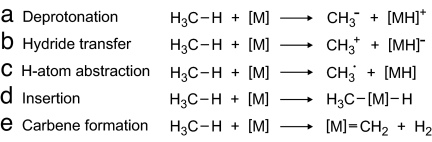Abstract
Motivated by the search for ways of a more efficient usage of the large, unexploited resources of methane, recent progress in the gas-phase activation of methane by ligated transition-metal ions is discussed. Mass spectrometric experiments demonstrate that the ligands can crucially influence both reactivity and selectivity of transition-metal cations in bond-activation processes, and the most reactive species derive from combinations of transition metals with the electronegative elements fluorine, oxygen, and chlorine. Furthermore, the collected knowledge about intramolecular kinetic isotope effects associated with the activation of C–H(D) bonds of methane can be used to distinguish the nature of the bond activation as a mere hydrogen-abstraction, a metal-assisted mechanism or more complex reactions such as formation of insertion intermediates or σ-bond metathesis.
Keywords: bond activation, kinetic isotope effects, mass spectrometry
The conversion of methane from fossil or biogenic resources to more valuable feedstock forms one of the central challenges for solving global energy problems (1). The first burden to master in this challenging task is the activation of methane under ambient conditions to subsequently enable a selective transformation of the intermediate to useful products, such as methanol.
Here, we describe various thermal reactions of methane with gaseous metal cations, which may be of relevance in these respects. If it would be possible to extend the reactions described below from the laboratory to large-scale industrial processes, the resources of methane could be used much more efficiently. Imagine, for example, a small plant that has methane and air as inputs with methanol dropping out as the product. Likewise, oxidative coupling of methane to larger hydrocarbons would be of considerable interest, provided a catalyst can achieve high selectivity and large conversion. At the very outset, we would like to point out that our contributions are basic research dealing with isolated, very much idealized systems and are thus quite remote from achieving these goals in a practical sense. Furthermore, because of Coulomb repulsion, gas-phase reactions with charged species comprise only several 106 molecules at best and are hence irrelevant for any kind of “production.” Nevertheless, gas-phase studies may serve to experimentally verify theoretical predictions and particularly to propose new concepts for the low-temperature C–H bond activation of methane.
Conceptually, there exist several ways for the activation of methane by a reactive metal species [M], where [M] stands for a bare or ligated, neutral, or charged metal atom or metal-containing fragment. Heterolysis of a C–H bond by either deprotonation (route a in Scheme 1) or hydride transfer (route b in Scheme 1) leads to methyl anion and cation, respectively, concomitant with the corresponding metal-hydride fragments. C–H bond homolysis, usually occurring as H-atom abstraction (route c in Scheme 1), leads to the production of a methyl radical, which constitutes the first step in the oxidative coupling of methane to ethane via recombination of two CH3• radicals. Transition metals capable to easily change their valence state are also able to undergo oxidative insertion into the C–H bond (route d in Scheme 1). Finally, particularly 5d elements can also dehydrogenate methane to afford metal-bound carbenes (route e in Scheme 1). Note, however, that the activation of methane is only the first step in the conversion of methane because occurrence of a catalytic process requires that the exothermicity released in the subsequent reaction of the free or coordinated methyl or methylene fragments with another partner (e.g., oxygen to finally afford methanol) is sufficiently large to also regenerate the initial metal species [M].
Scheme 1.
Early gas-phase studies of methane activation were focused on the reactions of bare metal cations. A landmark discovery was made by Irikura and Beauchamp (2, 3), who detected thermal reactions of methane with 5d transition-metal cations; Reaction 1 with M = Ta, W, Os, Ir, and Pt.
In the case of M = Pt, also a catalytic variant with molecular oxygen leading to oxidized products such as methanol, formaldehyde, and formic acid could be realized (4, 5), and for M = Ta, a (stoichiometric) coupling of the initially formed TaCH2+ with CO2 to afford ketene as a product was found (6, 7). More recently, the details of these reactions, including those metals that cannot activate methane under thermal conditions, were analyzed by combined experimental and theoretical approaches, e.g., for M = Fe (8), Ni (9), Zr (10), Hf (11), Mo (12), Ta (13), W (14), Re (15), Ir (16), and Au (17). Several metal dications were also found to activate methane in analogy to Reaction 1 (18).
