuro-adaptations in signaling, behavior, and neuronal morphology (3–7). Cdk5 also functions in synaptic plasticity and learning (8–10) in hippocampus. Striatal synaptic plasticity involves changes in Cdk5-dependent regulation of dopamine signaling (11), and conditional loss of Cdk5 in the striatum lowers the threshold dose required for cocaine to induce a place preference (3). However, dysregulation of Cdk5 in the striatum and its effects on striatal-mediated behavior and learning has remained largely unexplored.
Cdk5 activity is dependent on association with its cofactor p35. Cdk5 may be regulated through auto-phosphorylation of p35 (12, 13) and glutamatergic Ca2+ signaling (14, 15). However, the most prominent feature of Cdk5 regulation may be its hyper-activation upon removal of the first 100 amino acids of p35 by the Ca2+-dependent protease calpain. The resulting Cdk5/p25 complex engenders aberrant activity and is neurotoxic. P25 overexpression in mouse hippocampus and cortex results in dramatic neurodegeneration and memory loss (16, 17). Interestingly, transient p25 expression initially enhances synaptic plasticity and memory (18), raising the possibility of a physiological function for controlled levels of p25 that may be generated during excitatory neurotransmission. Here we evaluated the ability of NMDA receptor activation to produce p25 and then targeted the striatum for inducible transgenic overexpression of p25 as a model for dysregulation of Cdk5 in this brain region. The results reveal that the striatum is resistant to the neurodegenerative effects of aberrant Cdk5 activity but that p25 expression nonetheless causes deleterious behavioral, histological, and biochemical effects and impairs the function of this brain region.
Results
P25 Generation by Striatal NMDA Receptor Activation and Production of Transgenic Mice Overexpressing p25 in the Striatum.
Striatal glutamate released from the synaptic terminals of prefrontal cortical neurons elevates MSN intracellular Ca2+, and these neurons are susceptible to glutamatergic excitotoxicity. To assess the ability of glutamate to activate calpain and invoke endogenous synaptic p25 generation, acute dorsal striatum mouse slices were treated with NMDA (Fig. 1A). P25 production was dose- and time-dependent, with prominent amounts being produced in response to excitotoxic levels of NMDA. This was specific for NMDA receptor activation, as the antagonist MK801 blocked the effect [supporting information (SI) Fig. S1A]. Furthermore, treatment of primary cultured rat striatal neurons with NMDA caused p25 production throughout the neuronal soma and processes as detected by a novel p25-specific monoclonal antibody (Fig. 1B). These data demonstrate that p25 is produced by glutamatergic activity in striatal neurons.
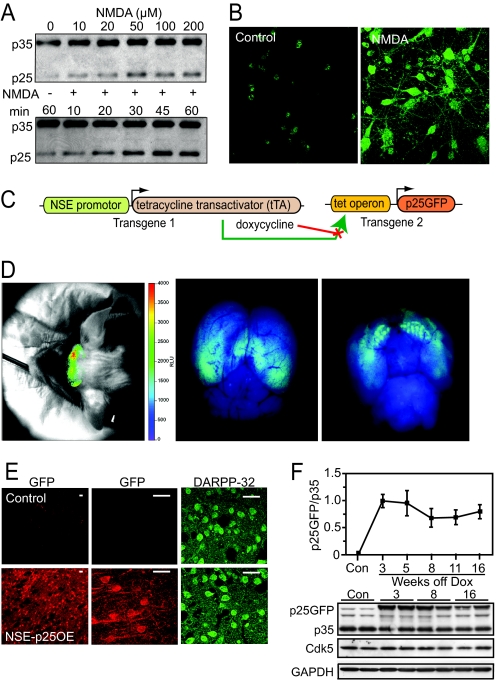
Fig. 1.
Excitotoxic generation of p25 and transgenic overexpression. (A) Dose- (Top) and time- (Bottom) dependent generation of p25 in acute striatal slices treated with NMDA for 30 min at the indicated concentration (Top) or at 100 μM for the indicated time intervals (Bottom). (B) Immunostains of p25 in primary rat striatal cultures under control or NMDA (100 μM, 5 min) treatment. (C) Schematic of the inducible bi-transgenic NSE-tTA/tetOp-p25GFP system. (D) Optical fluorescence image of p25GFP expression in vivo (Left) and in vitro (Middle, dorsal view; Right, ventral view) after dissection of the p25GFP-expressing brain. (E) Immunostains of p25GFP (anti-GFP, Left) and DARPP-32 (Right) in control and NSE-p25OE mouse striatum. (Scale bars, 25 μm.) (F) Temporal expression of p25GFP transgene versus endogenous p35 in control and NSE-p25OE mouse striatum (n = 5–7 per group), with immunoblots for control (Con, 16 weeks after doxycycline removal) and NSE-p25OE (3, 8, and 16 weeks after doxycycline removal) striatum shown. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serves as a loading control.
