Abstract
A functional memory T cell pool is critical for resistance to pathogen reinfection. Lymphopenia produces memory-like CD8+ T cells through homeostatic proliferation, and such “HP-memory” cells can control lethal bacterial infections similarly to conventional, antigen-experienced, memory T cells. These 2 pathways for memory T cell generation are quite distinct. We show here, however, that similar factors are required for production of protective memory CD8 T cells via both homeostatic and conventional pathways. Induction of protective HP-memory CD8 T cells requires CD4+ T cell “help,” which we show is antigen nonspecific yet requires CD40L–CD40 interactions with host cells. The functional competence of HP-memory CD8 T cells also requires release of endogenous bacterial components (which follows irradiation-induced lymphopenia), potentially mimicking the role of adjuvants in conventional immune responses. Lymphopenic environments lacking these key factors support similar CD8 T cell homeostatic proliferation and the acquisition of memory phenotype, yet the HP-memory cells generated are defective in pathogen elimination. These findings suggest unexpected parallels in the requirements for generating protective memory CD8 T cells by distinct pathways, and they suggest ways to bolster immune competence during recovery from lymphopenia.
Keywords: homeostasis, host defense, lymphopenia, CD4 T cells
The establishment of a sustained, functional CD8+ memory T cell pool is critical for protective immunity against many pathogens and tumors (1). Typically, memory T cells are generated after priming by a foreign antigen, with the memory population appearing as the effector pool declines. However, in response to T cell lymphopenia, naïve T cells slowly proliferate and transition to a memory-like state in a process termed “homeostatic proliferation” (HP) (2). Unlike conventional memory T cells, which are induced in response to foreign peptide–MHC complexes and inflammatory cues, “HP-memory” T cells are produced in response to homeostatic cytokines (IL-7, IL-15) and engagement with self-peptide–MHC complexes (1, 2). Furthermore, the conversion from naïve to HP-memory cells does not appear to pass through an effector cell stage or the expansion and contraction phases typical of conventional immune responses. Nonetheless, these HP-memory T cells display phenotypic and functional characteristics of antigen-experienced memory cells (2), including the ability to control lethal bacterial infection (3).
HP-memory T cells arise during lymphopenia induced by irradiation or genetic T cell deficiency, making this process relevant for therapeutic strategies designed to reconstitute the immune system, such as after cancer therapy. Indeed, lymphopenia can augment the antitumor response following adoptive T cell immunotherapy approaches (4–6). In addition, lymphopenia occurs during infection with various pathogens (7, 8), and some (for example, HIV) induce sustained T cell depletion. Finally, lymphopenia occurs in physiological situations: during ontogeny of the immune system, T cell numbers are low and there is evidence that very young mice support the production of HP-memory cells derived from naïve precursors (9–11). Hence, HP-memory T cells may be produced in response to various lymphopenic episodes and could contribute to future immune responses. Therefore, it is important to determine the functional characteristics of this pool and their therapeutic potential.
Much is known about the requirements for efficient generation of conventional memory CD8+ T cells. Naïve T cells require stimulation through the T cell receptor (TCR) by foreign peptide–MHC ligands, engagement of costimulatory molecules, and cytokines (such as IL-12 or type I IFN) that enhance the T cell response (12). Adjuvants, such as Toll-like receptor (TLR) agonists, are important for enhancing antigen presentation and production of cytokines, such as IL-12. Furthermore, it has become clear that CD4+ T cells play a key role in generating effective memory CD8+ T cells in response to cellular and microbial antigens.
The requirements for the production of effective HP-memory CD8+ T cells are much less well defined. We recently demonstrated a role for CD4+ T cells in the generation of protective HP-memory CD8+ T cells (3). We observed that proliferation of naïve CD8+ T cells in lymphopenic hosts occurs regardless of the presence of CD4+ T cells, but that the protective capacity of the resultant HP-memory CD8+ T cells was dramatically impaired when they were generated in the absence of CD4+ T cells (3). This finding may be especially relevant to situations of sustained lymphopenia and CD4 depletion (e.g., in HIV/AIDS), where the protective function of CD8+ T cells may be compromised. However, the mechanism by which CD4+ T cells offer “help” to CD8+ T cells during HP is not understood.
In this study we address the basis by which both CD4+ T cells and factors induced by lymphodepletion dictate the protective function of HP-memory CD8+ T cells. Our data suggest unexpected parallels in the requirements for regulating function of both HP- and conventional-memory CD8+ T cells, and suggest ways in which lymphopenia can be harnessed to enhance therapeutic approaches.
Results
CD4+ T Cell Help for HP-Memory CD8+ T Cells Is Not Antigen-Specific and Is Not Required Throughout Lymphopenia.