Bare metal cations are, however, rightly considered as a very extreme case, and most of them bear limited relevance with respect to processes in applied catalysis because the reduction of complexity is too large. In fact, most of the above-mentioned examples of methane activation lead to species that do not react further with methane; exceptions are found among 5d metals (2, 3, 13, 14, 19, 20). In addition, bare transition-metal ions correspond to the oxidation state (+I), which is unusual in condensed-phase chemistry. To approach slightly more realistic molecular models that are still amenable to theory, in this contribution, we describe recent progress with respect to the activation of methane by ligated transition-metal ions. Because they form a topic on their own, we note that, with a few exceptions, di- and oligonuclear metal clusters are not covered here. Generally, it has be concluded, however, that polynuclear metal cluster ions are not more reactive than their atomic brethren, because, despite a favorable thermochemistry, kinetic barriers in the entrance channels may hinder alkane activation (8, 21, 22).
Ligated Metal Cations
At the outset, let us make a general distinction of the ligands in terms of their electron shell. Mere coordinative, closed-shell ligands L, such as L = H2O, CO, alkenes, or arenes, do not change the formal oxidation state of the metal. In contrast, open-shell ligands X form (polarized) covalent bonds to the metal and thereby increase the formal oxidation state, e.g., X = F, OH, Cl, Br, I, CH2, NH, O, and N. Note that some open-shell ligands bear an intermediate behavior, e.g., NO and O2.
Closed-Shell Ligands L.
In general, coordination of metal ions to closed-shell ligands L lowers the reactivity compared with bare metal cations, which may, however, result in increased selectivities, provided the reactivity is not quenched completely (23, 24). In this respect, Tjelta and Armentrout (25) reported impressive examples for the ability of tuning selectivities in C–H- versus C–C-bond activation of small alkanes by Fe(L)+ cations with L = H2O and CO; the former ligand induces a preferential attack of C–H bonds, and the latter also promotes the cleavage of C–C-bonds in the substrate. Both ligated cations, Fe(H2O)+ and Fe(CO)+, are less reactive than bare Fe+, however, and none of these three cationic species allows a room-temperature activation of methane.
Albert et al. (26) found a spectacular example for the effect of ligation with a closed-shell ligand, in that Rh2Ar+ can activate methane, whereas the bare dirhodium cation Rh2+ does not. The authors explained the pronounced effect, exerted by no more than a single rare-gas atom, by the increased lifetime of the intermediate encounter complex formed upon exchange of argon by methane in the first step to yield excited [Rh2CH4]+* that subsequently undergoes dehydrogenation according to Reaction 2; in terms of the classification in Scheme 1, Reaction 2 is an example for route e.
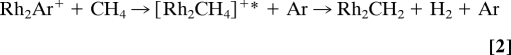 |
Similarly, PtAr+ is able to dehydrogenate methane, but here, the ligand damps the reactivity in that the rate constant for bare Pt+ is ≈7 times larger; higher PtArn+ clusters (n = 2–6) do not activate methane any more (27). These simple examples thereby illustrates the general consideration that ligated M(L)+ species are much less reactive than the corresponding bare metal cations and often even act as sinks of catalytic cycles.
The seemingly straightforward classification of ligands L encounters some ambiguity with transition metals that can easily change their oxidation state. A metal–olefin complex, for example, can also be viewed as a metallacyclopropane in which the metal formally undergoes a two-electron oxidation. Thus, in the triad of the coinage metals, Ag(C2H4)+ is a T-shaped π-complex with genuine Ag(I), whereas Au(C2H4)+ exhibits an auracyclopropane structure with a triangular bonding scheme and a significantly elongated C–C bond; for Cu(C2H4)+, the situation is in between these extremes (28). Recently, we also could generate the insertion intermediate HNi(OH)+, which is experimentally distinguishable from the water complex Ni(H2O)+ (29). Formally, HNi(OH)+ corresponds to the unusual oxidation state Ni(III), and it is thus not too surprising that the HNi(OH)+ cation is able to activate methane according to Reaction 3. Mechanistically, this process may be viewed as an example for either route c or route d in Scheme 1, depending on whether the formation of dihydrogen is considered as a formal abstraction (via σ-bond metathesis) or whether an insertion intermediate is involved.
