Abstract
Autoimmune polyendocrine syndrome type I (APS I) results in multiple endocrine organ destruction and is caused by mutations in the autoimmune regulator gene (AIRE). APS I is characterized by circulating tissue-specific autoantibodies, and the presence of these antibodies is often predictive of organ destruction. The importance of AIRE in ensuring central tolerance by regulating the negative selection of autoreactive T cells has been shown clearly. However, in Aire−/− mice the phenotype (i.e., autoantibodies, liver infiltrates of B cells, splenomegaly, and marginal zone B-cell lymphoma) is predominantly B-cell mediated, suggesting an exaggerated activation of B cells. We have studied T-cell-independent B-cell responses in the absence of AIRE and found that Aire−/− mice have an increased response against T-cell-independent type II antigens. We linked this exaggerated response to the elevated serum levels of the B-cell-activating factor of the TNF family (BAFF) that were found both in APS I patients and in Aire−/− mice. Transfer of Aire−/− bone marrow into irradiated nude mice resulted in increased percentage of BAFF-expressing antigen-presenting cells compared with wt bone marrow, suggesting a T-cell-independent mechanism behind our findings. Furthermore, in vitro experiments showed that AIRE-deficient murine bone marrow-derived dendritic cells produced significantly more BAFF than wt cells when stimulated with IFN-γ but not when stimulated with IL-10. Our results suggest a cell-intrinsic role for AIRE in peripheral dendritic cells by regulating IFN-γ-receptor signaling and point toward complementary mechanisms by which AIRE is involved in maintaining tolerance.
Keywords: autoimmunity, tolerance
Autoimmune polyendocrine, syndrome type I (APS I) (Online Mendelian Inheritance in Man 240300) is a monogenic disorder that results in a progressive autoimmune destruction of multiple endocrine organs and is characterized by circulating tissue-specific autoantibodies. The patients also develop chronic mucocutaneous candidiasis and non-endocrine gastrointestinal and skin diseases (1, 2). Several of the autoantigens in APS I have been identified (e.g., the cytochrome P450 cholesterol side-chain cleavage enzyme in the adrenal glands and glutamic acid decarboxylase 65 in the pancreas) (3, 4). The autoimmune regulator (AIRE) gene, mutations of which cause APS I, functions as a transcriptional regulator and is expressed mainly in medullary epithelial cells (MECs) of the thymus and peripheral antigen-presenting cells (APCs) (5, 6). MECs have a role in the negative selection of autoreactive thymocytes, a process that is impaired in the absence of AIRE (7, 8). In addition, AIRE deficiency leads to elevated numbers of peripheral APCs with an increased ability to activate T cells (9). Thus, AIRE has been implicated in both central and peripheral tolerance mechanisms.
In addition to multiple autoantibodies, Aire−/− mice engineered to mimic the most common Finnish mutation in APS I also develop marginal zone B-cell (MZB) lymphoma and liver infiltrates of B cells, indicating increased activation of B cells in the periphery (10–12). The MZBs are resident cells of the spleen located at the red pulp/white pulp junction. Specifically, MZBs can be activated rapidly to produce antibodies to bloodborne antigens in a T-cell-independent manner (13). The low activation threshold of MZBs and the fact that an increase in self-antigen B-cell receptor (BCR) signaling strength promotes MZB development in the periphery suggests that MZBs have a role in autoimmune antibody responses (14, 15). MZBs are particularly sensitive to stimulation of the cytokine B-cell-activating factor of the TNF family (BAFF) that is released primarily by dendritic cells (DCs) and radio-resistant stromal cells (16, 17). BAFF specifically binds to B cells and is required for maturation and plasma cell survival. In humans, increased serum levels of BAFF have been reported in several autoimmune diseases and correlate with titers of autoantibodies and disease activity (18). Overexpression of BAFF in a transgenic mouse system leads to the development of autoantibodies, splenomegaly, and expansion of the marginal zone, the same phenotype that can be observed in aged Aire−/− mice (19, 20). Furthermore, BAFF transgenic mice develop sialadenitis with lymphocytic cell infiltrates of salivary glands, a feature also reported in Aire−/− mice (7, 20, 21). The fact that the major manifestations in Aire−/− mice are caused by activated B cells together with the similarities in phenotype between BAFF transgenic mice and Aire−/− mice led us to investigate T-cell-independent B-cell responses and serum BAFF levels in the absence of AIRE.
Results
Aire−/− Mice Have an Increased Response to T-Cell-Independent Type II Antigens.