We previously reported that OT-I TCR-transgenic CD8+ T cells that have undergone HP in C57BL/6 (B6) hosts provide robust protective immunity against a high-dose infection with Listeria monocytogenes expressing ovalbumin (LM-OVA) that is comparable to that of conventional memory cells (primed by a foreign antigen) (3). However, HP-memory OT-I cells generated in MHC class II-deficient (MHCII-KO) hosts failed to control LM infection (3), suggesting a requirement for CD4+ T cell help during HP. This is not a generalized functional defect, because HP-memory CD8+ T cells generated in the presence or absence of CD4 cells exhibit a similar potential for IFN-γ, TNF, and IL-2 production (3) and for cytolysis [supporting information (SI) Fig. S1] after TCR stimulation.
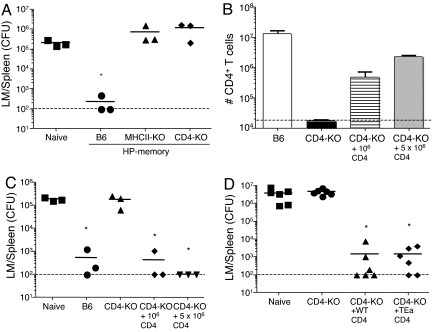
Some authors have proposed that OT-I T cells themselves might respond to MHC class II molecules (13), and it was possible that this interaction (rather than the presence of CD4+ T cells) was required for producing protective HP-memory T cells. To address this possibility, we tested the protective function of HP-memory OT-I T cells generated in CD4-deficient mice by using the same experimental approach as used previously (3) (illustrated schematically in Fig. S2). This approach allows assessment of protective function on a per-cell basis in the context of a normal animal. HP-memory OT-I T cells generated in B6 mice provided robust protective immunity, as expected (3). However, HP-memory OT-I cells produced in either MHCII-KO or CD4-KO mice failed to offer protective immunity beyond that of naïve OT-I T cells (Fig. 1A). These data suggested that the lack of host CD4+ T cells rather than class II MHC molecules was responsible for generation of dysfunctional HP-memory CD8+ T cells.
Fig. 1.
Protective function of OT-I HP-memory T cells requires CD4+ T cells. (A) HP-memory OT-I cells were generated in sublethally irradiated B6, MHCII-KO, or CD4-KO mice. After 3 weeks in these hosts, secondary transfers were performed into intact B6 mice. Mice were infected with LM-OVA 1 day later. Five days after infection, colony-forming units (CFU) of LM in the spleen were determined. Symbols represent individual mice. *, P = 0.0083, bacterial counts in test group vs. naïve group. (B–D) HP-memory OT-I cells were generated as described for A, except that some CD4-KO mice also received 106 or 5 × 106 polyclonal CD4+ T cells 1 day after sublethal irradiation. (B) Number of CD4+ T cells recovered from each of the indicated hosts. (C) HP-memory OT-I cells from the indicated groups were adoptively transferred into B6 mice and challenged with LM-OVA. Five days later, the number of CFU in the spleen was determined. Each symbol represents an individual mouse. *, P = 0.0008, bacterial counts in test group vs. naïve group. (D) HP-memory OT-1 cells were generated in CD4-KO mice. Some mice also received 106 polyclonal CD4+ T cells or TEa CD4+ T cells at the time of OT-1 cell transfer. *, P = 0.0079, bacterial counts in test group vs. naïve group.
This system also provided an opportunity to selectively reconstitute the CD4+ T cell pool during HP and test its effect on the protective function of OT-I HP-memory cells. We transferred naïve B6 CD4+ T cells together with naïve OT-I cells into irradiated CD4-KO hosts. This led to a partial restoration of the CD4 compartment 3 weeks later (Fig. 1B). As expected, transferred CD4+ T cells underwent HP in the irradiated CD4-KO hosts, as documented by uniform up-regulation of the CD44 marker and carboxyfluorescein succinimidyl ester (CFSE) dilution (data not shown). We also noted that the yield of OT-I T cells at this time point was slightly improved as the number of transferred CD4+ T cells was increased (data not shown). More importantly, we found that cotransfer of as few as 106 polyclonal CD4+ T cells led to acquisition of protective function by HP-memory OT-I T cells produced in CD4-KO hosts (Fig. 1C). These data show that “add-back” of CD4+ T cells into lymphopenic CD4-KO hosts can provide help for the protective function of HP-memory CD8+ T cells.
