Abstract
Combinatorial nuclear transformation is a novel method for the rapid production of multiplex-transgenic plants, which we have used to dissect and modify a complex metabolic pathway. To demonstrate the principle, we transferred 5 carotenogenic genes controlled by different endosperm-specific promoters into a white maize variety deficient for endosperm carotenoid synthesis. We recovered a diverse population of transgenic plants expressing different enzyme combinations and showing distinct metabolic phenotypes that allowed us to identify and complement rate-limiting steps in the pathway and to demonstrate competition between β-carotene hydroxylase and bacterial β-carotene ketolase for substrates in 4 sequential steps of the extended pathway. Importantly, this process allowed us to generate plants with extraordinary levels of β-carotene and other carotenoids, including complex mixtures of hydroxycarotenoids and ketocarotenoids. Combinatorial transformation is a versatile approach that could be used to modify any metabolic pathway and pathways controlling other biochemical, physiological, or developmental processes.
Keywords: induced mutation, metabolic engineering, transgenic plant, provitamin A
Plants produce a vast array of secondary metabolites that have advantageous ecological functions, and many of these molecules also have nutritional and/or pharmacological properties in humans (1). Carotenoids are secondary metabolites that play a role in light perception and photoprotection (2), but they are also required by animals as metabolic precursors and antioxidants, and some have specific health benefits such as the prevention of cancer (3, 4), maintenance of the immune system (4), and the prevention of blindness (5, 6). Animals are unable to synthesize carotenoids directly and must obtain them from their diets. Although fruits and vegetables are particularly good sources of certain carotenoids, cereal grains generally lack these compounds, leading to deficiency diseases in countries where cereals are the staple diet. Also, with the exception of Adonis aestivalis flowers, astaxanthin and other ketocarotenoids are scarce in plants, although they are abundant in fish and shellfish and are used in aquaculture to enhance the aesthetic qualities of salmon (7, 8). For these reasons there is much interest in studying carotenoid biosynthesis pathways in plants and modifying cereal crops to enhance the carotenoid content (6, 9, 10).
Studying and engineering secondary metabolism in plants can be compromised by the sheer complexity of the pathways, which may have multiple branches, multifunctional enzymes, cell type-specific and compartmentalized enzymes, and complex feedback mechanisms (11). One approach to overcome this challenge is to clone genes encoding pathway enzymes and modify their expression, but modulating single enzymes is often unhelpful because pathways are regulated at multiple points. It is becoming increasingly apparent that multistep engineering, where partial or complete pathways are reconstructed or extended by the expression of 2 or more enzymes simultaneously, is the most desirable way to study and modulate complex pathways such as carotenoid biosynthesis (12, 13). However, multigene engineering is a significant hurdle in complex pathway analysis because of the diminishing rate of return as more transgenes are introduced simultaneously (14). We have addressed this challenge by developing a combinatorial nuclear transformation strategy in maize, allowing us to generate a metabolic library for the investigation of carotenoid biosynthesis and the synthesis of specific combinations of carotenoids.
It has been acknowledged that “rational engineering of complicated metabolic networks involved in the production of biologically active plant compounds has been greatly impeded by our poor understanding of the regulatory and metabolic pathways underlying the biosynthesis of these compounds” (15). Targeted metabolite analysis combined with cDNA-amplified fragment-length polymorphism-based transcript profiling is emerging as a useful strategy for the creation of novel tools for metabolic engineering (16). In this context a population of transgenic plants expressing a combinatorial transgene complement provides an invaluable resource for targeted metabolic engineering.
We used as a model system the South African elite white maize variety M37W, which lacks carotenoids in the endosperm because of the absence of the enzyme phytoene synthase (PSY1) [supporting information (SI) Fig. S1] (17, 18). This system allowed us to carry out a preliminary screen for transgenic plants accumulating different carotenoids based on endosperm color. After transforming white maize embryos with 5 carotenogenic transgenes, we recovered plants carrying all combinations of the input genes. This combinatorial population was mined for phenotypes corresponding to the production of specific carotenoids, which in turn correlated with specific transgene expression and metabolic profiles.
Our approach provides a unique and surprisingly straightforward strategy for metabolic pathway analysis and multigene metabolic engineering in plants. It involves the introduction and coordinated expression of multiple transgenes followed by the selection of stable lines expressing the specific combination of transgenes required for particular metabolic outputs (Table S1). Individual lines, producing specific metabolites, can be goals in themselves if the aim is to engineer particular molecules. However, by examining the entire diverse population of plants, it becomes possible to dissect the pathway and subsequently reconstruct it either in its original form or with modifications, thus providing a basis for understanding and subsequently engineering the synthesis of novel metabolites. The broad significance of this approach is that it considerably simplifies the process of carotenoid metabolic engineering by making it analogous to screening a library of metabolic variants for the correct functional combination. This approach could be applied to any pathway (metabolic or otherwise) given a suitable template for combinatorial transformation.
Results
Combinatorial Nuclear Transformation Generates a Diverse Library of Plants with Distinct and Stable Phenotypes.