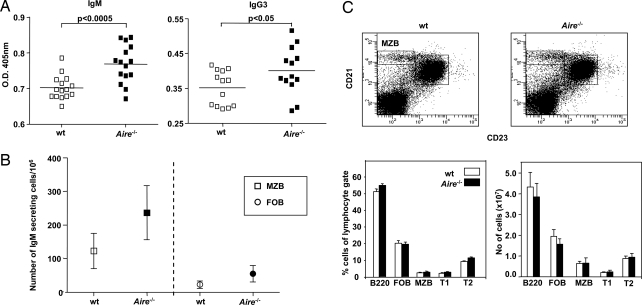
Immunization with the T-cell-independent type II (TI-II) antigen, 2,4,6-trinitrophenol (TNP)-Ficoll was performed in Aire−/− and wt mice to investigate in vivo innate B-cell responses in the absence of AIRE. The Ficoll-mediated response requires no T cells for its generation, illustrated by the fact that athymic (nu/nu) mice have a response similar to that of wt mice (22). In contrast, this response is highly dependent on MZBs, because mice lacking MZBs (i.e., Pyk-2-deficient mice) have a striking defect in TI-II responses (13). Aire−/− mice displayed significant increases in the production of TNP-specific antibodies of MZB-associated subclasses, typically IgG3 and IgM [Fig. 1A and supporting information (SI) Fig. S1]. This response was B-cell intrinsic and probably caused to a large extent by the MZBs, given the increased frequency of IgM-secreting MZBs sorted from Aire−/− mice after TNP-Ficoll stimulation in vitro, measured by enzyme-linked immunosorbent spot (ELISPOT) assay (Fig. 1B). Next, we characterized the B-cell compartment with flow cytometry to investigate whether the in vivo difference in TNP-Ficoll response was caused by variations in the size of the B-cell populations in Aire−/− and wt mice. However, the different B-cell subsets (i.e., mature follicular B cells [FOBs], MZBs, and precursor T1 and T2 cells) seemed to be proportionally and numerically normal in Aire−/− mice (Fig. 1C). Thus, the increased response against TNP-Ficoll was not caused by an abnormal B-cell compartment but rather by exaggerated activation of the MZBs.
Fig. 1.
Aire−/− mice have an increased specific response against TI-II antigen. (A) Serum levels of TNP-specific IgM and IgG3 on day 7 after immunization of Aire−/− mice and wt littermate controls with TNP-Ficoll, determined by ELISA. Each data point is from an individual mouse, and mean values are indicated by horizontal lines. (B) Number of IgM-secreting MZBs (squares) and FOBs (circles) measured after stimulation with 1 μg/ml TNP-Ficoll in vitro and detected by ELISPOT. Each data point represents the mean value of three different experiments, and in each experiment cells sorted from three Aire−/− and three wt mice were used. (C) FACS analysis of the B cell population in Aire−/− mice and wt littermates. Bars represent percent or absolute number of cells of the lymphocyte gate and mean value and SEM of six mice in each group. FOBs are defined as CD21posCD23posIgDbrightIgMlow, MZBs as CD21brightCD23neg, transitional 1 cells (T1) as CD21negCD23negIgMbrightIgDneg, and T2 cells as CD21posCD23posIgMbrightIgDbright. Representative FACS plots from Aire−/− and wt mice for CD23 and CD21 are shown.
AIRE Deficiency Leads to Increased Levels of BAFF Through a T-Cell-Independent Mechanism.
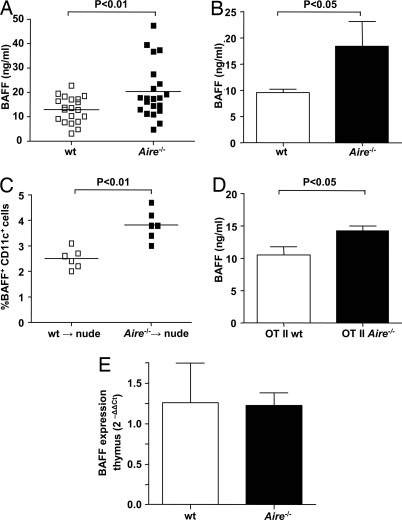
Next we wanted to elucidate the mechanisms behind the exaggerated activation of the MZBs in the absence of AIRE. AIRE-expressing cells in the periphery (i.e., monocyte-derived DCs) produce the cytokine BAFF that promotes B-cell survival and antibody responses (6, 23). BAFF transgenic mice develop autoantibodies and splenomegaly and have an expanded marginal zone, the same phenotype that can be observed in aged Aire−/− mice (19). These similarities prompted us to investigate serum BAFF in Aire−/− mice, and we found significantly higher levels than in wt mice (Fig. 2A). Increased serum BAFF also was found in a younger (4-week-old) cohort of mice, suggesting that our findings were not caused by a general activation of the immune system in response to an ongoing in vivo inflammation (Fig. 2B).
Fig. 2.