Formally, Reaction 3 may also be regarded as an example of route a in Scheme 1, i.e., the reaction of a metal hydride with the methane to yield the corresponding methanideconcomitant with dihydrogen. Interestingly, the metal-hydrido species HNi(OH)+ was generated by electrospray ionization of a NiI2 solution in the protic mixture of methanol and water. The details of ion generation are still not resolved, but use of nickel(II) iodide is essential, and the hydridic H-atom stems from the methoxy group of the methanol used as a cosolvent, whereas the hydroxy group stems from water (29, 30).
An even more extreme situation with regard to the ambivalent behavior of ligands evolves for dioxygen complexes of metal cations in which the (open-shell) dioxygen ligand can behave either as a mere π-type ligand or give rise to peroxide- or even dioxide structures (31–34).
Open-Shell Ligands X.
The prototype of this class of covalent ligands is X = F. Because of its large electronegativity and poor donor properties, fluorine forms strongly polarized covalent bonds to metals, and thus, each fluorine increases the metal's formal oxidation by one unit. Even in the very extreme situation of the diatomic trication UF3+ (35), fluorine still acts as an electronegative ligand, as demonstrated by population analysis that reveals a formal charge of 3.4 for uranium in UF3+. Other electronegative ligands such as X = Cl, Br, I, and OH behave similarly to fluorine in this respect, although the metal fluorides are most reactive.
Unlike coordinative ligands L, attachment of a covalent ligand X often increases reactivity. Bare Cr+ for example, is one of the least reactive transition-metal cations, whereas already CrCl+ is significantly more reactive (36), and CrF+ is even capable of dehydrogenating propane (37). With respect to the activation of methane, a single fluorine is not sufficient, however, and CrF2+ does not react with CH4, whereas CrF3+ and CrF4+ are able to activate the C–H bonds of methane (37). In the case of CrF3+, the major product of the reaction with methane corresponds to a methyl cation concomitant with neutral HCrF3 (or CrF2 + HF). This reaction hence is an example for hydride transfer according to route b in Scheme 1. Hydride transfer also occurs for bare and ligated transition-metal dications (18), but this is due less to the presence of a transition metal than a general feature in the bimolecular reactions of isolated dications (38). Similar to CrF+, FeF+ is also more reactive than other FeX+ species (X = OH, Cl, Br, I, etc.), but none of these cations can activate methane (39). In the case of NiX+, diatomic NiF+ promotes C–H bond activation of methane according to Reaction 4, whereas the nickel–halide cations NiCl+, NiBr+, and NiI+ react only with larger alkanes (40).
The trends in reactivity can be explained not only in terms of the electronegative character of X, but also by the corresponding reaction enthalpies that are mostly related to the formation of HX, whose bond-dissociation energies are much lower for the heavier halogens. Formation of HX as the thermochemical driving force also accounts for the strong preference of C–H over C–C bond activation in the case of larger alkanes (36, 37, 40).
Also the platinum-chloride and -bromide cations PtXn+ (n = 1, 2) are capable of methane activation under thermal conditions (41). For the chlorides PtCl+ and PtCl2+, exclusive losses of HX in analogy to Reaction 4 are observed, whereas in the case of PtBr+ formation of the corresponding carbene also takes place to some extent, i.e., Reaction 5, which is analogous to Reaction 1, with M = PtBr. Notably, the formal platinum(IV) species PtCl3+ does not react with methane, most probably because it is coordinatively saturated to an extent that there exists no sufficiently attractive vacant coordination site for the initial binding of methane before its activation within the collision complex.
Although the electron affinity of hydrogen is much lower, the corresponding metal-hydride cations MH+ behave similar to the metal halides in several respects (42–44). Thus, NiH+ and PtH+ promote C–H bond activation of methane according to Reaction 6 (29, 41). The obvious explanation for this behavior is the favorable thermochemical driving force associated with the formation of dihydrogen in conjunction with the energetic preference of M–C over M–H bonds. The investigation of the group 10 metal-hydride cations has recently been completed by a study of PdH+ (45). Despite the similarities of Reaction 6 for M = Ni, Pd, and Pt, however, the details of the potential-energy surfaces differ decisively, in that the reactions of NiH+ and PtH+ involve two spin surfaces, whereas σ-bond metathesis in the case of PdH+ exclusively proceeds on the singlet surface.