To better understand the consequences of potentially excessive striatal p25 generation, we derived an in vivo model of p25 overexpression by crossing tet operon (tetOp) p25GFP expressing mice (16) with a line containing the tetracycline transactivator (tTA) under the direction of the neuron-specific enolase (NSE) promoter (Fig. 1C), which produces high levels of transgene expression in the dorso-lateral striatum (19, 20). Thus we generated a bi-transgenic mouse line (NSE-p25OE) in which high expression of p25GFP could be selectively induced in the forebrain following removal of the tetracycline derivative doxycycline, beginning at 3 weeks of age, as shown in Figs. 1 D–F. Specific expression was detected via in vivo imaging of GFP-mediated optical fluorescence in live transgenic mice 8 weeks following removal of dietary doxycycline (Fig. 1D). Expression was prominent in forebrain regions (Fig. 1D, Left), and was confirmed by imaging of GFP-dependent fluorescence following removal of the mouse brain (Fig. 1D, Middle, Right). P25GFP expression was prominently detected in striatal MSNs of NSE-p25OE mice by immunohistochemistry (Fig. 1E), and was absent in the non-expressing litter-mate controls. Expression was also apparent in hippocampal (dorsal view; Fig. 1D, Middle) and striatal regions (ventral view; Fig. 1D, Right). Immunoblotting confirmed high levels in striatum 3 weeks following removal of doxycycline that peaked between 3 and 5 weeks after doxycycline removal, before reaching steady-state levels that were maintained up to 16 weeks after doxycycline removal. Lower levels of expression were observed in the hippocampus and cortex (Fig. S1C). Little or no expression was observed in the cerebellum of NSE-p25OE mice. In contrast to the induced high levels of p25GFP, no change occurred in striatal Cdk5 or p35 (Fig. 1F). Consequently, we derived a mouse that inducibly overexpressed fluorescent p25 in striatum.
Striatal p25 Overexpression Results in Loss of Cocaine Sensitization and Motor Coordination Deficits.
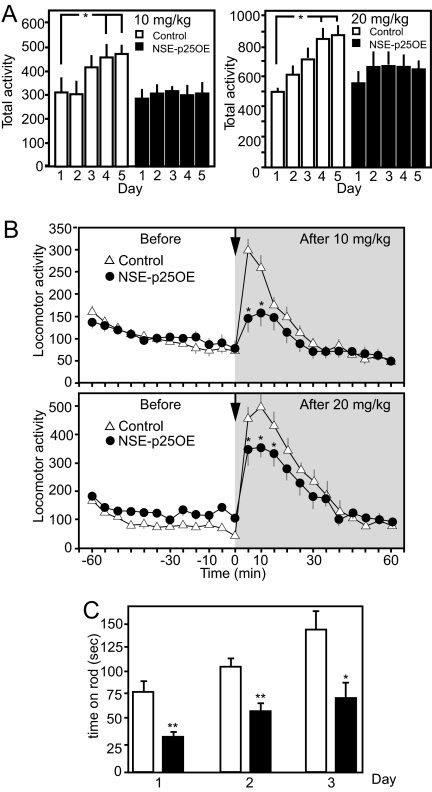
Cdk5-dependent regulation of dopamine signaling modulates behavioral effects of psychomotor stimulants (4, 5, 21). Whereas pharmacological or genetic inactivation of Cdk5 enhances cocaine responses (3, 7), the influence of p25-dependent dysregulation of Cdk5 on the behavioral effects of cocaine has not been examined. Therefore, baseline motor activity and locomotor responses to cocaine were evaluated for NSE-p25OE versus litter-mate control mice at 8 weeks after doxycycline removal (Fig. 2 A and B). NSE-p25OE mice exhibited normal baseline motor activity (21.8 ± 4.6 beam breaks, controls vs. 24.9 ± 1.9, NSE-p25OE mice, n = 7). Furthermore, locomotor responses to initial or acute doses of 10 mg/kg (i.e., low dose) or 20 mg/kg (i.e., high dose) of cocaine were the same in control and NSE-p25OE mice. However, in control mice, both high and low cocaine doses produced progressively greater increases in locomotor activity over 5 consecutive days (Fig. 2A). In contrast, the ability of prior drug exposure (low or high doses) to augment the effects of cocaine was completely absent in NSE-p25OE mice. In comparison to day 1, locomotor activity of controls was significantly greater on days 4 and 5 for both the low (1.4 ± 0.2 fold and 1.5 ± 0.2 fold) and high (1.7 ± 0.2 fold and 1.8 ± 0.1 fold) cocaine doses. However, no such increases occurred in NSE-p25OE mice. Consequently, locomotor responses on days 4 and 5 were significantly reduced in NSE-p25OE mice. Examination of the temporal features of the locomotor response for 60 min following cocaine administration in 5-min bins on day 5 revealed dramatic reduction in the effects of cocaine that were apparent over the initial 30 min, when the motor activity was greatest (Fig. 2B). Thus, dysregulation of Cdk5 in the striatum interfered with the development of locomotor sensitization to cocaine.