Aire−/− mice have elevated serum levels of BAFF. (A) Serum BAFF in Aire−/− mice and wt littermates, measured with ELISA. Each data point is from an individual mouse, and mean values are indicated by horizontal lines. (B) Serum BAFF in a younger (4-weeks-old) cohort of Aire−/− mice and wt littermates. Bars represent mean value and SEM of at least five mice in each group. (C) Percent BAFF-expressing DCs 6 weeks after Aire−/− or wt BM reconstitution into irradiated nude mice, measured in blood with flow cytometry; n = 6 in each group. (D) Serum BAFF in Aire−/− OT II TCR transgenic mice compared with wt OT II TCR littermates. Bars represent mean value and SEM of eight mice in each group. (E) BAFF expression in thymus of Aire−/− and wt mice, measured with QT-PCR. Bars represent mean values and SEM. Relative expression of BAFF was normalized to that of β-actin, and the ΔCt values from five Aire−/− mice were compared with the mean ΔCt value of five wt mice, using the 2−ΔΔCt method.
The expression of AIRE in thymic MECs and the role of AIRE in the negative selection of T cells led us to investigate whether the increased serum BAFF in the absence of AIRE was connected to presence of autoreactive T cells. Therefore we transferred Aire−/− or wt bone marrow (BM) into irradiated thymic-deficient nude mice. In this animal model, no mature T cells can develop from the received BM, thereby excluding a role for AIRE expression in the thymus. However, radiation induced excessive release of BAFF from the stromal compartment (Fig. S2), and therefore we were unable to compare BAFF production from the transferred BM-derived cells by measuring total serum levels. To circumvent this problem, we analyzed the expression of membrane-bound BAFF on CD11c+ blood DCs with flow cytometry 6 weeks after reconstitution. Nude mice receiving BM from Aire−/− mice had significantly higher percentages of BAFF-expressing DCs than mice receiving wt BM (Fig. 2C). To verify that no mature T cells were transferred with the BM into the nude mice recipients, the presence of CD3+ cells in the blood was analyzed 6 weeks after reconstitution. We could not detect any mature T cells, and thus the increased percentage of BAFF-expressing DCs was not caused by the presence of autoreactive T cells (Fig. S3A). The involvement of T cells was assessed further by measuring serum BAFF in Aire−/− mice crossed with a T-cell receptor (TCR) transgene recognizing an ovalbumin peptide (OTII mice) and thereby minimizing activation of autoreactive T cells in the system. In accordance with the previous finding, serum BAFF levels were higher in Aire−/− OTII mice than in wt OTII controls (Fig. 2D). The majority of the T cells in this animal model do not have the potential to recognize self-peptides and therefore should be unaffected by the absence of AIRE expression in the thymus. However, 10% to 15% of the CD4+ T cells in the OT II mice are not TCR transgenic and potentially could give rise to an autoimmune response. To exclude this possibility, we analyzed the expression of the activation markers CD69 and CD25 on the nontransgenic T cells and could not detect any up-regulation (Fig. S3B). Furthermore, BAFF expression was quantified in thymus from Aire−/− and wt mice with QT-PCR, and no differences could be detected (Fig. 2E). These results strongly suggest that autoreactive T cells are not involved in the mechanisms causing elevated serum BAFF in the absence of AIRE.
Serum Levels of BAFF But Not of a Proliferation-Inducing Ligand (APRIL) Are Increased in APS I Patients.
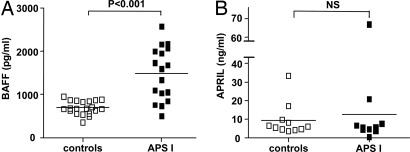
To substantiate further our finding that AIRE deficiency leads to excess BAFF production, we measured serum BAFF in 17 APS I patients and found it to be significantly higher than in healthy controls (Fig. 3A). The BAFF levels in APS I patients were comparable to those found in patients who have other autoimmune diseases in which autoantibodies are involved in the pathology (e.g., anti-double-stranded DNA antibodies in systemic lupus erythematosus, anti-SSA antibodies in primary Sjögren's syndrome, and rheumatoid factors in rheumatoid arthritis) (18, 24–26). In contrast, serum levels of a proliferation-inducing ligand (APRIL), another member of the TNF family that shares the transmembrane activation and calcium-modulator and cytophilin ligand interaction (TACI) and B-cell maturation antigen (BCMA) receptors with BAFF, were not elevated in APS I patients (Fig. 3B). These results show that the TNF family member BAFF is specifically up-regulated in the absence of functional AIRE both in patients and in mice.
Fig. 3.
The levels of serum BAFF but not of APRIL are increased in APS I patients. (A) Serum levels of BAFF in APS I patients compared with healthy controls measured with ELISA. Each data point represents one individual patient or healthy control. Mean values are indicated by horizontal lines. (B) Serum levels of APRIL in APS I patients and healthy controls measured with ELISA. NS = not significant.
AIRE Is Involved in the IFN-γ Receptor Signaling Pathway in Peripheral Dendritic Cells.