Metal-oxide cations comprise an important class of compounds with ligands of the type X that are able to activate methane. Methane activation by gaseous metal-oxide cations MO+ generally leads to three different kinds of products, bare metal cations concomitant with methanol (Reaction 7), metal hydroxides via loss of a methyl radical (Reaction 8), and formation of metal-carbene cations, concomitant with water as a neutral product (Reaction 9).
Reactions 7 and 8 are of direct relevance for the conversion of methane to more valuable products. The former leads to methanol, and the latter is the first step in the oxidative coupling of methane to ethane. Therefore, the reactions of metal-oxide cations have received great attention and have been reviewed repeatedly (46–48). Here, we concentrate on a few key features that are of particular relevance for the activation of methane. Reactions 7 and 9 proceed via the insertion species (H3C)M(OH)+ as the key intermediates. Although the metal has the formal oxidation state M(III) in MO+ and in (H3C)M(OH)+, there exist a fundamental difference in the bonding situations, which has important consequences. Thus, at least 3d and 4d metals cannot effectively support π-type double bonds in MO+, which consequently leads to high-spin ground states of the MO+ monocations. In contrast, the insertion intermediates primarily involve σ-type bonding, for which perfect pairing and thus low-spin ground states are preferred. This leads to a situation in which the spin surfaces cross each other such that the reaction pathway of lowest energy demand involves a cross-over from the high- to the low-spin surface and eventually back to high spin in the final products. In fact, the reactions of bare metal-oxide cations in the gas phase inter alia led to the development to the concept of two-state reactivity (49), which is now widely accepted in various areas of science far beyond gas-phase ion chemistry, as, for example, in organometallic chemistry and also in the context of metalloenzymes (50). In contrast, Reaction 8 formally corresponds to a simple abstraction hydrogen-atom and therefore does not need to involve a spin change. As outlined further below, these differences in the types of reaction mechanism are also reflected in the kinetic isotope effects associated with the C–H bond activation of methane.
So far, thermal methane activation has been achieved by the mononuclear transition-metal oxide cations MnO+ (51), FeO+ (52, 53), NiO+ (54), OsO+ (55), and PtO+ (5). Interestingly, the branching ratios of Reactions 7–9 very much depend on the position of the metal in the periodic table. Thus, MnO+ leads to exclusive hydrogen-atom abstraction (Reaction 8), for FeO+ Reactions 7 and 8 compete, whereas NiO+ only undergoes Reaction 7. In OsO+, oxygen serves as a mere spectator ligand, and the osmium center activates methane in analogy to Reaction 1, with M = OOs (55, 56). Finally, PtO+ reacts with a collision rate with a competition of Reactions 7 and 9 in favor of the latter because of the operation of relativistic stabilization of the PtCH2+ product (57). Furthermore, several high-valent, higher metal-oxide cations were found to activate methane in the gas phase under thermal conditions, i.e., CrO2+ (33, 58), HOFeO+ (59), MoO3+ (60), OsOn+ with n = 2, 4 (55), and PtO2+ (61). Also notable is the main-group metal-oxide cation MgO+, which efficiently activates methane with a strong preference for hydrogen-atom abstraction to afford MgOH+ according to Reaction 8 (62).
Metal-imido cations MNH+ and metal carbenes MCH2+ are formally isolobal to MO+ species; in general, however, the latter are found to be much more reactive. FeNH+, for example, can activate methane to afford Fe+ and methylamine (63), but the reaction is much less efficient than for FeO+. Bare metal-nitrido cations MN+, which could potentially activate methane, could so far not be generated experimentally in yields sufficient for subsequent reactivity studies.
Combinations of L and X.
With regard to bridging the gap to condensed-phase catalysts, more promising systems are perhaps those in which the metal species bear additional coordinative ligands and yet have preserved their intrinsic reactivity. Unfortunately, precisely the opposite behavior is often observed. For example, the reactive FeO+ cation, which oxidizes almost any substrate, including such inert compounds as acetonitrile or C6F6 (64), does not activate alkanes any more if attached to an arene ligand, e.g., (C6H6)FeO+ (65–67). These arene complexes of FeO+ react only with activated compounds such as olefins. A related, but conceptually different, example is represented by the cation (phen)CuO+ (phen = 1,10-phenanthroline), which is a formal Cu(III) compound (68). In this case, the bare CuO+ cation itself is too reactive to be generated in yields sufficient for gas-phase reactivity studies. The phen ligand can stabilize the Cu(III) center and (phen)CuO+ is one of the few ligated metal-oxide cations that is capable of activating small alkanes, although methane itself does not react with (phen)CuO+. The key step in the gas-phase synthesis of (phen)CuO+ is the homolytic decomposition of the nitrato ligand in the copper(II) precursor species (phen)Cu(NO3)+ according to Reaction 10.