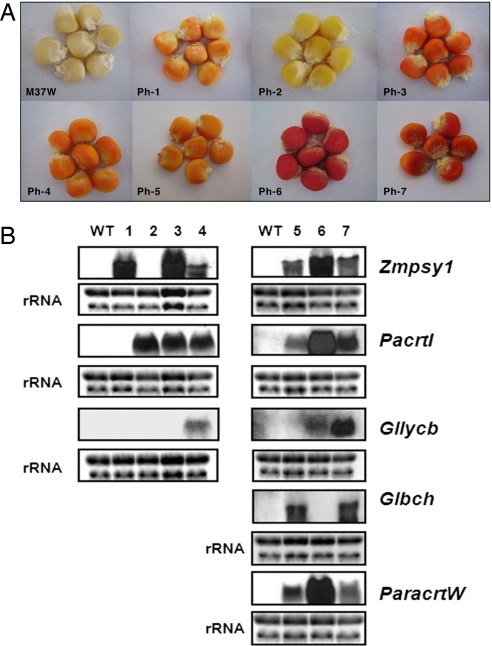
We transformed 13-day-old immature zygotic embryos of South African elite white maize variety M37W by bombarding them with metal particles coated with 6 constructs (Fig. S2), the selectable marker bar and 5 carotenogenic genes: Zmpsy1 (Zea mays phytoene synthase 1), PacrtI (Pantoea ananatis phytoene desaturase), Gllycb (Gentiana lutea lycopene β-cyclase), Glbch (G. lutea β-carotene hydroxylase, a plant-type β-ring nonheme di-iron monooxygenase introducing hydroxy groups at C-3), and ParacrtW (Paracoccus β-carotene ketolase). Each gene was driven by a different endosperm-specific promoter (respectively, the low molecular weight wheat glutenin, barley hordein, rice prolamin, rice glutelin-1, and maize γ-zein promoters; Tables S2 and S3). A population of regenerated plants was screened by genomic PCR revealing many different combinations of transgenes, including 9 lines (13%) containing all 5 carotenoid input genes. Multiple independent transgenic lines containing and expressing the same transgene complement were identified (Table S4). All of the transgenic plants showed normal morphology and development, reflecting the restriction of transgene expression to the seed endosperm. Visual inspection of the endosperm tissue revealed 7 distinct phenotypes based on endosperm color (Ph-1 to Ph-7; Fig. 1A). Analysis of steady-state mRNA levels revealed which transgenes were expressed in individual transgenic plants (Fig. 1B).
Fig. 1.
Phenotypes and genotypes of 7 combinatorial transformants. (A) Endosperm colors of 7 different transgenic maize phenotypes. Ph-1 expressing Zmpsy1 only accumulates zeaxanthin and has a bright yellow color. Ph-2 expressing only PacrtI has a phenotype similar to WT M37W with a slight increase in total carotenoids. Ph-3 (Zmpsy1 and PacrtI) accumulates a significant amount of lycopene and has an orange–red color. Ph-4 expresses Gllycb in addition to Zmpsy1 and PacrtI and accumulates β-carotene, hence the orange color. Ph-5, Ph-6, and Ph-7 express Zmpsy1+PacrtI+Glbch, Zmpsy1+PacrtI+Gllycb, and Zmpsy1+PacrtI+Gllycb+Glbch, respectively, in addition to ParacrtW, thus showing a range of colors from orange to red depending on the accumulation of ketocarotenoids. (B) Northern blot analysis (30 μg of total RNA per lane) to monitor transgene expression in WT M37W and the 7 transgenic phenotypes (lanes 1–7). Staining of rRNA with ethidium bromide was used as a loading control.
We found a precise correlation between the phenotypes and expressed transgenes. Phenotype 1 (Ph-1), expressing Zmpsy1 alone, appeared similar in color to WT yellow maize, whereas Ph-2, expressing PacrtI alone, was very pale yellow in color. The combination of Zmpsy1 and PacrtI in Ph-3 generated an orange–red phenotype, whereas the combination of Gllycb in addition to Zmpsy1 and PacrtI in Ph-4 produced a distinct orange–yellow color. Ph-5, Ph-6, and Ph-7 were more complex phenotypes resulting from the expression of a bacterial ketolase gene, ParacrtW, in addition to Zmpsy1+PacrtI+Glbch, Zmpsy1+PacrtI+Gllycb, or Zmpsy1+PacrtI+Gllycb+Glbch, respectively. These lines also showed distinguishable orange to red phenotypes (Fig. 1A).
Reconstruction of the Carotenoid Pathway in White Maize Leads to the Accumulation of Extraordinary Levels of Metabolic Intermediates and End Products.
HPLC analysis showed that the different color phenotypes reflected the accumulation of different metabolites, confirming a direct correspondence between genotype and carotenoid accumulation. The metabolic profiles of each transgene complement and resulting phenotype were qualitatively consistent in the multiple transgenic events tested, despite variation in the absolute levels of particular compounds (Fig. 2 and Table S1). Ph-1 (Zmpsy1 alone) showed a 53-fold increase of total carotenoids over white maize [58.21 vs. 1.10 μg/g dry weight (DW)], whereas Ph-2 (PacrtI alone) and Ph-3 (Zmpsy1+PacrtI) showed 2.5- and 142-fold increases, respectively (2.69 and 156.14 μg/g DW) (Table S1). The total carotenoid content in Ph-4 (Zmpsy1+PacrtI+Gllycb) reached 148.78 μg/g DW. These data suggest that PSY1 is the key enzyme limiting carotenoid accumulation in the endosperm of white maize.
Fig. 2.
HPLC analysis of carotenoids in the endosperm of white maize (M37W), yellow maize (A632), and the 7 transgenic lines (T2 mature seeds). Extracts from Ph-1 and Ph-4 were separated on a Nucleosil C18 column; all other extracts were separated on a Hypersil C18 column.