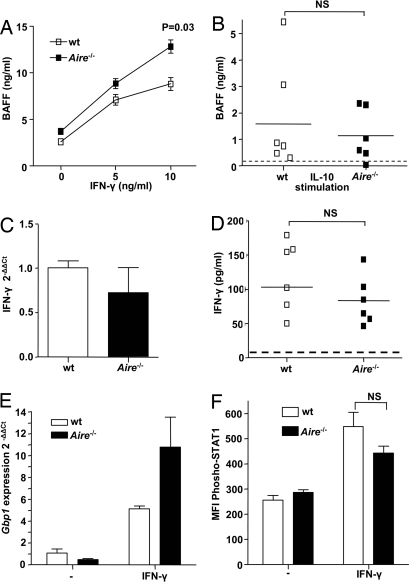
BAFF is reported to be secreted by myeloid DCs upon stimulation with cytokines such as IFN-γ or IL-10, whereas APRIL is not induced by IFN-γ stimulation (23, 27). Thus, we speculated that the increased levels of BAFF in the absence of AIRE were a consequence of dysregulation of the IFN-γ receptor signaling. To test for this possibility, we measured in vitro production of BAFF from Aire−/− and wt BM-derived DCs (BMDCs) stimulated with IFN-γ or IL-10 and adjusted for numerical comparison. The BMDCs were cultured from mouse femurs for 6 days before stimulation, enabling us to evaluate the consequence of AIRE deficiency in this specific cell type. In line with our hypothesis, Aire−/− BMDCs expressed significantly higher levels of BAFF upon IFN-γ stimulation in a dose-dependent manner (Fig. 4A). The same effect could not be seen after IL-10 stimulation, pointing toward an IFN-γ-dependent mechanism (Fig. 4B). The Aire−/− BMDCs displayed a normal phenotype regarding common surface markers, indicating that our findings were not a consequence of defective maturation or viability (Fig. S4).
Fig. 4.
AIRE is involved in the IFN-γ receptor signaling pathway in peripheral DCs. (A) In vitro production of BAFF from Aire−/− or wt BMDCs after stimulation with different concentrations of IFN-γ (horizontal axes), measured with ELISA. Data represent mean values and SEM of four mice in each group and are representative of two separate experiments. (B) BAFF production from Aire−/− or wt BMDCs after stimulation with 0.5 μg/ml rmIL-10, measured with ELISA. The dotted line represents the mean value of IL-10 production from nonstimulated BMDCs from Aire−/− and wt mice. Each data point represents one individual mouse; mean values are indicated by horizontal lines. (C) Expression of IFN-γ mRNA in spleen from Aire−/− (filled bar) and wt (open bar) mice measured with quantitative RT-PCR. Bars represent fold change of IFN-γ transcript from three Aire−/− mice relative to the mean ΔCt value of three wt littermates. All values were normalized using the 2−ΔΔCt method and Gapdh as the reference gene. (D) IFN-γ production from Aire−/− and wt BMDCs after LPS stimulation measured with ELISA. The dotted line represents the mean value of IFN-γ production from nonstimulated BMDCs from Aire−/− and wt mice. (E) Expression of the STAT-1 target gene Gbp1 in Aire−/− or wt adherent splenocytes, stimulated with IFN-γ for 4 h and measured with quantitative RT-PCR. Bars represent mean fold change of Gbp1 transcript from three Aire−/− mice or wt mice stimulated with IFN-γ, compared with the mean ΔCt value of unstimulated cells from wt mice. All values were normalized using the 2−ΔΔCt method and β-actin as the reference gene. (F) Flow cytometric analysis of phospho-STAT1 (Y701)-positive mononuclear myeloid cells from Aire−/− (filled bar) and wt (open bar) spleens. Bars represent mean fluorescence intensity values and SEM of phosphorylated STAT1, gated on myeloid mononuclear cells with five mice in each group. NS = not significant.
To evaluate other possible sources of excess BAFF production in the absence of AIRE, we measured the in vitro production from purified murine CD4+ T cells and from peritoneal macrophages. Both these cell types have been reported to express low levels of BAFF mRNA, but when the T cells were stimulated with anti-CD3 or concanavalin A, we could detect only minor levels of BAFF in the supernatant with no difference between Aire−/− and wt mice (Fig. S5). Nor could we detect BAFF production from the peritoneal macrophages after IFN-γ stimulation (data not shown). Thus, we concluded that the elevated serum BAFF in the absence of AIRE was caused to a large extent by augmented production from peripheral DCs.