This approach allowed us to address whether CD4 T cell TCR specificity was important for their provision of help to HP-memory OT-I cells. CD4-KO mice were irradiated and adoptively transferred with naïve OT-I cells and either polyclonal B6 CD4+ T cells or TEa TCR transgenic CD4+ T cells (specific for the Eα52–68 peptide in the context of I-Ab; ref. 14). Both polyclonal and TEa transgenic T cells proliferated in CD4-KO hosts and up-regulated CD44 (data not shown). Furthermore, the protective function of the resultant HP-memory OT-I cells was similar regardless of whether polyclonal or TCR transgenic CD4+ T cells were used (Fig. 1D). Similar findings were obtained in preliminary experiments using OT-II (15) rather than TEa TCR transgenic CD4+ T cells (data not shown). These data suggested that the TCR specificity of the CD4+ T cell pool is irrelevant to its ability to help drive development of protective HP-memory OT-I.
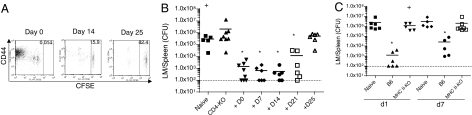
The timing of CD4 help is important in conventional CD8+ T cell immune responses. For example, cognate help by CD4+ T cells is thought to occur during the initial activation of naïve CD8+ T cells by antigen-presenting cells (APCs), and it may involve coordinated interaction of both T cell types with the same dendritic cell (DC) (16). On the other hand, CD4+ T cells can influence survival and function of conventional memory CD8+ T cells during the memory maintenance phase (17). Hence, we asked whether the induction of CD8+ T cell HP and the provision of help could be temporally separated. OT-I CD8+ T cells were transferred on day 0 (sublethal irradiation being on day −1), and polyclonal wild-type CD4+ T cells were transferred at various time points during the remaining lymphopenic phase. As expected, CD4+ T cell numbers and the extent of proliferation and CD44 up-regulation were dependent on the time spent in the lymphopenic hosts, such that all of the CD4+ T cells had divided multiple times if transferred on day 0, and few had divided if transferred on day 25 (Fig. 2A). Surprisingly, after transfer of equal numbers of HP-memory OT-I T cells into new hosts and infection with LM, we found that as little as 1 week of contact with CD4+ T cells (i.e., transfer on day 21) was sufficient to significantly improve the protective capacity of the HP-memory CD8+ T cells (Fig. 2B).
Fig. 2.
Short-term exposure to CD4+ T cells in a lymphopenic environment leads to functional HP-memory OT-I T cells. HP-memory OT-I cells were generated in irradiated CD4-KO mice. At various times after irradiation, polyclonal CFSE-labeled CD4+ T cells were also adoptively transferred. Crosses indicate mice that did not survive to day 5. (A) At 28 days after OT-I transfer, spleen and lymph nodes were harvested and the CD4 T cell pool was analyzed for CFSE dye dilution and CD44 expression. (B) HP OT-I cells from each group in A were adoptively transferred into B6 hosts, which then were challenged with LM-OVA. Data represent splenic CFU from individual mice in 2 separate experiments, measured at day 5 after infection. *, P = 0.0014 [day 0 (D0)], P = 0.0057 (D7, D14), and P = 0.0071 (D21), bacterial counts in test group vs. naïve group. (C) HP-memory OT-I cells were generated in B6 or MHCII-KO mice. Secondary transfer was performed, and the host mice were challenged with LM-OVA either 1 day later or 7 days after cell transfer. The number of CFU of LM in the spleen was determined 5 days after infection. Data points represent individual mice from 2 separate experiments. The limit of detection (≈100 organisms) is indicated by a dashed line.
We also asked the distinct question of whether the function of “unhelped” HP-memory OT-I cells could be restored by CD4+ T cells in nonlymphopenic hosts. In our standard approach (Fig. S2), we tested the protective function of HP-memory OT-I cells 1 day after adoptive transfer into normal B6 mice, which may not be sufficient time for CD4+ T cells to rescue the function of unhelped HP-memory cells. However, we found that unhelped HP- memory OT-I T cells were still nonprotective after being “parked” for 7 days in normal B6 mice (Fig. 2C). Together, these experiments suggest that the lymphopenic setting is important for delivery of CD4+ T cell help in this system. Our data demonstrate that reintroduction of CD4+ T cells into the lymphopenic setting, regardless of specificity and even late in the proliferative response by CD8+ T cells, has a positive impact on the functional development of HP-memory OT-I cells.
CD40–CD40L Interactions Are Required for Optimal HP-Memory T Cell Protective Function.