Fig. 2.
Effects of p25 overexpression on responses to repeated cocaine administration and motor learning. (A) Total locomotor activity (beam breaks) for the first 10 min following injection of 10 mg/kg (Left, n = 12 controls, nine NSE-p25OE) or 20 mg/kg (Right, n = 5 per group) cocaine on 5 successive days for control and NSE-p25OE mice (*, P < 0.05, one-way ANOVA followed by Student-Neumann-Keuls post-hoc test). (B) Time course of locomotor activity in response to administration of 10 mg/kg (Top) or 20 mg/kg (Bottom) of cocaine with activity over 60 min before (white background) and after (gray background) injection of control and NSE-p25OE on day 5. Arrow marks injection point. (C). Performance of control and NSE-p25OE mice on the rotarod apparatus (*, P < 0.05 and **, P < 0.01, two-way ANOVA followed by Bonferroni post-hoc test, n = 7).
Dopamine neurotransmission functions in motor performance (22), and cortico-striatal circuitry is thought to modulate aspects of motor learning (23, 24). To assess the effect of striatal p25 overexpression upon motor coordination, control and NSE-p25OE mice were evaluated for performance on the “rotarod” task. NSE-p25OE mice at 8 weeks after doxycycline removal exhibited a noticeable lack of motor coordination as evidenced by an inability to stay mounted during the task (Fig. 2C). Furthermore, two-way ANOVA (genotype × day) revealed that, although all mice increased the average time spent on the rod each day (F [2, 22] = 21.5, P < 0.001) with no interaction between genotype and average time spent on the rod (P > 0.1), there was a main effect of genotype such that NSE-p25OE mice spent significantly less time on the rod than controls (F [1, 22] = 13.53, P < 0.01). Thus, performances on two measures of dopamine- and striatal-dependent motor behavior were compromised by Cdk5 dysregulation.
Histological, Morphological, and Biochemical Changes in NSE-p25OE Striatum.
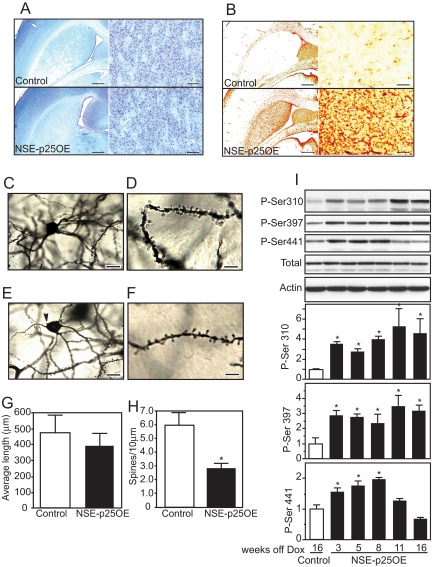
Activation of Cdk5 by p25 overexpression has been shown to induce neuronal cell death evident by 5 weeks in the hippocampus and cortex (16). However, histological evaluation of the effects of p25 overexpression in NSE-p25OE mice revealed no detectible evidence of neurodegeneration up to 20 weeks off doxycycline, as assessed by fluoro-gold or silver staining of NSE-p25OE mouse tissue (data not shown). Furthermore, no obvious loss of neurons was evident in Nissl-stained striatal sections at 20 weeks off doxycycline (Fig. 3A). Despite the lack of neurodegeneration, high levels of glial fibrillary acidic protein (GFAP), a marker of activated astrocytes, were evident by 12 weeks after doxycycline removal (Fig. 3B), indicating that p25 overexpression produced a potent neuro-inflammatory response. This effect was accompanied by significant changes in neuronal morphology as determined by Golgi staining. Control and NSE-p25OE MSN somas were similar in size (19.6 ± 4.5 μm vs. 17.5 ± 1.5 μm in diameter, respectively) and dendritic length was not significantly affected by p25 overexpression (Fig. 3G). However, NSE-p25OE somas lost the typical smooth oval appearance (Fig. 3C, control MSN) and were more often characterized by a tapered balloon shape with prominent somal blebbing (Fig. 3E). In addition, NSE-p25OE MSNs exhibited significantly decreased spine density (Fig. 3 D and F; 47.1% ± 6.6% of controls; 5.97 ± 0.92 spines/10 μm of dendrite for control vs. 2.81 ± 0.40 spines/10 μm for NSE-p25OE; P < 0.05, Student t test, n = 7 animals, 3–5 neurons per animal) with no significant effect upon dendritic length (479.9 ± 107.3 μm for control vs. 393.0 ± 70.2 μm for NSE-p25OE). Furthermore, p25 overexpression had no effect on the number of dendritic branches or somal area. Thus, p25-dependent dysregulation of Cdk5 results in detrimental changes in MSN morphology and possibly synaptic connectivity, corresponding with the observed behavioral deficits.