Next we wanted to assess whether the increased production of BAFF in Aire−/− mice and APS I patients was a consequence of stronger IFN-γ stimulation, caused either by higher levels of this cytokine or by dysregulation of IFN-γ receptor signaling. The IFN-γ levels in serum are reported to be normal in Aire−/− mice (9), and when the expression of splenic IFN-γ mRNA was quantified, we found no difference between Aire−/− and wt mice (Fig. 4C). In addition, in vitro LPS-stimulated BMDCs from Aire−/− and wt mice produced similar amounts of IFN-γ, as measured with ELISA (Fig. 4D). Thus, our findings suggested that the IFN-γ stimulation caused augmented signaling via the IFN-γ receptor in AIRE-deficient DCs, leading to excess production of BAFF. Interestingly, a recent publication demonstrates a functional interaction between AIRE and the transcriptional co-activator protein inhibitor of activated STAT1 (PIAS1) in the nucleus (28). Upon IFN-γ receptor stimulation, STAT1 becomes tyrosine phosphorylated and translocates into the nucleus where it activates transcription. The PIAS1 protein interferes with the recruitment of STAT1 to the gene promoter, thereby inhibiting STAT1 signaling (29). We wanted to examine whether AIRE deficiency caused a dysregulation of STAT1 signaling similar to that seen in the absence of PIAS1. Therefore we treated splenic adherent cells from Aire−/− and wt mice with IFN-γ and quantified the induction of Gbp1 expression, a STAT1 target gene that is two-fold up-regulated in Pias1−/− mice (29). Indeed, Aire−/− cells also had a two-fold increase in Gbp1 expression upon IFN-γ stimulation, further supporting a role for AIRE in STAT1 signaling (Fig. 4E). Although the STAT1 signaling was affected, the phosphorylation status of STAT1 in myeloid mononuclear cells did not differ between Aire−/− and wt mice ex vivo or after stimulation with IFN-γ (Fig. 4F). This finding indicates that the excessive BAFF production in the absence of AIRE in vivo was caused by defective signaling via the IFN-γ receptor downstream of STAT1 phosphorylation.
Higher Levels of BAFF Lead to Exaggerated Activation of Marginal Zone B Cells.
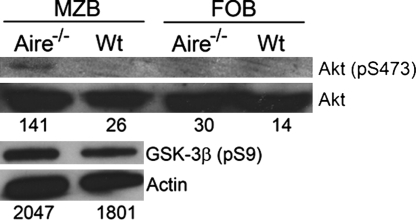
Finally, to investigate the in vivo consequence of increased BAFF in the absence of AIRE, we sorted MZBs and FOBs and performed Western blot analysis on proteins involved in the BAFF receptor signaling pathway. Binding of BAFF to the BAFF receptor triggers phosphorylation of the anti-apoptotic serine/threonine kinase Akt, which also is involved in the BCR signaling pathway (25). The phosphorylation status of Akt and the downstream element glycogen synthase kinase (GSK)-3β was increased in Aire−/− MZBs (Fig. 5). This finding shows that MZBs from Aire−/− mice display an increased state of activation through the BAFF receptor pathway before BCR stimulation. Thus, the increased BAFF levels in the absence of AIRE lead to exaggerated stimulation of B cells, particularly of BAFF-sensitive MZBs, making them more prone to produce autoantibodies and to mediate other functions such as antigen presentation, leading, in the long term, to the development of MZB lymphoma.
Fig. 5.
Increased phosphorylation status of survival kinases in Aire−/− B cells. Akt phosphorylation (S437) and GSK-3β phosphorylation (S9) was measured by immunoblot analysis on protein from sorted MZBs and FOBs from Aire−/− and wt mice, using the respective phosphospecific antibodies. Numbers represent normalized levels of p-Akt to the Akt signal and GSK-3β to the actin signal, reported as intensity/mm2.
Discussion
In this report we show that T-cell-independent B-cell responses are increased in absence of AIRE, a finding that can be linked to the significantly higher serum levels of BAFF displayed in both Aire−/− mice and APS I patients. We demonstrate here that AIRE-deficient murine BMDCs produce excess BAFF upon IFN-γ stimulation, indicating a cell-intrinsic effect of AIRE in the periphery. The recently published functional interaction between AIRE and PIAS1, a negative regulator of STAT1 signaling, supports our finding that the absence of AIRE can result in augmented signaling downstream of the IFN-γ receptor (28). As a consequence, excess BAFF is produced by Aire−/− DCs, leading to increased activation of B cells in the periphery (Fig. 6). In accordance with this finding, we found that the STAT1-target gene Gbp1 had a two-fold up-regulation in Aire−/− cells stimulated with IFN-γ. In addition, APS I patients are shown to have significantly elevated serum levels of CXCL10, another STAT1 target. Interestingly, both Gbp1 and cxcl10 are up-regulated in IFN-γ-stimulated BMDCs from Pias1−/− mice (29, 30). As in AIRE deficiency, deficiency of suppressor of cytokine signaling (SOCS1) leads to higher DC production of BAFF and to systemic autoimmune-like disease in mice. SOCS1 is crucial for negative regulation of IFN-γ by suppressing Janus kinase tyrosine kinase activity, resulting in the hyperresponsiveness of SOCS1-deficient DCs when stimulated with IFN-γ (31).
Fig. 6.