The predominant carotenoids accumulating in Ph-1 endosperm were zeaxanthin (18.25 μg/g DW, 31.35% of total carotenoids), lutein (14.95 μg/g DW, 25.68%) and β-carotene (7.10 μg/g DW, 12.20%), whereas those accumulating in Ph-3 were β-carotene (57.35 μg/g DW, 36.73%) and lycopene (26.69 μg/g DW, 17.09%) (Fig. 2). These findings suggest that both lycopene cyclases may be rate-limiting enzymes in the synthesis of cyclic carotenes in Ph-3. Simultaneous expression of Zmpsy1, PacrtI, and Gllycb in Ph-4 dramatically reduced the levels of lycopene (11.50 μg/g DW, 7.73%) but increased the levels of zeaxanthin (34.53 μg/g DW, 23.21%) compared with Ph-3 (Fig. 2). Phytoene, which is not present in WT M37W endosperm, accumulated in Ph-1, Ph-3, and Ph-4 (which express Zmpsy1) but not in Ph-2 (which does not express Zmpsy1) (Table S1). This finding indicates that the conversion of phytoene to lycopene is a subsequent limiting step for carotenoid biosynthesis, revealing where phytoene synthesis is enhanced. Phenotypes and carotenoid content remained stable for at least 2 generations (homozygous T2 plants). Multiple independent transgenic plants expressing the same transgene complement exhibited identical qualitative metabolite profiles (Tables S1 and S4).
Competition Between Bacterial Ketolase and β-Carotene Hydroxylase Extends the Carotenoid Pathway and Allows the Synthesis of Complex Mixtures of Ketocarotenoids.
Ph-4 endosperm (Zmpsy1+PacrtI+Gllycb) accumulated not only β-carotene but also xanthophylls such as lutein and zeaxanthin. The pathway can be extended even further to ketocarotenoids such as astaxanthin by expressing ParacrtW. We generated 3 unique phenotypes in which the ParacrtW transgene was expressed in combination with Zmpsy1 and PacrtI, differing in the additional expression of Glbch (Ph-5), Gllycb (Ph-6), or both Glbch and Gllycb (Ph-7, which expressed all 5 carotenogenic input transgenes).
In terms of ketocarotenoid synthesis, HPLC analysis (Fig. 2) revealed the presence of adonixanthin (4-ketozeaxanthin) in Ph-5, adonixanthin, echinenone (4-keto-β-carotene), and 3-hydroxy-echinenone in Ph-7, and these 3 carotenoids plus astaxanthin (3,3′-dihydroxy-4,4′-diketo-β-carotene) in Ph-6. The predominant carotenoids accumulating in Ph-5 were zeaxanthin (27.47 μg/g DW; 28.37% of total carotenoids) and lutein (18.11 μg/g DW; 18.71%) in addition to ketocarotenoids (10.62 μg/g DW; 10.96%) (Table S1). Ph-5 is “based on” Ph-3, having the Ph-3 genotype with additional genes Glbch and ParacrtW (Fig. S1). Ph-5 accumulated less β-carotene than Ph-3 (8.72 vs. 57.35 μg/g DW), probably reflecting the conversion of β-carotene into β-cryptoxanthin and zeaxanthin by GlBCH (Fig. S1). Similarly, Ph-7 is based on Ph-4, having the Ph-4 genotype with additional genes Glbch and ParacrtW (Fig. S1), and it produced mainly β-carotene (25.78 μg/g DW; 25.24%) and zeaxanthin (16.78 μg/g DW; 16.43%) in addition to ketocarotenoids (17.98 μg/g DW; 17.61%) (Table S1).
Ph-6 (Zmpsy1+PacrtI+Gllycb+ParacrtW) accumulated significant amounts of ketocarotenoids (35.85 μg/g DW; 24.29%) and β-carotene (34.81 μg/g DW; 23.72%), but less zeaxanthin (13.71 μg/g DW; 9.34%), most likely because of the absence of GlBCH (Fig. S1). Ph-7 accumulated the 3 mono-ketocarotenoids echinenone, 3-hydroxy-echinenone, and adonixanthin, whereas Ph-5 produced only adonixanthin. The missing echineneone and 3-hydroxy-echineneone in Ph-5 may be caused by a shortage of β-carotene (8.72 μg/g DW), reflecting the absence of lycopene β-cyclase (Fig. S1). In contrast, Ph-6 produced not only the 3 mono-ketocarotenoids, but also the important di-ketocarotenoid astaxanthin. The shared difference, when Ph-6 is compared with Ph-5 and Ph-7, is the lack of Glbch, which is therefore likely to explain their distinct metabolic profiles.
Discussion
Gene transfer to plants provides 1 way to study and modify metabolic pathways precisely, and multigene engineering allows entire pathways to be reconstructed free of endogenous regulation. This in turn requires strategies to introduce multiple transgenes into plants and ensure their coordinated expression over many generations (12). Expressing multiple transgenes stably is one of the most significant hurdles currently limiting progress in plant molecular biology (14, 19), because the chances of failure for at least 1 of the transgenes increases with the number of genes introduced, requiring the generation of very large populations to ensure complete pathway reconstruction. Alternative approaches such as individual transformation followed by crossing to “stack” transgenes are unworkable for large numbers of transgenes because of the time taken to stack all transgenes in 1 line and the risk of segregation of unlinked genes in later generations.