Fig. 3.
Astrogliosis, morphological changes, and actin regulation in NSE-p25OE mice. Nissl stain of coronal sections from control (Top) and NSE-p25OE (Bottom) mouse striatum at 20 weeks after doxycycline removal at low and high magnification. (Scale bars, 2 mm [Left] and 100 μm [Right].) (B) Immunohistochemical stain of GFAP in control (Top) and NSE-p25OE (Bottom) mouse striatum. (Scale bars, 2 mm [Left] and 100 μm [Right].) (C–F) Bright-field micrographs of Golgi-Cox stained striatal MSNs from control (C and D) and NSE-p25OE (E and F) mice at low (Left) and high (Right) magnification. (Scale bars, 20 μm in C and E; 8 μm in D and F). Arrow (E) marks typical somal blebbing seen in NSE-p25OE MSNs. (G and H) Quantitation of dendritic length and spine density in control and NSE-p25OE mice (*, P < 0.05, Student t test). (I) Immunoblots and quantitation of Cdk5-dependent phosphorylation of WAVE1 in control and NSE-p25OE mouse striatum (*, P < 0.05, one-way ANOVA followed by Student-Neumann-Keuls post-hoc test).
Neuro-adaptive responses to chronic cocaine exposure include increases in dendritic spines that are dependent upon Cdk5 (6) and involve regulation of synaptic actin cytoskeletal dynamics. WAVE1 is an important Cdk5 effector that regulates Arp2/3 complex-dependent synaptic actin polymerization (25, 26). Cdk5-dependent phosphorylation of WAVE1 at Ser-310, 397, and 441 inhibits its role in actin polymerization and spine formation. Previous studies raise the possibility that NMDA-dependent inactivation of Cdk5 and reduction in the phosphorylation of these sites may be necessary for spine morphogenesis (14, 15, 26). Interestingly, Cdk5-dependent phosphorylation of WAVE1 at all three sites was elevated in response to overexpression of p25 in NSE-p25OE mice (Fig. 3I). WAVE1 phosphorylation at Cdk5-dependent sites was increased by 3 weeks after doxycycline removal and remained elevated to 16 weeks. At 8 weeks after doxycycline removal, Cdk5-mediated phosphorylation of WAVE1 was increased significantly at Ser-310 (4.0 ± 0.3 fold), Ser-397 (2.4 ± 0.6 fold), and Ser-441 (2.0 ± 0.04 fold).
Alterations in Cdk5-Dependent Signaling in NSE-p25OE Mice.
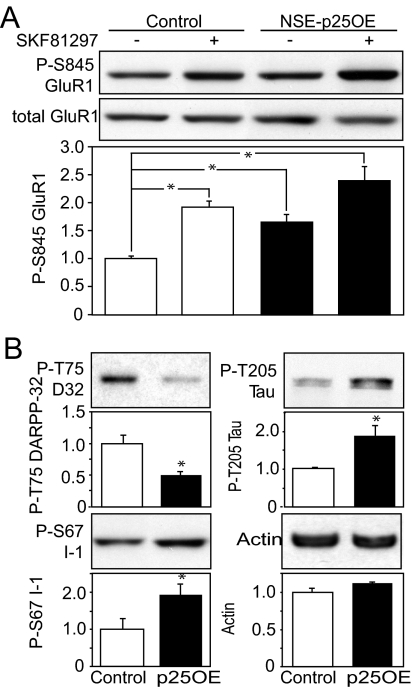
Dopamine-dependent regulation of glutamate receptor signaling is an important mechanism contributing to responses to drugs of abuse and motor coordination (27–29). Because NSE-p25OE mice show deficits in both locomotor sensitivity to cocaine administration and general motor coordination and learning, it is possible that they have impairments in either dopamine or glutamate signaling. Therefore, the regulation of protein kinase A (PKA)-dependent phosphorylation of Ser-845 of the GluR1 subunit of the AMPA receptor by D1 dopamine receptor activation was evaluated by immunoblotting acute striatal slice lysates. Interestingly, 8 weeks after doxycycline removal, NSE-p25OE mice exhibited significantly increased basal phosphorylation at Ser-845 of the glutamate GluR1 receptor subunit (Fig. 4A; 1.65 ± 0.1 fold higher than control) and, consequently, a blunted response to the dopamine D1-type dopamine receptor agonist SKF-81297 (1.45 ± 0.2 fold increase) compared with control mice (1.95 ± 0.1 fold increase).