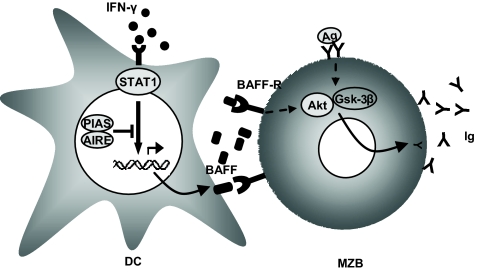
Schematic picture of the possible role for AIRE in IFN-γ receptor signaling. In the absence of AIRE, PIAS1 cannot mediate inhibition of STAT1 signaling upon IFN-γ receptor stimulation, resulting in excessive BAFF production. Subsequently, the increased levels of BAFF result in overly activated B cells, primarily the MZBs that produce autoantibodies and ultimately develop into MZB lymphoma. Thus, BAFF contributes to the phenotype seen in Aire−/− mice and APS I patients.
It has been shown clearly that AIRE deficiency leads to increased numbers of surviving autoreactive T cells caused by insufficient negative selection in the thymus. The suggested mechanism behind this defect is the lack of self-antigen expression in Aire−/− MECs (7). However, Aire−/− mice display autoimmunity against antigens that are not repressed in Aire−/− MECs, indicating alternative mechanisms by which AIRE maintains tolerance (21). In addition, several reports have demonstrated a function for AIRE in peripheral tolerance: the absence of AIRE leads to increased DC-mediated activation of T cells, independent of central tolerance mechanisms (9). Noticeably, the main phenotype seen in aged Aire−/− mice (autoantibodies, splenomegaly, liver infiltrates of B cells and MZB lymphoma) is caused by activated B cells, indicating that not only T cells contribute to autoimmunity in the absence of AIRE (11). Our results show that a cell-intrinsic effect of AIRE deficiency in peripheral DCs leads to higher serum BAFF in both mice and patients and that autoreactive T cells are not involved in these mechanisms. The higher BAFF levels can explain the enhanced activation of B cells, in particular of the BAFF-sensitive MZBs, making them more prone to produce autoantibodies and leading, in the long term, to the development of MZB lymphoma. Elevated serum BAFF levels are found in several autoimmune diseases and correlate with the titers of autoantibodies. Thus, BAFF is likely to affect the activation of the immune system and to contribute to autoimmunity in APS I.
In conclusion, our findings that AIRE deficiency leads to DC hyperresponsiveness upon IFN-γ stimulation and as a consequence to elevated serum BAFF levels point toward complementary mechanisms by which AIRE is involved in maintaining tolerance. At present, blockade of BAFF, together with the use of agents that interfere with the BAFF activation pathway, is viewed as promising therapy in several autoimmune diseases. We suggest that high BAFF levels influence the disease phenotype and etiology of APS I and therefore can be an attractive target in the treatment of these patients.
Methods
Mice and Patients.
The generation and breeding of congenic C57BL/6 Aire−/− and of OT-II TCR transgenic mice have been described previously (9, 32). BM transfer was performed by i.v. injection of 5 × 106 donor-derived BM cells into recipient mice irradiated with 900 rad or 450 rad for BALB/c nude mice at least 4 h before injection. Mice were maintained at the animal facility of the Department of Microbiology, Tumor and Cell Biology at Karolinska Institutet and were used at the age of 8–16 weeks or 4 weeks (Fig. 2B). Peripheral blood from 17 patients with APS I and from 20 matched healthy controls was used with the participants' informed consent. The work was approved by the local ethics committees.
Cell Cultures.
BM cells were isolated by flushing mouse femurs with RPMI-1640 (HyClone). To generate dendritic cells, 2 × 106 marrow cells were plated in complete RPMI-1640 (10% heat-inactivated FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, 5 μM mercaptoethanol, and 25 μg/ml gentamycin) supplemented with 10 ng/ml rmGM-CSF and 10 ng/ml rmIL-4 (Invitrogen). Cells were stimulated with 5 or 10 ng/ml rmIFN-γ, 100 ng/ml LPS, or 0.5 μg/ml rmIL-10 (all from Sigma). Flow cytometric analysis showed that the cultured cells contained 93% to 95% CD11c+ cells. Splenic CD4+ T cells were separated by magnetic activated cell sorting (MACS) using a CD4 T-cell negative selection kit (Miltenyi Biotech) according to the manufacturer's instructions. T cells were stimulated with 5 or 10 μg/ml concanavalin A or with 7.5 μg/ml anti-CD3 antibodies for 72 h, and activation was assessed by visual examination and flow cytometry for CD69 expression.
Immunizations and ELISA.
To measure specific immunoglobulin response, we collected serum from the mice on day 7 after immunization with 100 μg 2,4,6 TNP-Ficoll i.v. and captured specific antibodies on plates coated with BSA-TNP (5 μg/ml). The 4-(hydroxy-3-nitro-phenyl) acetyl (NP)-specific antibodies were detected with alkaline phosphatase (AP)-conjugated goat anti-mouse IgM and IgG subclass antibodies (Southern Biotechnology Associates) at OD405. Cytokine levels in cell culture supernatants and in serum from mice and humans were measured using specific ELISA kits for mouse BAFF (Alexis), human BAFF and APRIL (R&D Systems), and mouse IFN-γ (Mabtech) according to the manufacturers' instructions. Each sample was tested in duplicate.