A prominent mechanism of CD4+ T cell help for conventional CD8+ T cell responses (especially during responses to nonreplicating antigens) is through the CD40–CD40L pathway, in which CD40L on responding CD4+ T cells engages CD40 on DCs, prompting DC maturation and “licensing” for naïve CD8+ T cell activation (18–21). To explore the importance of the CD40–CD40L pathway in HP-memory generation, we initially tested the protective function of HP-memory OT-I cells created in CD40-KO hosts. These studies revealed a profound functional defect in HP-memory OT-I T cells generated in CD40-KO hosts (Fig. 3A). It is worth noting that CD40-KO animals have a normal-sized CD4 pool (data not shown), suggesting that the mere presence of CD4+ T cells is not adequate to provide help for the HP-memory CD8+ T cells. In an alternative experimental approach, we tested the impact of agonistic anti-CD40 antibodies on OT-I HP-memory cells generated in MHCII-KO hosts. Interestingly, this treatment led to acquisition of protective function of “helpless” HP-memory OT-I cells, suggesting that CD4 help could be replaced by direct stimulation of CD40 on host cells during HP. Consistent with these data, HP-memory OT-I cells generated in CD40L-KO mice also failed to provide protection against LM-OVA infection (Fig. 3B).
Fig. 3.
CD40 stimulation is required for help to HP-memory OT-I cells. (A) HP-memory OT-I cells from B6, MHCII-KO, and CD40-KO hosts were generated. Some irradiated B6 mice and MHCII-KO mice were treated weekly with agonist anti-CD40. After 3 weeks, OT-I cells were adoptively transferred from lymphopenic hosts to new B6 mice. Mice were challenged with LM-OVA 1 day later, and the CFU of LM in the spleen were determined 5 days after infection. *, P < 0.0001, bacterial counts in test group vs. naïve group. (B) HP-memory OT-I cells from B6, CD4-KO, and CD40L-KO hosts were generated. After 3 weeks, OT-I cells were adoptively transferred from lymphopenic hosts into normal B6 mice and assessed for LM-OVA control. *, P < 0.0001, bacterial counts in test group vs. naïve group. (C) HP-memory OT-I T cells from B6 or CD4-KO mice were generated. Some CD4-KO mice also received a transfer of 106 (high) or 2 × 105 (low) polyclonal CD4+ T cells from wild-type (B6) or CD40L-KO mice. After 3 weeks, OT-I cells were adoptively transferred into normal B6 hosts and challenged with LM-OVA. Data points represent individual mice from 2 or 3 experiments. *, P < 0.0001 (B6), P = 0.0002 (+WT high), P = 0.0005 (+WT low), and P = 0.0006 (+KO high), bacterial counts in test groups vs. naïve group.
These studies did not address whether CD40L expression on CD4+ T cells themselves was important for helping HP-memory CD8+ T cells. Using the CD4 add-back approach, we cotransferred OT-I T cells together with either wild-type or CD40L-KO CD4+ T cells into irradiated CD4-KO mice and tested the protective function of the resultant HP-memory OT-I cells. These studies indicated that CD40L-deficient CD4+ T cells were markedly impaired compared with their wild-type counterparts in providing help to HP-memory OT-I cells (Fig. 3C). However, CD40L CD4+ T cells could offer some help when transferred at high numbers, implying additional CD40L-independent functions. Together, these data argue that CD40–CD40L interactions are important for CD4 help during the development of functional HP-memory CD8+ T cells.
Exogenous IL-12 Substitutes for CD4 Help in Inducing Protective HP-Memory CD8+ T Cells.
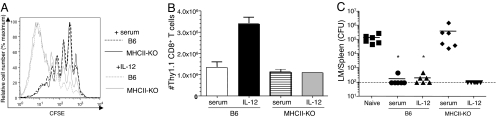
One mechanism by which CD40–CD40L interactions can enhance immune responses is to cooperate with other signals to induce production of IL-12 by DCs (22, 23). Furthermore, homeostatic expansion is enhanced by increasing levels of IL-12 (24). Hence, we tested whether exogenous IL-12 could substitute for CD4 help during CD8+ T cell HP. CFSE-labeled OT-I cells were adoptively transferred into sublethally irradiated B6 or MHCII-KO hosts. Mice were then treated for 3 days with IL-12. As previously reported (24), we found a large increase in both the number of OT-I cell divisions (Fig. 4A) and the total number of recovered OT-I cells in B6 mice treated with IL-12 (Fig. 4B). There was also enhanced proliferation of OT-I cells in MHCII-KO mice (Fig. 4A) although, surprisingly, the total number of recovered OT-I cells was not increased (Fig. 4B), suggesting impairment in HP-memory OT-I survival in the MHCII-KO hosts. Nevertheless, the protective function of unhelped HP-memory OT-I cells was restored by IL-12 treatment during HP (Fig. 4C), suggesting that IL-12 can substitute for CD4-mediated help to HP-memory CD8+ T cells.
Fig. 4.