Fig. 4.
Modulation of D1 receptor signaling, PKA-dependent phosphorylation, and Cdk5-dependent phosphorylation of physiological and aberrant substrates. (A) Effect of a D1 receptor agonist on phospho-Ser-845 GluR1. Immunoblots of lysates from untreated (−) and SKF81297-treated (+) acute striatal slices from control and NSE-p25OE mice are shown (Top) with quantitation normalized for total GluR1 (*, P < 0.05, one-way ANOVA followed by Student-Neumann-Keuls post-hoc test, n = 6). (B) Immunoblots and quantitation of Cdk5-dependent phosphorylation of Thr-75 DARPP-32 (P-T75), Thr-205 Tau (P-T205), Ser-67 l-1 (P-S67 I-1), and actin in control and NSE-p25OE (p25OE) mouse striatum (*, P < 0.05, Student t test, n = 6).
Cdk5 modulates dopamine signaling via the phosphorylation of the protein phosphatase 1 inhibitor, dopamine, and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) at Thr-75, thereby converting it to an inhibitor of PKA (1). Basal levels of phospho-Thr-75 DARRP-32 were decreased in NSE-p25OE striatal lysates to 42.0% ± 6.0% of controls (Fig. 4B), consistent with Cdk5 activity being shifted away from DARPP-32 and, as a result, basal PKA activity being elevated in these mice. Cdk5 also phosphorylates the DARPP-32 homologue protein phosphatase 1 inhibitor-1 (I-1) at Ser-67 (30). However, phosphorylation at this site in NSE-p25OE mice appears to be regulated differently than phospho-Thr-75 DARPP-32, as phospho-Ser-67 was elevated (1.93 ± 0.3 fold; P < 0.05, via Student t test) at 8 weeks after doxycycline removal.
Aberrant Cdk5 activity toward a number of substrates may have deleterious effects and contribute to neurodegeneration (16). In examining the effects of p25 overexpression in the striatum, Cdk5-dependent phosphorylation of the microtubule binding protein tau at Ser-205 was found to be elevated 1.89 ± 0.3 fold. In contrast, Cdk5-dependent phosphorylation of the microtubule binding protein stathmin at Ser-38 (31) and the amyloid precursor protein at Thr-668 (32) were unaffected (data not shown). These findings indicate that dysregulation of Cdk5 in the striatum may alter dopamine signaling and the specificity of Cdk5 in a manner that is consistent with behavioral and morphological deficits that do not extend to neuronal death. Cdk5 also regulates presynaptic release of dopamine by decreasing the rate of dopamine production via phosphorylation of tyrosine hydroxylase (TH) at Ser-40 (33). It is possible that this is a contributing factor to the behavioral and biochemical changes in the NSE-p25OE mice, because changes in presynaptic release have been shown to contribute to decreased sensitivity to cocaine in a model of Huntington disease (HD) in which Cdk5 activity has been implicated (34, 35). However, phosphorylation at Ser-40 TH was not significantly different in NSE-p25OE striatum from controls (83.9% ± 14%; P > 0.5, Student t test) (data not shown).
Discussion
Dysregulation of striatal dopamine signaling is associated with neurological and neuropsychiatric disorders. We have demonstrated that Cdk5 regulates dopamine neurotransmission, but the ability of over-activation of Ca2+-permeable ionotropic glutamate receptors to generate p25 and the long-term consequences of Cdk5 dysregulation by p25 in the striatum have not been previously evaluated. Here we showed that glutamate receptor activation caused p25 production. Although appreciable p25 levels were generated in striatal tissue in response to excitotoxic levels of NMDA, some p25 was detected when striatal tissue or primary cultures were exposed to glutamate at lower concentrations or for only brief periods. So far, p25 production has been primarily associated with neurotoxicity and neurodegeneration. However, low levels of p25 may be produced during intense synaptic activity such as that which accompanies learning. To date, no physiological role for p25 has been assigned, and whether limited amounts of Cdk5/p25 are necessarily sequestered or have a specific function in mediating synaptic remodeling remains to be determined.
We found that transgenic overexpression of p25 in the striatum resulted in the loss of locomotor sensitization. The ability of cocaine to produce progressively greater increases in behavioral responses provides demonstration of the neuro-adaptations that may impart reinforcing properties upon this addictive drug. In contrast to the effects of aberrant dysregulation and hyper-activation of Cdk5 in the NSE-p25OE mice, pharmacological as well as transgenic inhibition of striatal Cdk5 enhances locomotor and other responses to cocaine as well as natural reward, and the development of locomotor sensitization (3, 5, 7). It should also be noted that the low levels of p25 expression induced in the cortex and hippocampus could contribute to the observed behavioral deficits
The behavioral deficits induced by p25 expression are consistent with biochemical studies that showed reduced D1 receptor-mediated activation of the PKA/phospho-Ser-845 GluR1 pathway. P25 overexpression also reduced phosphorylation of Thr-75 DARPP-32 and increased phosphorylation of Ser-67 I-1. Apparently the change in substrate specificity that accompanies formation of the Cdk5/p25 complex directs the kinase away from DARPP-32. Although this is surprising, it is consistent with previous reports of reduced phosphorylation of some physiological substrates (16) and with the elevations in basal levels of PKA-dependent phosphorylation of Ser-845 GluR1 that corresponds with reduced phospho-Thr-75 levels (1). It also raises the possibility that this signaling pathway is invoked during selective synaptic excitatory neurotransmission.