We have devised a strategy to simplify the process of metabolic analysis and engineering in plants based on combinatorial nuclear transformation to create metabolically diverse transgenic libraries. To demonstrate the principle we generated a carotenoid metabolic library in white maize variety M37W, which normally lacks carotenoids in the endosperm because of the absence of the enzyme PSY1 (Fig. S1). We constructed individual expression vectors for 5 carotenogenic genes each under the control of a different endosperm-specific promoter to avoid potential gene silencing caused by promoter homology (20) and to ensure the coordinated, restricted expression of all transgenes, a feat that has not been achieved in previous reports of multigene transfer using marker genes to our knowledge (21). Direct DNA transfer with separate vectors usually results in transgene integration at a random single locus, in the form of a multigene array (22, 23) containing any number of transgenes from 1 to n, with the distribution within the transgenic population tending to describe a normal curve as would be expected from random sampling (24). Input transgenes once integrated remain linked and do not segregate in subsequent generation as is the norm for direct DNA transfer of multiple transgenes irrespective of whether these are on 1 cointegrate vector or independent plasmids (cotransformation) (19–24). Plants carrying 1–5 transgenes in a roughly normal distribution could be identified from colored seed phenotypes arising from the accumulation of specific carotenoids, and these could be correlated with mRNA and metabolite profiles to identify and quantify the specific carotenoid molecules present in the endosperm. The genotypes and phenotypes were inherited stably from generation to generation once homozygosity had been achieved. Multiple independent transgenic lines containing and expressing the same transgene complement exhibited similar qualitative carotenoid profiles, indicating that the combination of expressed transgenes was responsible for the overall metabolic profile, but the amounts varied from line to line showing that microvariation in the expression levels of individual transgenes influenced the levels of each specific compound (see Table S1).
The comparative analysis of these plants provides unequivocal confirmation that PSY1 is the key enzyme-limiting carotenoid biosynthesis in maize endosperm (17, 25). The overexpression of phytoene synthase has a potent effect on carotenoid levels in storage organs generally, e.g., resulting in a 1.9-fold increase in tomato fruits (26), a 50-fold increase in canola seeds (27), and an 8-fold increase in potato tubers (28). In our equivalent maize plants (Ph-1, overexpressing Zmpsy1), the total endosperm carotenoid levels were elevated 53-fold, but the expression of 2 sequential enzymes (ZmPSY1 and PaCRTI in Ph-3) resulted in a further tripling of the carotenoid levels. In addition, the expression of ZmPSY1 and PaCRTI in Ph-3 led to an accumulation of lycopene, the product of the enhanced phytoene synthase and desaturase branch of the pathway. The addition of lycopene β-cyclase in Ph-4 alleviated this partial pathway limitation by converting lycopene preferentially to β-carotene and further derivatives (Fig. 2).
The ratio of β- to ε-ring derivatives was 1.42 in Ph-1 (Zmpsy1), 3.28 in Ph-3 (Zmpsy1+PacrtI), and 3.11 in Ph-4 (Zmpsy1+PacrtI+Gllycb). As observed in transgenic canola (29) and rice (30, 31), the β,β branch of the pathway appears to be favored, perhaps implying the existence of a rate-limiting step in the β,ε branch. Phytoene was not detected in WT M37W endosperm, but it accumulated in all of the transgenic varieties with the exception of Ph-2 (the only 1 lacking Zmpsy1). This finding suggests that the conversion of phytoene to lycopene (catalyzed by endogenous desaturases and isomerases in Ph-1, and by PaCRTI in addition to endogenous desaturases and isomerase in the other phenotypes) is a rate-limiting step for carotenoid biosynthesis in these phenotypes. The impact of endogenous enzyme activities on the product of a transgenic extended metabolic pathway has been discussed in the case of golden rice (32). However, contrary to our Ph-3 maize line, rice grains expressing the same 2 genes (Zmpsy1 and PacrtI) did not accumulate phytoene in endosperm tissue (32).
Significant amounts of lycopene accumulated in maize lines Ph-3 and Ph-4, in contrast to results obtained in rice expressing similar complements of genes (30, 31), similarly to transgenic canola seeds expressing bacterial crtB (the P. ananatis (formerly Erwinia uredovora) phytoene synthase gene) and crtI, or crtB, crtI and crtY/Bnlycb (29), and unlike transgenic potato tubers expressing bacterial crtB and crtI, or crtB, crtI and crtY (33), where lycopene could not be detected. In the case of rice, lack of lycopene was caused by its complete conversion to downstream products (32). These results demonstrate that lycopene β-cyclase can be a rate-limiting step in the conversion of lycopene to cyclic carotenes in transgenic white maize endosperm, in contrast to rice. The introduced transgenes had no effect on the expression of endogenous carotenogenic genes, including psy1 and psy2 (phytoene synthase 1 and 2), pds (phytoene desaturase), zds (ζ-carotene desaturase) crtiso (carotenoid isomerase), lyce (lycopene ε-cyclase), lycb (lycopene β-cyclase), and bch1 and bch2 (β-carotene hydroxylase), as measured by mRNA blot and semiquantitative RT-PCR analysis (data not shown).