Exogenous IL-12 results in the development of functional HP-memory cells in MHCII-KO hosts. HP-memory OT-I cells were generated in B6 or MHCII-KO mice by adoptive transfer of naïve Thy-1.1 OT-I cells into sublethally irradiated hosts. Mice were also given daily doses of either IL-12 or vector alone (mouse serum) for 3 days after adoptive transfer. (A) CFSE dye dilution was examined 22 days after transfer. Profiles are gated on Thy-1.1 OT-I cells from the spleen and lymph nodes. (B) Numbers of recovered Thy-1.1 OT-I T cells recovered per spleen and lymph nodes 22 days after transfer. Means ± SD are shown from 2 independent experiments. (C) Secondary adoptive transfer of OT-I HP-memory cells from the indicated groups into intact B6 mice was performed followed by challenge with LM-OVA. CFU of LM in the spleen was determined 5 days after infection. Individual mice from 2 experiments are shown. *, P = 0.0041, bacterial counts in test groups vs. naïve group.
Bacterial Release After Sublethal Irradiation Provides a Signal for Functional HP-Memory CD8+ T Cell Development.
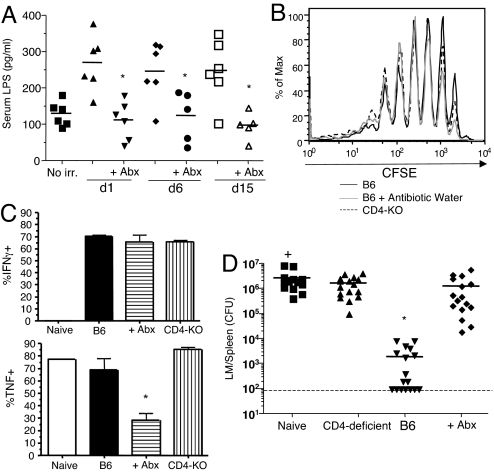
We reported previously that HP-memory CD8+ T cells generated in genetically T-deficient animals (e.g., Rag-KO or TCRα-KO hosts) were not protective against LM, unlike HP-memory cells produced in irradiated hosts (3). Although this difference relates partly to the lack of CD4+ T cells in genetically T-deficient hosts, we considered that there could also be positive effects of irradiation on the differentiation of HP-memory CD8+ T cells. Indeed, a recent report showed that sublethal irradiation leads to a transient appearance of bacterial products in the blood, DC activation, production of inflammatory cytokines, and increased potency of T cells in tumor immunotherapy (4). This was correlated with release of commensal microbes into the bloodstream, and treatment with antibiotics before irradiation blunted the T cell response in those studies (4). Similarly, we noted elevated levels of LPS in the serum of mice on days 1, 6, and 15 after irradiation, whereas inclusion of broad-spectrum antibiotics before irradiation prevented this rise in serum LPS (Fig. 5A). We next tested whether such antibiotic treatment influenced the protective function of HP-memory OT-I cells. Naïve OT-I T cells proliferated similarly in antibiotic-treated and untreated groups of irradiated mice (Fig. 5B) and were phenotypically indistinguishable (data not shown). Unexpectedly, in vitro peptide stimulation and analysis of cytokine production revealed that fewer cells from antibiotic-treated mice were able to produce TNF (Fig. 5C), although similar numbers of HP-memory OT-I cells made IFN-γ (Fig. 5C) and IL-2 (data not shown). We also noted a decreased mean fluorescence intensity in the TNF+ population from antibiotic-treated mice. (Fig. S3A). More importantly, the HP-memory OT-I T cells generated in antibiotic-treated hosts showed a dramatic impairment in protective immunity against LM-OVA (Fig. 5D). Regardless of treatment, the HP-memory OT-I T cells expanded similarly by day 5 after infection (Fig. S3B), and at this stage there were equal numbers of IFN-γ-producing and TNF-producing cells (data not shown). These data suggest that transient bacterial release after sublethal irradiation treatment contributes to the generation of functional HP-memory CD8+ T cells.
Fig. 5.
Antibiotic treatment of sublethally irradiated mice prevents development of fully functional HP-memory T cells. Beginning 3 days before sublethal irradiation, B6 mice were given either normal water or water containing broad-spectrum antibiotics (Abx). CFSE-labeled OT-I T cells were transferred into both groups of mice 1 day after irradiation. (A) Serum levels of LPS on the indicated days after irradiation. Data points represent individual mice from 2 separate experiments. *, P = 0.0019 [day 1 (d1)], P = 0.0248 (d6), and P = 0.0057 (d15), serum LPS in antibiotic-treated groups vs. no antibiotics. (B) OT-I T cell CFSE dye dilution 24 days after adoptive transfer of OT-I cells into irradiated hosts. (C) Percentage of HP-memory OT-I cells from the indicated lymphopenic hosts that made cytokines after in vitro stimulation with OVA peptide. Bars represent the mean ± SD from 2 separate experiments. *, P = 0.0179, frequency of TNF+ HP-memory OT-I cells in antibiotic-treated group vs. B6 group. (D) HP-memory OT-I cells were transferred into intact B6 mice and challenged with LM-OVA 1 day later. The CFU of LM in the spleen were determined 5 days after the challenge. Data points represent individual mice from 4 separate experiments. *, P = 0.0003, bacterial counts in test group vs. naïve group.