Loss of dendritic spines accompanied p25 overexpression. Although hyper-phosphorylation of WAVE1 implicates this regulatory mechanism of the synaptic cytoskeleton, it is possible that the spine density reduction was also mediated by additional direct or indirect mechanisms. Overexpression of p25 resulted in astroglial activation (i.e., astrogliosis) as evidenced by enhanced expression of GFAP. Astrogliosis is a hallmark of CNS damage, and robust and sustained astrogliosis accompanies excessive glutamate signaling and dendritic spine loss in other brain regions (36, 37). It remains to be determined precisely how the aberrant activation of Cdk5, altered actin regulation, and loss of spines leads to astrogliosis. However, that p25 overexpression induced this response independent of neuronal cell loss suggests that it may serve as an immediate “perpetrator” linking early or subtle aspects of excitotoxicity to the astroglial activation response.
P25 overexpression has been shown to rapidly induce the death of neurons (17, 18). Somewhat remarkably, MSNs remained alive, albeit apparently compromised in function, during p25 overexpression. Although striatum is susceptible to neurotoxic insults, including necrotic death accompanying ischemia and stroke-related brain injury, it remains relatively resistant to the neurodegenerative effects of Alzheimer disease. In contrast, MSNs are selectively targeted by HD, and HD model mice exhibit nearly identical deficits in responses to cocaine (34, 35) as NSE-p25OE mice. Numerous neuropsychiatric illnesses and disorders including schizophrenia, attention deficit-hyperactivity disorder, and drug addiction likely involve dysregulation of signaling pathways that integrate glutamate and dopamine signaling. That p25 overexpression induced such dramatic deficiencies but did not cause loss of striatal neurons raises the possibility that it may serve as a contributing factor to non-neurodegenerative neuropsychiatric disorders.
Materials and Methods
NSE-p25OE Mouse Generation.
A bi-transgenic mouse line expressing p25GFP selectively in the forebrain was generated by crossing mice containing the p25GFP transgene under the direction of the tetOp (16) with a line containing the tetracycline tTA coupled to the NSE promoter (19, 20). Both breeding lines, tetOp and NSE-tTA, were produced on mixed C57BL/6 backgrounds, and non-p25GFP-expressing litter-mates were used in all experiments to control for any possible strain-specific effects. Breeding dams and neonates were kept on doxycycline to suppress transcription of the p25GFP transgene, and doxycycline administration was discontinued in pups at 3 weeks of age. Animals were maintained and all experiments were conducted according to institutional animal care and use committee guidelines (University of Texas Southwestern Medical Center, Dallas, TX).
Immunoblot Analysis.
SDS/PAGE and immunoblotting were conducted as previously described (1). Antibodies for p35 (C19) and Cdk5 (J-3) were from Santa Cruz Biotechnology. Anti-phospho-Ser-845 GluR1 and phospho-Ser-40 TH were from PhosphoSolutions; total GluR1 from Chemicon; anti-GAPDH and phospho-Thr-205 Tau from Sigma; anti-Tau (Tau-5) and phospho-Thr-668 Tau from Biosource. Anti-phospho-Thr-75 DARPP-32 (1), phospho-Ser-67 I-1 (30), and anti-phospho-WAVE1 (25) antibodies have been previously described. Anti-phospho-Ser-38 stathmin (31) was provided by André Sobel and Patrick Curmi (INSERM, Paris, France). Anti-beta actin and anti-GFP antibodies were from Abcam, Inc. The p25-specific monoclonal antibody was generated in mice injected with pure Cdk5/p25 and was determined to only recognize p25 and not p35 by immunoblotting and immunohistochemistry.
Striatal Cultures and Immunohistochemistry.
Embryonic striatal neurons (E18) from Long Evans rats (Charles River Labs) were cultured 14 to 21 days in vitro on 12-mm coverslips and incubated under either control or 100 μM NMDA treatment conditions for 5 min at 37 °C. Cells were then fixed and labeled with a mouse monoclonal antibody that specifically recognizes p25, but not p35 (Fig. S1B) and imaged as previously described (33).
In Vivo Imaging.