Although the natural end point of the β,β branch of the carotenoid pathway in maize is zeaxanthin, in vitro (34, 35) and in vivo (36) studies in certain bacteria have shown that the pathway may continue to astaxanthin as a final product (Fig. S1). The formation of all 7 theoretically possible ketolated intermediates and products in maize endosperm expressing ParacrtW and combinations of Zmpsy1, PacrtI, Gllycb, and Glbch is shown in Fig. 3. Astaxanthin is formed from β-carotene by the addition of keto groups at the 4 and 4′ positions and hydroxyl groups at the 3 and 3′ positions of the β-ionone rings. These reactions are catalyzed by β-carotene ketolase and β-carotene hydroxylase, respectively (Fig. 3). In the first step, each enzyme can carry out its reaction independently, but further events depend critically on which reaction occurs first (34). Paracoccus β-carotene ketolase has a strong preference for carotenoids with at least 1 nonhydroxylated β-ionone ring, e.g., β-carotene, β-cryptoxanthin, echinenone, and 3-hydroxy-echinenone. In contrast, 3-hydroxylated β-ionone rings like zeaxanthin, 3′-hydroxy-echinenone, and adonixanthin are poor substrates for this enzyme (35). We generated 3 transgenic maize phenotypes producing ketocarotenoids, all of which produced the mono-ketocarotenoid adonixanthin (4-ketozeaxanthin). Ph-5 accumulated adonixanthin alone (100% of total ketocarotenoids), whereas in Ph-6 and Ph-7, adonixanthin represented 62.72% and 69.41% of total ketocarotenoids, respectively (Table S1). ParacrtW has also been expressed simultaneously with a β-carotene hydroxylase gene in tomato and tobacco plants (37), resulting in the accumulation of 1 major ketocarotenoid (echinenone) in tomato leaves and fruits, and several ketocarotenoids including echinenone, 3-hydroxy-echinenone, 3′-hydroxy-echinenone, canthaxanthin, adonixanthin, and astaxanthin in tobacco leaves and nectary tissues. Constitutive expression of ParacrtW in Lotus japonicus caused the accumulation of echinenone, canthaxanthin, adonixanthin, and astaxanthin in flower petals (38).
Fig. 3.
Significant reaction steps involved in the biosynthetic pathway of individual ketocarotenoids from β-carotene in transgenic maize transformed with ParacrtW. The dotted ovals indicate the different substrates competitively modified by CRTW and BCH, leading to the formation of monoketocarotenoids and diketocarotenoids.
The product of the ParacrtW transgene uses the same substrate as β-carotene hydroxylase, an unsubstituted β-ionone ring. The hydroxylase and ketolase thus compete at 4 stages for different substrates in the extended carotenoid pathway: for β-carotene, for the unsubstituted sides of β-cryptoxanthin, and for echinenone and 3-hydroxyechinenone (Fig. 3). Thanks to the restricted ketolation of a 3-HO-β-ionone ring (35), nonketolated zeaxanthin and the monohydroxy-diketo carotenoid adonixanthin represent by-products of the pathway to astaxanthin. Therefore, we propose that the astaxanthin in maize endosperm is derived mostly from adonirubin (3-hydroxycanthaxanthin) via 3-hydroxyechinenone (the product of echinenone hydroxylation) or canthaxanthin (the product of echinenone ketolation) (Fig. 3). The ketolase has to overcome the hydroxylase twice, first by the ketolation of β-carotene, and then by the ketolation of either echinenone or 3-hydroxy echinenone; otherwise astaxanthin cannot be formed. Therefore, the accumulation of astaxanthin is determined by the balance of the ketolase relative to the hydroxylase. Only plants expressing ParacrtW produce a surplus of ketolase, ensuring the formation of astaxanthin. Otherwise, the hydroxylase activity is too high and adonixanthin is the only final keto-hydroxy product of the pathway (together with nonketolated zeaxanthin). High hydroxylase activity appears to be the case in Ph-5 and Ph-7, where total concentrations of ketolated carotenoids are much lower than in Ph-6, and the pathway stops without the second ketolation at the level of adonixanthin (Fig. 3). Ph-6 had the highest ketocarotenoid levels and was the only line to synthesize astaxanthin, probably reflecting the relatively low hydroxylase levels (only endogenous hydroxylase but no GlBCH activity, because of the absence of the transgene) and high ketolase levels (high ParaCRTW activity) (Fig. 1B). ParacrtW mRNA levels were substantially lower in Ph-5 and Ph-7, both of which also expressed Glbch and thus had greater hydroxylase levels, driving flux away from the di-ketolated carotenoids. To summarize, we did not expect 100% conversion of β-carotene to astaxanthin, in agreement with previous studies expressing the same ketolase gene (37, 38). In Ph-5, Ph-6, and Ph-7, all expressing ParacrtW, we expected and obtained high ketolase activity, resulting in ketocarotenoids levels representing 11–24% of total carotenoids (Table S1). The accumulation of astaxanthin in Ph-6 (and no additional hydroxylase expressed in the form of GlBCH) compared with Ph-7 (expressing Glbch), supports the proposed competition between the ketolase and hydroxylase activities in our transgenic lines (Fig. 3) in agreement with the previous in vitro (34, 35) and in vivo (36) studies.