Our data to this point indicated that 2 factors were critical for development of functional HP-memory CD8+ T cells: CD4+ T cell-delivered CD40L signals, and acute bacterial release resulting from sublethal irradiation. Genetically T-deficient mice, such as Rag-KO animals, would lack both components, and they offered an opportunity to test this model by reconstitution of each factor.
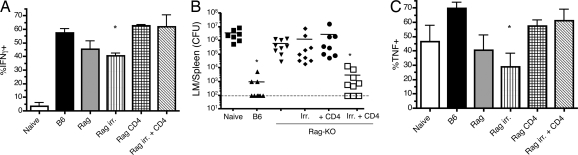
Naïve OT-I cells were transferred into Rag-KO animals, some of which were irradiated and/or adoptively transferred with polyclonal CD4+ T cells. OT-I proliferation was robust in all of these hosts, although it was enhanced in the irradiated animals (data not shown). Assessment of in vitro HP-memory function revealed defects in IFN-γ and TNF production by OT-I HP-memory cells produced in Rag-KO hosts and Rag-KO hosts that were sublethally irradiated (Fig. 6A, 6C, and Fig. S4). Consistent with our previous observation (3), HP-memory cells from Rag-KO mice failed to provide protection against LM-OVA challenge in secondary hosts (Fig. 6B). Alteration of the lymphopenic hosts by irradiation or CD4+ T cell transfer alone did not significantly enhance the ability of HP-memory CD8+ T cells to provide protective immunity, but the combination of irradiation and CD4+ T cell transfer led to dramatic enhancement of protective immunity, and several challenged mice had no detectable bacteria in the spleen (Fig. 6B). Similarly, protective HP-memory cells were generated in Rag-KO mice that had been irradiated and injected with IL-12 (Fig. S5). Interestingly, IL-12 treatment of unirradiated Rag-KO hosts did not yield protective HP-memory CD8 T cells. Together, these data support the conclusion that CD4+ T cells (and/or IL-12) cooperate with signals induced by released bacterial components (arising from sublethal irradiation) to drive generation of fully functional HP-memory CD8+ T cells.
Fig. 6.
CD4 transfer and sublethal irradiation allow for functional HP-memory T cell development in Rag-KO mice. OT-I cells were transferred into Rag-KO or sublethally irradiated B6 mice. Some Rag-KO mice were also sublethally irradiated and/or given adoptive transfer of polyclonal CD4+ T cells on day 0. After 3–4 weeks in lymphopenic hosts, spleen and lymph node cells were harvested. (A and C) Cells were stimulated in vitro with OVA peptide, and the percentage of OT-I cells making (A) IFN-g or (C) TNF was determined. Bars represent the mean ± SD from 3 separate experiments. (B) Harvested OT-I cells were adoptively transferred in equal numbers into intact B6 mice. One day later, mice were challenged with LM-OVA, and the CFU of LM in the spleen were determined 5 days after challenge. Data points represent individual mice from 3 separate experiments. *, P = 0.0039, bacterial counts in test groups vs. naïve group.
Discussion
In this report, we examined what is required in the lymphopenic environment for development of fully functional, protective HP-memory CD8+ T cells. At its minimum, HP involves proliferation and conversion of T cells from naïve to memory phenotype (2). However, our findings here and in previous studies (3) demonstrate that HP-memory CD8+ T cells with similar division histories and phenotypic profiles can exhibit drastically different capacities for protective immunity. The data in this report suggest that optimal protective function of HP-memory CD8+ T cells depends on specific components in the lymphopenic environment, including the presence of CD40L-expressing CD4+ T cells and exposure to bacterial components (which can be induced by sublethal irradiation).
We determined that optimal CD4+ T cell help for HP-memory CD8+ T cells was not limited by antigen specificity (since both monoclonal and polyclonal CD4+ T cells were similarly potent), but did require CD40L expression and could be replaced by antibody-mediated stimulation of CD40. These data suggest that, similar to conventional immune responses, CD40L–CD40 interactions between CD4+ T cells and APCs (perhaps DCs) are likely involved during help for HP-memory CD8+ T cells. CD40L is induced after CD4+ T cell activation, yet we did not observe up-regulation of CD40L during HP in lymphopenic hosts (data not shown). However, a recent study demonstrated that even naïve CD4+ T cells express cell surface CD40L (which in normal mice is down-modulated by engagement with CD40; ref. 25). Hence, we speculate that the basal CD40L–CD40 interaction, which occurs at a steady state in lymphoreplete animals, may become functionally important during lymphopenia.