Imaging of p25GFP fluorescence in NSE-p25OE mouse brains was conducted as previously described (38). Digitized images were stored offline and post-processed using ImageJ (National Institutes of Health) and Photoshop (Adobe Systems) software.
NSE-p25OE Mouse Behavioral Experiments.
Spontaneous locomotor activity cocaine response assays were conducted and analyzed as previously described (3). For the rotarod motor coordination test, mice were placed on a rotarod (IITC Life Science) that accelerated from 5 to 45 rpm over the 5-min test period. The time at which mice fell from the rod, or after mice had rotated two full rotations without regaining balance, was recorded as the test end point. Mice were tested five times a day for 3 days and the average time on rod for each day was reported. Average time on the rod was used to analyze rotarod data.
Histological Analysis.
Brains were fixed using a standard paraformaldehyde fixation format, cryoprotected, and sectioned in the horizontal plane (25 μm). Selected sections were stained to evaluate neurodegeneration using Nissl and Fluoro-Jade B as previously described (39). To examine reactive gliosis, additional sections were immunostained for GFAP with the modification that the dilutions of primary and secondary antibodies were 1:10,000. Golgi-Cox staining was performed as previously described (40). Full reconstructions were made by tracing individual neurons using Neurolucida (MicroBrightfield). The dendrites from individual MSNs were digitally reconstructed and morphological data were analyzed using NeuroExplorer. Spine density was calculated by taking a random 100- to 150-μm section after the third branch point on each major dendrite. The values from each dendrite were averaged, and this value was used to determine the average spine density of each analyzed MSN. Images were obtained using a ×100 oil emersion objective via an Optronics Microfire CCD attached to an Olympus BX51 microscope.
Pharmacological Experiments.
Striatal experiments were conducted as previously described (1). For generation of p25 by NMDA exposure, normal Krebs buffer was replaced by Krebs buffer containing 3 mM Ca2+ and 0 mM Mg2+ to increase Ca2+ current through NMDARs and enhance calpain-mediated cleavage of p35. For D1 agonist experiments, slices were treated with 1 μM SKF81297 for 5 min. All tissue samples were fast-frozen on dry ice and kept frozen at −80 °C until immunoblotting.
Supplementary Material
Acknowledgments.
We thank Eric Nestler for the NSE line, and Cathy Steffen and Shari Birnbaum for breeding, genotyping, and behavioral studies assistance. This work was supported by basic science training program T32-DA7290 in drug abuse (D.A.M.); National Institute on Drug Abuse grants DA16672 (to J.A.B.) and DA10044 (to P.G. and A.C.N.); National Institute of Mental Health grants MH079710–0 (to J.A.B.) and MH074866 (to P.G. and A.C.N.); and National Heart, Lung, and Blood Institute grant HL077101 (to J.A.B.). Support was also provided via a Whitehall Foundation Grant (C.W.C.) and the Picower Foundation (P.G.). Generation of the p25-GFP mouse line and feasibility studies were made possible by the support of the Howard Hughes Medical Institute and National Institutes of Health grant NS051874 (L.-H.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806078105/DCSupplemental.
References
- 1.Bibb JA, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signaling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 2.Chergui K, Svenningsson P, Greengard P. Cyclin-dependent kinase 5 regulates dopaminergic and glutamatergic transmission in the striatum. Proc Natl Acad Sci USA. 2004;101:2191–2196. doi: 10.1073/pnas.0308652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benavides DR, et al. Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J Neurosci. 2007;27:1967–12976. doi: 10.1523/JNEUROSCI.4061-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibb JA. The role of Cdk5 in neuronal plasticity and drug abuse. Neurosignals. 2003;12:191–199. doi: 10.1159/000074620. [DOI] [PubMed] [Google Scholar]
- 5.Bibb JA, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- 6.Norholm S, et al. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor JR, et al. Inhibition of cyclin dependent kinase 5 in the nucleus accumbens enhances the locomotor activating and incentive motivational effects of cocaine. Proc Natl Acad Sci USA. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelo M, Plattner F, Giese KP. Cyclin-dependent kinase 5 in synaptic plasticity, learning and memory. J Neurochem. 2006;99:353–370. doi: 10.1111/j.1471-4159.2006.04040.x. [DOI] [PubMed] [Google Scholar]