For many plants transformed with a ketolase gene such as ParacrtW the conversion of adonixanthin to astaxanthin appears to be an important limiting step for astaxanthin biosynthesis. Our results demonstrate that the avoidance of adonixanthin accumulation was crucial for astaxanthin production in transgenic maize endosperm. A strategy that may help to overcome this problem is the use of crtW gene from Brevundimonas sp. SD212, which appears to be more efficient in ketolating 3-hydroxy-β-ionone end groups (39). Alternatively, it might be possible to use the astaxanthin synthase (ASY) gene from Xanthophyllomyces dendrohous, a multifunctional enzyme catalyzing all steps from β-carotene to astaxanthin formation by the oxygenation of carbons 3 and 4 (40). The reaction involves the 4-ketolation of β-carotene followed by 3-hydroxylation without the accumulation of 3-hydroxy intermediates.
As well as gaining insight into the mechanisms underlying carotenoid biosynthesis in plants, particularly bottlenecks in the endogenous pathway and the competition between β-carotene hydroxylase and β-carotene ketolase for substrates in the extended pathway leading to astaxanthin, the combinatorial library approach provides a convenient strategy for the rapid identification of metabolic variants with desirable modified properties. For example, we identified plants with extraordinarily high levels of unsubstituted carotenoids (carotenes) and plants with complex mixtures of hydroxycarotenoids and ketocarotenoids (xanthophylls). This approach is much simpler than traditional methods for the modification of the carotenoid pathway because it relies on probability and random sampling to generate a library of metabolic variants and a rapid visual selection process to identify lines of interest. Although the metabolic library in this case relied on the availability of a suitable null background (white maize) and the convenience of color-based visual screening for the rapid evaluation of metabolically-diverse transgenic plants, there is no reason the same approach should not be used to dissect and modify other pathways as long as suitable screening methods can be found. The approach is analogous to standard mutagenesis screens although the “mutants” are generated not by random mutagenesis to create loss-of-function phenotypes, but by random multiplex transgene insertion to create partially reconstructed pathways. Combinatorial transformation therefore provides an efficient approach for the dissection of complex metabolic pathways (including those where competition between enzymes at multiple stages leads to intricate combinations of different metabolites) while simultaneously allowing the same pathways to be modified in a multitude of ways to facilitate metabolic engineering approaches.
Methods
Maize Combinatorial Transformation.
Maize plants (Zea mays L., cv. M37W) were grown in the greenhouse or growth room with a 10-h photoperiod (28/20 °C day/night temperature) and 60–90% relative humidity for 50 days, followed by a 16-h photoperiod (21/18 °C day/night temperature) thereafter. Thirteen-day-old immature zygotic embryos were bombarded with 10 mg of gold particles coated with the carotenogenic constructs and the selectable marker bar (41) at a molar ratio of 3:1 as reported (42) and returned to osmoticum medium for 12 h before selection. Bombarded callus was selected on phosphinothricin-supplemented medium as described (43, 44), and transgenic plantlets were regenerated and hardened off in soil. Seventy independent events were selected for further analysis and either pollinated with nontransformed white maize (M37W) pollen or self-pollinated to produce T1 seeds.
DNA Analysis of Transgenic Plants.
Transgenic maize lines were characterized by PCR using 3 primer sets for each transgene, amplifying segments of the 5′ end, middle, and 3′ end of the transgene (Table S4). The corresponding plasmids were used as positive controls. PCRs were carried out under standard conditions with 100 ng of genomic DNA and 0.5 units of GoTaq DNA polymerase in a 20-μl reaction volume. The reactants were denatured at 95° C for 3 min, followed by 30 cycles of 94 °C for 45 s, 60 °C for 45 s, 72 °C for 90 s, and a final extension at 72 °C for 10 min.
Northern Blot Analysis.
Total RNA was isolated from near-mature endosperm tissue by using a RNeasy Plant Min Kit (Qiagen), and 30-μg aliquots were fractionated on a denaturing 1.2% (wt/vol) agarose gel containing formaldehyde and blotted by using standard methods (45). The membrane was probed with digoxigenin-labeled partial cDNAs at 50 °C overnight using DIG Easy Hyb (Roche Diagnostics). The partial cDNAs were converted into probes by using the DIG probe synthesis kit (Roche Diagnostics) and primer set 2 (Table S5). After washing and immunological detection with anti-DIG-AP (Fab-Fragments; Roche Diagnostics) according to the manufacturer's instructions, chemiluminescence by disodium 3-(4-methoxyspiro {1,2-dioxetane-3,2′-(5′-chloro) tricyclo[3.3.1.13,7]decan}-4-yl)phenyl phosphate (CSPD) (Roche) was detected on Kodak BioMax light film (Sigma–Aldrich).
HPLC Analysis.
Total carotenoids were extracted from freeze-dried endosperm in 20 mL of 50/50 (vol/vol) tetrahydrofuran and methanol at 60 °C for 15–20 min, and total carotenoids were quantified by measuring absorbance at 465 nm. For HPLC separation, the solvent was evaporated under a stream of N2 gas at 37 °C and redissolved in 50 μl of acetone, and 20 μL of aliquot was injected immediately. Three different HPLC systems were used at flow rates of 1 mL/min. For ketocarotenoid samples, a Hypersil HyPurity Elite C18 5μ column was used with a mobile phase of acetonitrile/methanol/2-propanol (85:10:5 vol/vol/vol) and a column temperature of 32 °C (40). All other samples were separated on a Nucleosil C18 3μ column with the same mobile phase at 25 °C. In each case zeaxanthin and lutein were separated in parallel runs on a C18 Vydac 218TP54 column with methanol as the mobile phase (46). Samples were monitored with a Kontron DAD 440 photodiode array detector with on-line registration of the spectra. All carotenoids were identified by cochromatography with authentic reference compounds and comparison of their spectra. Those standards were also used for quantitation in combination with the extinction coefficients (47).