A second signal was influenced by the nature of the lymphopenic environment. Others have demonstrated that bacterial release after low-dose irradiation regulates T cell control of tumors during immunotherapy (4). Here, we provided additional evidence for this conclusion and demonstrated its relevance for homeostatic (as opposed to antitumor) responses, because the presence of bacterial products in the lymphopenic host supports generation of protective HP-memory CD8+ T cells. Presumably, other cytoablative approaches, including chemotherapy, may exert similar effects, although this possibility needs to be determined directly. Such findings suggest that there may be crucial differences in the “quality” of the HP-memory pool depending on the “type” of lymphopenic environment in which it was generated. Importantly, our data suggest that the signals generated by irradiation-induced release of bacterial products are distinct from the contribution of CD4 T cells or IL-12. For example, we found that either CD4 T cells or IL-12 could drive production of protective HP-memory CD8 T cells in irradiated Rag-KO hosts, but not in unirradiated Rag-KO hosts (Fig. 6 and Fig. S5). The pathway activated by irradiation-induced bacterial release is unclear, although studies in the tumor immunotherapy model suggest an important role for the LPS receptor TLR4 (4). In this context, lymphopenia induced by irradiation or by acute infection might have similar efficacies in supporting the production of functional HP-memory CD8+ T cells by introduction of microbial products into the bloodstream. On the other hand, in situations where lymphopenia is not accompanied by acute release of microbial components, such as in genetically T cell-deficient hosts (and perhaps in neonatal mice), HP-memory CD8+ T cells may exhibit an impaired protective capacity.
Taken together, our data suggest that production of functionally protective HP-memory CD8+ T cells involves many of the same factors required for functional conventional-memory CD8+ T cells. During typical conventional immune responses to infection, APCs (chiefly DCs) are activated by pathogen-derived molecules (e.g., TLR ligands) and by CD40L–CD40 interactions (requiring CD4 help), which produce optimal DC activation (including production of functional IL-12) (22), leading to effective CD8 responses (1, 18). Similarly, we show that CD4 help, CD40–CD40L interactions, and release of bacterial components after sublethal irradiation contribute to production of protective HP-memory CD8+ T cells and that exogenous IL-12 could “bypass” the requirement for CD4+ T cells. Whether DCs themselves are required for generation of functional HP-memory cells is unclear. CD11c+ cells are sufficient (26) to induce the proliferation of T cells in a lymphopenic environment, but it has not been shown conclusively that they are required (27). In preliminary experiments, we have seen that a portion of CD11c+ cells in the lymph node up-regulate CD40 and CD86 after irradiation (data not shown). Future work will be necessary to address the requirement for DCs both in respect to the induction of T cell proliferation and the acquisition of functional memory characteristics.
An unresolved question is the nature of the defect in HP-memory CD8+ T cells that are unable to mediate protection. As we show here, this defect is not unique to unhelped HP-memory T cells. We note more extensive functional defects in T cells generated in Rag-KO animals and in antibiotic-treated irradiated mice, because in both cases these cells show reduced efficacy in production of inflammatory cytokines after TCR activation. However, we found similar cytokine production by helped and unhelped HP-memory CD8+ T cells (3), and both pools are competent for differentiation into cytolytic cells (Fig. S1), yet these pools differ dramatically in protective immunity (Figs. 5 and 6) (3). Our previous studies (3) indicated a lack of CD4+ T cell help could be compensated by TNF-related, apoptosis-inducing ligand deficiency in the OT-I T cells, but the mechanism by which TNF-related, apoptosis-inducing ligand functions in this model is not clear. The systems defined here for dissecting the requirements for establishing protective HP-memory CD8+ T cell function will allow for future studies of this important issue.
Overall, our data suggest that provision of the same cues that stimulate conventional CD8+ T cell responses (CD4 help, DC activation, inflammatory cues) promote protective function in HP-memory CD8+ T cells. This information is relevant for enhancing (or inhibiting) CD8+ T cell immune function in situations of lymphopenia, such as chemotherapy or radiotherapy and tissue grafts, as well as an adjunct in treatment of infectious diseases that induce lymphopenia.
Materials and Methods
Mice.
Mice deficient for I-Aβb (“MHCII-KO”), Rag-2 (“Rag-KO”), CD4 (“CD4-KO”), CD40 (“CD40-KO”), and CD154 (“CD40L-KO”) were used as indicated. OT-I.PL (Thy1.1+) mice were generated and maintained at the University of Minnesota. TEa TCR transgenic (14) Rag−/− mice were a kind gift from Marc Jenkins (University of Minnesota, Minneapolis, MN). Animals were maintained under specific pathogen-free conditions, and experimental procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee.
Bacterial Infections and Clearance.