A gas-phase activation of methane itself at room temperature has recently been achieved by related complexes of platinum(II) with aromatic nitrogen ligands (phen and bipy, where the latter stands for 2,2′-bipyridyl). These complexes of the type (H3C)Pt(L)+ (69) react with methane via degenerate σ-bond metathesis that is detected only by use of isotopic labeling, Reaction 11. Within the lifetime of the encounter complex [(H3C)Pt(L)(CD4)]+*, C–H(D) bond activation of methane can occur repeatedly, leading to a substantial amount of H/D scrambling in the overall reaction. The intermediate steps in Reaction 11 are partially reversible such that the evaluation of the respective kinetic parameters requires explicit kinetic modeling (70, 71) of the sequential steps that reveals moderate kinetic isotope effects for the C–H(D) bond activation of methane by this cationic platinum complex.
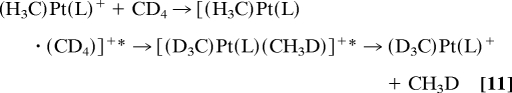 |
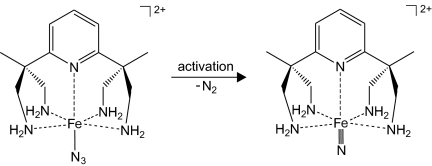
Similar to the generation of the reactive Cu(III) species (phen) CuO+, an attempt was made recently to generate a free metal–nitrido complex in the gas phase, where a polydendate cyclamacetato ligand was used for the stabilization of the FeN+ center containing formal iron(V). Analysis of the experimental findings suggests that the nitren [(cyclamacetato)FeN]+ was indeed generated initially upon decomposition of the azidocomplex [(cyclamacetato)Fe(N3)]+ used as the precursor, but before any subsequent bimolecular reactions, the intermediate nitrido complex promotes sacrificial self-oxidation of the ligand (72). Notably, the elusive [(cyclamacetato)FeN]+ cation could be trapped in the condensed phase via matrix-isolation techniques (73). Very recently, a FeN2+ unit with formal iron(V), stabilized by a polydendate aminoligand, could be generated in the gas phase in a similar manner (Scheme 2). The resulting species is able to transfer the nitrido-nitrogen to organic substrates such as dienes, yet it does not react with alkanes (74). Future work in this direction will show whether the design of other multiply ligated systems for the room-temperature activation of methane can be realized successfully.
Scheme 2.
In the context of multiply ligated metal species, we further note that with V4O10+ the first transition-metal oxide cluster was also found to activate methane via hydrogen-atom abstraction (75). Most recently, H-atom abstraction from methane has also been achieved with the cationic aluminum-oxide clusters Al2nO3n+ (n = 3–5) (76).
Intramolecular Kinetic Isotope Effects (KIEs) as a Mechanistic Probe for Methane Activation.
Finally, let us address the intramolecular KIEs associated with the C–H(D) bond activation of methane by cationic metal fragments in the gas phase; hence, KIE = kC–H/kC–D; investigation of CH2D2 is most suited for this purpose. Before a more detailed discussion of the results, it is important to point out that intramolecular KIEs provide direct mechanistic insight, whereas intermolecular KIEs (e.g., the rate constants for oxidation of CH4 and CD4, respectively) might be affected by a difference in collision rates, diffusion effects, etc. (53, 77, 78). This is particularly true for cases of two-state reactivity in which the cross-over between two spin surface can be the rate-determining step, such that the resulting intermolecular KIEs may be rather low, whereas intramolecular KIEs can still be significant. With the increased knowledge gathered in recent years, both experimentally and theoretically, some mechanistic guidelines for the interpretation of the primary, intramolecular KIEs associated with the C–H(D) bond activation of methane can be provided, however.