Supplementary Material
Acknowledgments.
We thank Dr. N. Misawa (Marine Biotechnology Institute of Japan, Kanaishi, Japan) for the P. ananatis crtI gene; Dr. P. D. Fraser (University of London, London) for the Paracoccus crtW gene; Dr. D. Ludovid (Consejo Superior de Investigaciones Cientificas/Institut de Recercha Tecnologia Agroalimentaries, Barcelona) for the rice prolamin and maize γ-zein promoters; the Council for Scientific and Industrial Research (Pretoria, South Africa) for the WT M37W maize; Dr. P. Beyer (University of Freiburg, Freiburg, Germany), and Prof. J. Hirschberg (The Hebrew University of Jerusalem, Jerusalem) for critical comments on the manuscript. This work was supported by Ministry of Education and Science Grant BFU2007-61413 and the Ramon y Cajal program of the Ministry of Education and Science. S.N. is the recipient of Ministry of Education and Science Ph.D Fellowship BES-2005-9161.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809737105/DCSupplemental.
References
- 1.Seigler DS. Plant Secondary Metabolism. Dordrecht, The Netherlands: Kluwer; 1998. [Google Scholar]
- 2.Frank HA, Cogdell RJ. Carotenoids in photosynthesis. Photochem Photobiol. 1996;63:257–264. doi: 10.1111/j.1751-1097.1996.tb03022.x. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E. Lycopene and prostate cancer risk: Methodological considerations in the epidemiologic literature. Pure App Chem. 2002;74:1427–1434. [Google Scholar]
- 4.Chew BP, Park JS. Carotenoid action on the immune response. J Nutr. 2004;134:257S–261S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- 5.Landrum JT, Bone AR. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385:28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- 6.Fraser PD, Bramley PM. The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res. 2004;43:228–265. doi: 10.1016/j.plipres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Van den Berg H, et al. The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J Sci Food Agric. 2000;80:880–912. [Google Scholar]
- 8.Cunningham FX, Jr, Gantt E. A study in scarlet: Enzymes of ketocarotenoid biosynthesis in the flowers of Adonis aestivalis. Plant J. 2005;41:478–492. doi: 10.1111/j.1365-313X.2004.02309.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhu C, et al. Transgenic strategies for the nutritional enhancement of plants. Trends Plant Sci. 2007;12:548–555. doi: 10.1016/j.tplants.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano G, Tavazza R, Diretto G, Beyer P, Taylor MA. Metabolic engineering of carotenoid biosynthesis in plants. Trends Biotechnol. 2008;26:139–145. doi: 10.1016/j.tibtech.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Verpoorte R, van der Heijden R, Memelink J. Engineering the plant cell factory for secondary metabolite production. Transgenic Res. 2000;9:323–343. doi: 10.1023/a:1008966404981. [DOI] [PubMed] [Google Scholar]
- 12.Capell T, Christou P. Progress in plant metabolic engineering. Curr Opin Biotechnol. 2004;15:148–154. doi: 10.1016/j.copbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Sandmann G, Römer S, Fraser PD. Understanding carotenoid metabolism as a necessity for genetic engineering of crop plants. Metabol Eng. 2006;8:291–302. doi: 10.1016/j.ymben.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Halpin C. Gene stacking in transgenic plants: The challenge for 21st century plant biotechnology. Plant Biotechnol J. 2005;3:141–155. doi: 10.1111/j.1467-7652.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- 15.Rischer H, et al. Gene-to-metabolite networks for terpenoid indole alkaloid biosynthesis in Catharanthus roseus cells. Proc Natl Acad Sci USA. 2006;103:5614–5619. doi: 10.1073/pnas.0601027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goossens A, et al. A functional genomics approach toward the understanding of secondary metabolism in plant cells. Proc Natl Acad Sci USA. 2003;100:8593–8600. doi: 10.1073/pnas.1032967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckner B, San-Miguel P, Bennetzen JL. The y1 gene of maize codes for phytoene synthase. Genetics. 1996;143:479–488. doi: 10.1093/genetics/143.1.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallagher GE, Mattews PD, Li F, Wurtzel ET. Gene duplication in the carotenoid biosynthetic pathway preceded evolution of the grasses. Plant Physiol. 2004;135:1776–1783. doi: 10.1104/pp.104.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dafny-Yelin M, Tzfira T. Delivery of multiple transgenes to plant cells. Plant Physiol. 2007;145:1118–1128. doi: 10.1104/pp.107.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhullar S, et al. Functional analysis of cauliflower mosaic virus 35S promoter: Re-evaluation of the role of subdomains B5, B4, and B2 in promoter activity. Plant Biotechnol J. 2007;5:696–708. doi: 10.1111/j.1467-7652.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Nandi S, Chen L, Rodriguez RL, Huang N. Expression and inheritance of nine transgenes in rice. Transgenic Res. 2002;11:533–541. doi: 10.1023/a:1020331608590. [DOI] [PubMed] [Google Scholar]
- 22.Altpeter F, et al. Particle bombardment and the genetic enhancement of crops: Myths and realities. Mol Breed. 2005;15:305–327. [Google Scholar]