Flow Cytometric Analysis and Cell Sorting.
Cells were stained with the following anti-mouse antibodies: FITC-conjugated: anti-CD25, anti-CD21, anti-IgD, anti-CD5, anti-B220, and anti-CD11c; phycoerythrin (PE)-conjugated: CD80, CD81, CD83, anti-TCRVβ5, anti-CD21, and anti-B220; allophycocyanin-conjugated: CD11b, anti-IgM; biotin-conjugated: anti-CD23, BAFF (clone IC9); PerCp-conjugated: streptavidin; PE-Cy7-conjugated: anti-CD69; allophycocyanin-Cy7-conjugated: CD3. All antibodies and streptavidin conjugates were purchased from BD Biosciences Pharmingen except for the BAFF antibody, which was from Alexis. Data were collected with a FACSAria flow cytometer, and data analysis was performed using FACSDiva software (BD Biosciences). For cell sorting CD43− splenic B cells were enriched using MACS with anti-CD43 beads (Miltenyi Biotech). FITC-conjugated anti-CD21 and PE-conjugated anti-CD23 antibodies (BD PharMingen) were used to sort out the MZBs and FOBs on the FACSAria (BD Biosciences). The MZBs were identified as CD43negCD21brightCD23neg, and the FOBs were identified as CD43negCD21posCD23pos. The purity for both populations was >99% after sorting. For the phosphorylation status of STAT1, splenocytes were stimulated with 100 ng/ml rmIFN-γ (Sigma) and analyzed with flow cytometry using an anti-phopho-STAT1 (Y701):PE (BD Biosciences) antibody according to the manufacturer's instructions.
ELISPOT Assay for IgM Antibodies.
Sorted MZBs and FOBs (1.5 × 105 cells/well) were stimulated with 1 μg/ml TNP-Ficoll in RPMI-1640 culture medium (10% heat-inactivated FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM mercaptoethanol) with 100 U/ml IL-1β (Biosource) and 100 U/ml IL-2 (Biosite) in a 96-well plate at 37 °C. On day 5, the cells were subjected to an ELISPOT plate (MultiScreen-IP, Millipore) coated with 5 μg/ml goat anti-mouse Ig (H+L) (Southern Biotechnology Associates), and incubated at 37 °C. AP-conjugated anti-mouse IgM antibodies (Southern Biotechnology Associates) was added after 48 h, and the plate was incubated with AP conjugate substrate kit (Bio-Rad) at room temperature until spots emerged. Each sample was tested in duplicate.
Western Blot Analysis.
MZB and FOB lysates were subjected to SDS/PAGE, and the following antibodies were used for detection: pAkt(Ser-473), pGSK3β(Ser-9), and Akt (Cell Signaling Technology) followed by an HRP-conjugated antibody to rabbit IgG (Cell Signaling). Actin was detected with an HRP-conjugated antibody (Santa Cruz Biotechnology). The proteins were visualized with a commercial ECL kit (Bio-Rad).
Quantitative RT-PCR.
Total RNA was extracted using a conventional TRIzol-extraction (Invitrogen). Reverse transcriptase reaction was performed using iScriptTMcDNA Synthesis Kit (Bio-Rad) according to the manufacturer's instructions. Quantitative RT-PCR was conducted with iCyclerIQ using 2X IQTM SYBR Green Supermix (Bio-Rad) and specific primer pairs for Ifn-γ (5′-AGCTCTTCCTCATGGCTGTTTC-3′, 5′-TTATGTTGTTGCTGATGGCCTGATTG-3′); Gapdh (5′-GCTCTCTGCTCCTCCCTGTT-3′, 5′-TCCGTTCACACCGACCTTCAC-3′); Baff (5′-TGTTGTCCAGCAGTTTCAC-3′, 5′-CTGCAGACAGTCTTGAA TGA-3′); Gbp1 (5′-ATCATATCCAAACTTCAGGAACAG-3′, 5′-GTGGAAACAGGGTAGAGAGCTTTAGT-3′); and β-actin (5′-TGTGGTGGTGAAGCTGTAGC-3′, 5′-GACGACATGGAGAA GATCTGG-3′).
Cycle thresholds were obtained with iCycler IQTM Optical System Software Version 3.1 (Bio-Rad). Expression levels were normalized to that of Gapdh or β-actin, and Aire−/− ΔCt values were compared with the mean ΔCt value of unstimulated wt mice, using the 2−ΔΔCt method.
Statistical Analysis.
The data were analyzed with the Student t-test and Wilcoxon rank sum test to ascertain the difference between the two groups. Figure bars represent mean values and SEM. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments.
The authors thank A. Patke and A. Tarakhovsky for valuable discussions and F. Wermeling, C. Möller, M. Blennow, P. Janson, G. Nilsson, and S. Gabrielsson for providing reagents and for technical assistance. This work was supported by the Swedish Research Council, the Lundberg Foundation, the Grönwalls Foundation, the Magnus Bergvall Foundation, the Swedish Diabetes Association, the Swedish Medical Society, the Agnes and Mac Rudbergs Foundation, the Swedish Juvenile Diabetes Foundation, and the Swedish Cancer And Allergy Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808205105/DCSupplemental.