For the platinum species listed in the left side of Table 1, the KIEs are generally quite low, which is consistent with the view that for platinum not the activation step itself, but the release of the products is rate-determining (5, 27, 79). In contrast, a sizable KIE occurs in the case of the nickel(II) fluoride cation NiF+, which is consistent with the view that this reaction proceeds as a σ-bond metathesis in which C–H(D) bond cleavage and H(D)-F bond formation contribute to the rate-determining step (40). For the metal-oxide cations (right side of Table 1), the reaction of the cluster cation V4O10+ proceeds as a genuine H-atom abstraction (75) that is close to the KIE of ≈1.3 reported for H-atom abstraction by free OH radicals (80). H-atom abstraction from methane by MnO+ (51) and MgO+ (62) is also promoted by the radical character of the oxygen atom, although theory implies a significant participation of the metal and the formation of an insertion intermediate (62, 81), as is reflected by the significant KIEs. MoO3+ exhibits a KIE of the same magnitude, thus pointing to the operation of a similar mechanism (60). In the case of OFeOH+ (59) and FeO+ (52, 53), even larger KIEs are observed that, at least in the latter case, can be ascribed to the formation of an intermediate insertion species in conjunction with spin cross-over (81–85).
Table 1.
Intramolecular kinetic isotope effects associated with the room-temperature C–H(D) bond activation of CH2D2 by selected ligated metal cations in the gas phase
| Metal hydrides and halides | KIE | Metal oxides | KIE |
|---|---|---|---|
| PtBr+ + CH4 → PtCH3+ + HBr | 1.03 | V4O10+ + CH4 → V4O10H+ + CH3• | 1.35 |
| PtH+ + CH4 → PtCH3+ + H2 | 1.11 | MnO+ + CH4 → MnOH+ + CH3• | 1.8 |
| PtCl+ + CH4 → PtCH3+ + HCl | 1.16 | MgO+ + CH4 → MgOH+ + CH3• | 2.1 |
| PtBr+ + CH4 → BrPtCH2+ + H2 | 1.25 | MoO3+ + CH4 → MoO3H+ + CH3• | 2.3 |
| PtCl2+ + CH4 → ClPtCH3+ + HCl | 1.38 | OFeOH+ + CH4 → FeOH+ + CH3OH | 3.5 |
| NiF+ + CH4 → NiCH3+ + HF | 3.3 | FeO+ + CH4 → FeOH+ + CH3• | 4.6 |
In a more general perspective, the knowledge collected so far allows the consideration of the experimentally measured intramolecular KIEs associated with the activation of CH2D2 as a mechanistic probe for the activation of methane. Thus, KIEs <1.5 can be attributed either to reactions in which the initial C–H(D) bond cleavage does not contribute to the rate-determining step (i.e., the Pt species in Table 1) or to very exothermic reactions that proceed as simple H-atom abstraction (i.e., V4O10+). Medium KIEs (1.5–2.5) indicate the occurrence of a metal-assisted mechanism (i.e., MnO+, MgO+, and MoO3+), which is, however, still governed by the radical character of the attacking oxygen atom. In contrast, large KIEs (>2.5) are indicative of concerted reactions with the formation of a covalently bound insertion intermediate (i.e., FeO+) and/or occurrence of σ-bond metathesis (i.e., NiF+).
Conclusions
Recent research has demonstrated that gas-phase model studies of the methane activation by transition-metal cations are not limited to the bare, atomic cations, but also comprise several ligated metal ions. These research efforts provide valuable insight with respect to basic research about the interplay between theory and experiment. In this context, the development of new mechanistic paradigms, such as two-state reactivity (TSR), is a particular highlight, and TSR scenarios are meanwhile considered relevant in several areas of chemical and biological sciences far beyond the field of gas-phase ion chemistry (50, 86). Inclusion of ligated systems does not only extend beyond the world of idealized “naked” atom chemistry (87), they also serve as more realistic models for active metal species capable of activating methane in the condensed phase. In the case of platinum, for example, the studies of the platinum-halide cations described above relate the chemistry observed in the gas phase with that of the famous Shilov system (88) and more recently proposed condensed-phase variants for the activation of methane (89–92), all of which involve platinum in the oxidation states (+II) and (+IV).