- 23.Kohli A, et al. The quest to understand the basis and mechanisms that control expression of introduced transgenes in crop plants. Plant Signal Behav. 2006;1:185–195. doi: 10.4161/psb.1.4.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohli A, et al. Transgene integration, organization, and interaction in plants. Plant Mol Biol. 2003;52:247–258. doi: 10.1023/a:1023941407376. [DOI] [PubMed] [Google Scholar]
- 25.Palaisa KA, Morgante M, Williams M, Rafalski A. Contrasting effects of selection on sequence diversity and linkage disequilibrium at two phytoene synthase loci. Plant Cell. 2003;15:1795–1806. doi: 10.1105/tpc.012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser PD, et al. Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc Natl Acad Sci USA. 2002;99:1092–1097. doi: 10.1073/pnas.241374598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY. Seed-specific overespression of phytoene synthase: Increase in carotenoids and other metabolic effects. Plant J. 1999;20:401–412. doi: 10.1046/j.1365-313x.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- 28.Ducreux LJ, et al. Metabolic engineering of high carotenoid potato tubers containing enhanced levels of β-carotene and lutein. J Exp Bot. 2005;56:81–89. doi: 10.1093/jxb/eri016. [DOI] [PubMed] [Google Scholar]
- 29.Ravanello MP, Ke D, Alvarez J, Huang B, Shewmaker CK. Coordinate expression of multiple bacterial carotenoid genes in canola leading to altered carotenoid production. Metabol Eng. 2003;5:255–263. doi: 10.1016/j.ymben.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Ye X, et al. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science. 2000;287:303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- 31.Paine JA, et al. Improving the nutritional value of golden rice through increased pro-vitamin A content. Nat Biotechnol. 2005;23:482–487. doi: 10.1038/nbt1082. [DOI] [PubMed] [Google Scholar]
- 32.Schaub P, Al-Babili S, Drake R, Beyer P. Why is golden rice golden (yellow) instead of red? Plant Physiol. 2005;138:441–450. doi: 10.1104/pp.104.057927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diretto G, et al. Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. PLoS ONE. 2007;2:e350. doi: 10.1371/journal.pone.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraser PD, Miura Y, Misawa N. In vitro characterization of astaxanthin biosynthetic enzymes. J Biol Chem. 1997;272:6128–6135. doi: 10.1074/jbc.272.10.6128. [DOI] [PubMed] [Google Scholar]
- 35.Fraser PD, Shimada H, Misawa N. Enzymic confirmation of reactions involved in routes to astaxanthin formation elucidated using a direct substrate in vitro assay. Eur J Biochem. 1998;252:229–236. doi: 10.1046/j.1432-1327.1998.2520229.x. [DOI] [PubMed] [Google Scholar]
- 36.Misawa N, et al. Functional expression of the Erwinia uredovora carotenoid biosythesis gene crtI in transgenic plants showing an increase of β-carotene biosynthesis activity and resistance to the bleaching herbicide norflurazon. Plant J. 1993;4:833–840. doi: 10.1046/j.1365-313x.1993.04050833.x. [DOI] [PubMed] [Google Scholar]
- 37.Ralley L, et al. Metabolic engineering of ketocarotenoid formation in higher plants. Plant J. 2004;39:477–486. doi: 10.1111/j.1365-313X.2004.02151.x. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki S, et al. Flower color alteration in Lotus japonicus by modification of the carotenoid biosynthetic pathway. Plant Cell Rep. 2007;26:951–959. doi: 10.1007/s00299-006-0302-7. [DOI] [PubMed] [Google Scholar]
- 39.Choi SK, et al. Characterization of β-carotene ketolases, CRTW, from marine bacteria by complementation analysis in Escherichia coli. Mar Biotechnol. 2005;7:515–522. doi: 10.1007/s10126-004-5100-z. [DOI] [PubMed] [Google Scholar]
- 40.Ojima K, et al. Cloning of the astaxanthin synthase gene from Xanthophyllomyces dendrorhous (Phaffia rhodozyma) and its assignment as a β-carotene 3-hydroxylase/4-ketolase. Mol Genet Genom. 2006;275:148–158. doi: 10.1007/s00438-005-0072-x. [DOI] [PubMed] [Google Scholar]
- 41.Christensen AH, Quail PH. Ubiquitin promoter based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Trans Res. 1996;5:213–218. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]
- 42.Christou P, Ford T, Kofron M. Production of transgenic rice (Oryza sativa L.) plants from agronomically important indica and japonica varieties via electric discharge particle acceleration of exogenous DNA into immature zygotic embryos. Bio/Technology. 1991;9:957–962. [Google Scholar]
- 43.Drakakaki G, et al. Endosperm specific coexpression of recombinant soybean ferritin and Aspergillus phytase in maize results in significant increases in the levels of bioavailable iron. Plant Mol Biol. 2005;59:869–880. doi: 10.1007/s11103-005-1537-3. [DOI] [PubMed] [Google Scholar]
- 44.Ramessar K, et al. Cost-effective production of a vaginal protein microbicide to prevent HIV transmission. Proc Natl Acad Sci USA. 2008;105:3727–3732. doi: 10.1073/pnas.0708841104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1989. [Google Scholar]
- 46.Römer S, et al. Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and cosuppression of carotenoid epoxidation. Metabol Eng. 2002;4:263–272. doi: 10.1006/mben.2002.0234. [DOI] [PubMed] [Google Scholar]
- 47.Davies BH. Carotenoids. In: Goodwin TW, editor. Chemistry and Biochemistry of Plant Pigments. London: Academic; 1976. pp. 38–366. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.