- 9.Cheung ZH, Fu AK, Ip NY. Synaptic roles of Cdk5: implications in higher cognitive functions and neurodegenerative diseases. Neuron. 2006;50:13–18. doi: 10.1016/j.neuron.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Hawasli AH, et al. Cdk5 Governs Learning, Memory and Synaptic Plasticity via NMDA Receptors. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calabresi P, et al. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J Neurosci. 2000;20:8443–8451. doi: 10.1523/JNEUROSCI.20-22-08443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamei H, et al. Suppression of the calpain-dependent cleavage of Cdk5 activator p35 to p25 by site-specific phosphorylation. J Biol Chem. 2007;282:1687–1694. doi: 10.1074/jbc.M610541200. [DOI] [PubMed] [Google Scholar]
- 13.Saito T, Hisanaga S. [Regulation of Cdk5 activity in post-mitotic neurons] Seikagaku. 2001;73:276–278. [PubMed] [Google Scholar]
- 14.Nguyen C, Hosokawa T, Ip N, Hisanaga S, Bibb JA. Differential regulation of the Cdk5-dependent phosphorylation sites of inhibitor-1 and DARPP-32 by depolarization. J Neurochem. 2007;103:1582–1593. doi: 10.1111/j.1471-4159.2007.04868.x. [DOI] [PubMed] [Google Scholar]
- 15.Wei FY, et al. Downregulation of Cdk5/p35 kinase activity in ionotropic NMDA/kainate receptors and its possible association with long term potentiation. J Neurochem. 2005;93:502–512. [Google Scholar]
- 16.Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 17.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 18.Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, et al. Transgenic animals with inducible, targeted gene expression in the brain. Mol Pharmacol. 1998;54:495–503. doi: 10.1124/mol.54.3.495. [DOI] [PubMed] [Google Scholar]
- 20.Sakai N, et al. Inducible and brain region-specific CREB transgenic mice. Mol Pharmacol. 2002;61:1453–1464. doi: 10.1124/mol.61.6.1453. [DOI] [PubMed] [Google Scholar]
- 21.Lindskog M, et al. The stimulant action of caffeine is mediated by an increase in the state of phosphorylation at the Cdk5 site of DARPP-32. Nature. 2002;428:774–778. doi: 10.1038/nature00817. [DOI] [PubMed] [Google Scholar]
- 22.Campanella G, Roy M, Barbeau A. Drugs affecting movement disorders. Annu Rev Pharmacol Toxicol. 1987;27:113–136. doi: 10.1146/annurev.pa.27.040187.000553. [DOI] [PubMed] [Google Scholar]
- 23.Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 24.Dang MT, et al. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci USA. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Y, et al. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- 26.Sung JY, et al. WAVE1 controls neuronal activity-induced mitochondrial distribution in dendritic spines. Proc Natl Acad Sci USA. 2008;105:3112–3116. doi: 10.1073/pnas.0712180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Lapish CC, Seamans JK, Judson Chandler L. Glutamate-dopamine cotransmission and reward processing in addiction. Alcohol Clin Exp Res. 2006;30:1451–1465. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 30.Bibb JA, et al. Phosphorylation of protein phosphatase inhibitor-1 by Cdk5. J Biol Chem. 2001;276:14490–14497. doi: 10.1074/jbc.M007197200. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi K, et al. Phosphorylation of the tubulin binding protein stathmin by Cdk5 and MAP kinases in the brain. J Neurochem. 2006;99:237–250. doi: 10.1111/j.1471-4159.2006.04113.x. [DOI] [PubMed] [Google Scholar]
- 32.Iijima K, et al. Neuron-specific phosphorylation of Alzheimer's beta-amyloid precursor protein by cyclin-dependent kinase 5. J Neurochem. 2000;75:1085–1091. doi: 10.1046/j.1471-4159.2000.0751085.x. [DOI] [PubMed] [Google Scholar]
- 33.Kansy JW, et al. Identification of tyrosine hydroxylase as a physiological substrate of Cdk5. J Neurochem. 2004;91:374–384. doi: 10.1111/j.1471-4159.2004.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hickey MA, Reynolds GP, Morton AJ. The role of dopamine in motor symptoms in the R6/2 transgenic mouse model of Huntington's disease. J Neurochem. 2002;81:46–59. doi: 10.1046/j.1471-4159.2002.00804.x. [DOI] [PubMed] [Google Scholar]
- 35.Johnson MA, Rajan V, Miller CE, Wightman RM. Dopamine release is severely compromised in the R6/2 mouse model of Huntington's disease. J Neurochem. 2006;97:737–746. doi: 10.1111/j.1471-4159.2006.03762.x. [DOI] [PubMed] [Google Scholar]
- 36.Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osterweil E, Wells DG, Mooseker MS. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J Cell Biol. 2005;168:329–338. doi: 10.1083/jcb.200410091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paroo Z, et al. Validating bioluminescence imaging as a high-throughput, quantitative modality for assessing tumor burden. Mol Imaging. 2004;3:117–124. doi: 10.1162/15353500200403172. [DOI] [PubMed] [Google Scholar]
- 39.Benkovic SA, O'Callaghan JP, Miller DB. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 2004;1024:59–76. doi: 10.1016/j.brainres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 40.Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse—Reverse computer microscopy: application of a new, high clarity Golgi—Nissl stain. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.