Challenge with LM-OVA was performed as described previously (3) (also see SI Methods). Briefly, mice were infected i.v. with ≈2 x 105 CFU. Spleen and liver CFU were determined 5 days post-infection. The limit of detection (approximately 100 organisms) is indicated on graphs by a dashed line, and crosses indicate mice that died before day 5.
Adoptive Transfers and Flow Cytometry.
Methods were as described previously (3). The general adoptive transfer scheme is summarized in Fig. S2, and details are provided in SI Methods. Briefly, naïve (CD44low) OT-I cells were transferred into indicated recipient mice, and in some cases CD4+ T cells were cotransferred and/or the donor cells CFSE labeled. At 3–4 weeks, cells were recovered and a secondary transfer of “HP-memory” OT-I cells (or an equal number of naïve OT-I cells) was performed into intact B6 hosts, which were challenged with LM-OVA 1 day later.
In Vivo Treatments.
After adoptive transfer, indicated host mice received anti-CD40 clone 1C10 (from eBioscience) (50 μg administered i.p. on days 0, 7, and 14) or recombinant mouse IL-12 (a kind gift from Wyeth) (1 μg administered i.v. on days 1, 2, and 3). Treatment with antibiotic-laced water (13 mg/L polymyxin B sulfate and 36.8 mg/L neomycin sulfate, both from Sigma) commenced on day −4 relative to irradiation.
Supplementary Material
Acknowledgments.
We thank Jason Vevea and Xiaojie Ding for technical support; members of the Jamequist labs for critical input; and Dave Masopust, Vaiva Vezys, and Matt Mescher for comments on the manuscript. This work was supported in part by grants from the Centers for Disease Control (CI00100) and the National Institutes of Health (075168) to S.C.J. S.E.H. was supported by the American Cancer Society–Biogen Idec/Genentech/Ronald Levy Postdoctoral Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806487105/DCSupplemental.
References
- 1.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 2.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 4.Paulos CM, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dummer W, et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumpey TM, Lu X, Morken T, Zaki SR, Katz JM. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J Virol. 2000;74:6105–6116. doi: 10.1128/jvi.74.13.6105-6116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang J, Lau LL, Shen H. Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J Immunol. 2003;171:4352–4358. doi: 10.4049/jimmunol.171.8.4352. [DOI] [PubMed] [Google Scholar]
- 9.Min B, et al. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 10.Le Campion A, et al. Naive T cells proliferate strongly in neonatal mice in response to self-peptide/self-MHC complexes. Proc Natl Acad Sci USA. 2002;99:4538–4543. doi: 10.1073/pnas.062621699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuler T, Hammerling GJ, Arnold B. Cutting edge: IL-7-dependent homeostatic proliferation of CD8+ T cells in neonatal mice allows the generation of long-lived natural memory T cells. J Immunol. 2004;172:15–19. doi: 10.4049/jimmunol.172.1.15. [DOI] [PubMed] [Google Scholar]
- 12.Mescher MF, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 13.Hao Y, Legrand N, Freitas AA. The clone size of peripheral CD8 T cells is regulated by TCR promiscuity. J Exp Med. 2006;203:1643–1649. doi: 10.1084/jem.20052174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 15.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 16.Castellino F, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 17.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bevan MJ. Helping the CD8+ T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 19.Bennett SR, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 20.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 21.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 22.Schulz O, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 23.Filatenkov AA, et al. CD4 T cell-dependent conditioning of dendritic cells to produce IL-12 results in CD8-mediated graft rejection and avoidance of tolerance. J Immunol. 2005;174:6909–6917. doi: 10.4049/jimmunol.174.11.6909. [DOI] [PubMed] [Google Scholar]
- 24.Kieper WC, Prlic M, Schmidt CS, Mescher MF, Jameson SC. IL-12 enhances CD8 T cell homeostatic expansion. J Immunol. 2001;166:5515–5521. doi: 10.4049/jimmunol.166.9.5515. [DOI] [PubMed] [Google Scholar]
- 25.Lesley R, Kelly LM, Xu Y, Cyster JG. Naive CD4 T cells constitutively express CD40L and augment autoreactive B cell survival. Proc Natl Acad Sci USA. 2006;103:10717–10722. doi: 10.1073/pnas.0601539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruber A, Brocker T. MHC class I-positive dendritic cells (DC) control CD8 T cell homeostasis in vivo: T cell lymphopenia as a prerequisite for DC-mediated homeostatic proliferation of naive CD8 T cells. J Immunol. 2005;175:201–206. doi: 10.4049/jimmunol.175.1.201. [DOI] [PubMed] [Google Scholar]
- 27.Zaft T, Sapoznikov A, Krauthgamer R, Littman DR, Jung S. CD11chigh dendritic cell ablation impairs lymphopenia-driven proliferation of naive and memory CD8+ T cells. J Immunol. 2005;175:6428–6435. doi: 10.4049/jimmunol.175.10.6428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.