References
- 1.Aaltonen J, Bjorses P, Sandkuijl L, Perheentupa J, Peltonen L. An autosomal locus causing autoimmune disease: Autoimmune polyglandular disease type I assigned to chromosome 21. Nat Genet. 1994;8(1):83–87. doi: 10.1038/ng0994-83. [DOI] [PubMed] [Google Scholar]
- 2.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91(8):2843–2850. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- 3.Winqvist O, Gustafsson J, Rorsman F, Karlsson FA, Kampe O. Two different cytochrome P450 enzymes are the adrenal antigens in autoimmune polyendocrine syndrome type I and Addison's disease. J Clin Invest. 1993;92(5):2377–2385. doi: 10.1172/JCI116843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuomi T, et al. Antibodies to glutamic acid decarboxylase and insulin-dependent diabetes in patients with autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 1996;81(4):1488–1494. doi: 10.1210/jcem.81.4.8636356. [DOI] [PubMed] [Google Scholar]
- 5.Nagamine K, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17(4):393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 6.Kogawa K, et al. Expression of AIRE gene in peripheral monocyte/dendritic cell lineage. Immunol Lett. 2002;80(3):195–198. doi: 10.1016/s0165-2478(01)00314-5. [DOI] [PubMed] [Google Scholar]
- 7.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the AIRE protein. Science. 2002;298(5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 8.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4(4):350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey C, et al. Increased antigen presenting cell-mediated T cell activation in mice and patients without the autoimmune regulator. Eur J Immunol. 2006;36(2):305–317. doi: 10.1002/eji.200535240. [DOI] [PubMed] [Google Scholar]
- 10.Wang CY, Shi JD, Davoodi-Semiromi A, She JX. Cloning of Aire, the mouse homologue of the autoimmune regulator (AIRE) gene responsible for autoimmune polyglandular syndrome type 1 (ASP1) Genomics. 1999;55(3):322–326. doi: 10.1006/geno.1998.5656. [DOI] [PubMed] [Google Scholar]
- 11.Hassler S, et al. Aire deficient mice develop hematopoietic irregularities and marginal zone B cell lymphoma. Blood. 2006;108(6):1941–1948. doi: 10.1182/blood-2006-04-019679. [DOI] [PubMed] [Google Scholar]
- 12.Ramsey C, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11(4):397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 13.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1(1):31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 14.Wen L, et al. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 2005;23(3):297–308. doi: 10.1016/j.immuni.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 16.Schneider P, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189(11):1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorelik L, et al. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198(6):937–945. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pers JO, et al. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann N Y Acad Sci. 2005;1050:34–39. doi: 10.1196/annals.1313.004. [DOI] [PubMed] [Google Scholar]
- 19.Batten M, et al. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192(10):1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackay F, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190(11):1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuroda N, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174(4):1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 22.Sharon R, McMaster PR, Kask AM, Owens JD, Paul WE. DNP-Lys-ficoll: A T-independent antigen which elicits both IgM and IgG anti-DNP antibody-secreting cells. J Immunol. 1975;114(5):1585–1589. [PubMed] [Google Scholar]
- 23.Nardelli B, et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97(1):198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 24.Mariette X, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren's syndrome. Ann Rheum Dis. 2003;62(2):168–171. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC{beta}- and Akt-dependent mechanism. J Exp Med. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker-Merok A, Nikolaisen C, Nossent HC. B-lymphocyte activating factor in systemic lupus erythematosus and rheumatoid arthritis in relation to autoantibody levels, disease measures and time. Lupus. 2006;15(9):570–576. doi: 10.1177/0961203306071871. [DOI] [PubMed] [Google Scholar]
- 27.Krumbholz M, et al. Interferon-beta increases BAFF levels in multiple sclerosis: Implications for B cell autoimmunity. Brain. 2008;131(Pt 6):1455–1463. doi: 10.1093/brain/awn077. [DOI] [PubMed] [Google Scholar]
- 28.Ilmarinen T, et al. Functional interaction of AIRE with PIAS1 in transcriptional regulation. Mol Immunol. 2008;45(7):1847–1862. doi: 10.1016/j.molimm.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 29.Liu B, et al. PIAS1 selectively inhibits interferon-inducible genes and is important in innate immunity. Nat Immunol. 2004;5(9):891–898. doi: 10.1038/ni1104. [DOI] [PubMed] [Google Scholar]
- 30.Kisand K, et al. Interferon autoantibodies associated with AIRE-deficiency decrease the expression of IFN-stimulated genes. Blood. 2008;112(7):2657–2666. doi: 10.1182/blood-2008-03-144634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanada T, et al. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19(3):437–450. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 32.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.