Unlike the activation of higher alkanes, that of methane continues to constitute a challenge that can neither be “planned” or engineered in advance. Aspects like the preparation of the reactive reagents, the control over competing pathways for C–H bond activation or overoxidation of intermediates are only a few of the obstacles to be coped with. Continuous work on this topic is therefore not only of considerable importance with regard to future energy resources but also a fascinating field of chemistry in its own right.
Acknowledgments.
This work was supported by the Cluster of Excellence “Unifying Concepts in Catalysis” (Technischen Universität Berlin), Czech Academy of Sciences Grant Z40550506, Deutsche Forschungsgemeinschaft Grant SFB 658, Agency of the Academy of Sciences of the Czech Republic Grants KJB400550704 and 203/08/1487, and the Fonds der Chemischen Industrie.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Olah GA, Goeppert A, Prakash GKS. Beyond Oil and Gas: The Methanol Economy. Weinheim, Germany: Wiley–VCH; 2006. [Google Scholar]
- 2.Irikura KK, Beauchamp JL. Methane oligomerization in the gas phase by 3rd-row transition metal ions. J Am Chem Soc. 1991;113:2769–2770. [Google Scholar]
- 3.Irikura KK, Beauchamp JL. Electronic-structure considerations for methane activation by 3rd-row transition-metal ions. Phys Chem. 1991;95:8344–8351. [Google Scholar]
- 4.Wesendrup R, Schröder D, Schwarz H. Design and realization of a catalytic cycle for the Pt+ mediated oxidation of methane by molecular oxygen in the gas phase. Angew Chem Int Ed Engl. 1994;33:1174–1176. [Google Scholar]
- 5.Pavlov M, et al. Pt+-catalyzed oxidation of methane: Theory and experiment. J Phys Chem A. 1997;101:1567–1579. [Google Scholar]
- 6.Wesendrup R, Schwarz H. Tantalum-mediated coupling of methane and carbon dioxide in the gas phase. Angew Chem Int Ed Engl. 1995;34:2033–2035. [Google Scholar]
- 7.Sändig N, Koch W. A quantum chemical view on the mechanism of the Ta+-mediated coupling of carbon dioxide with methane. Organometallics. 1998;17:2344–2351. [Google Scholar]
- 8.Chiodo S. Activation of methane by the iron dimer cation. A theoretical study. J Phys Chem A. 2006;110:12501–12511. doi: 10.1021/jp064611a. [DOI] [PubMed] [Google Scholar]
- 9.Liu F, Zhang X-G, Armentrout PB. Activation of CH4 by gas-phase Ni+ and the thermochemistry of Ni-ligand complexes. Phys Chem Chem Phys. 2005;7:1054–1064. doi: 10.1039/b500639m. [DOI] [PubMed] [Google Scholar]
- 10.Armentrout PB, Sievers MR. Activation of CH4 by gas-phase Zr+ and the thermochemistry of Zr-ligand complexes. J Phys Chem A. 2003;107:4396–4406. doi: 10.1021/jp056804o. [DOI] [PubMed] [Google Scholar]
- 11.Parke LG, Hinton CS, Armentrout PB. Why is hafnium so unreactive? Experimental and theoretical studies of the reaction of Hf+ with methane. Int J Mass Spectrom. 2006;254:168–182. [Google Scholar]
- 12.Armentrout PB. Activation of CH4 by gas-phase Mo+, and the thermochemistry of Mo-ligand complexes. J Phys Chem A. 2006;110:8327–8338. doi: 10.1021/jp056804o. [DOI] [PubMed] [Google Scholar]
- 13.Parke LG, Hinton CS, Armentrout PB. Experimental and theoretical studies of the activation of methane by Ta. J Phys Chem C. 2007;111:17773–17787. [Google Scholar]
- 14.Armentrout PB, Shin S, Liyanage R. Guided-ion beam and theoretical study of the potential energy surface for activation of methane by W+ J Phys Chem A. 2006;110:1242–1260. doi: 10.1021/jp052732p. [DOI] [PubMed] [Google Scholar]
- 15.Armentrout MM, Li F-X, Armentrout PB. Is spin conserved in heavy metal systems? Experimental and theoretical studies of the reaction of Re+ with methane. J Phys Chem A. 2004;108:9660–9672. [Google Scholar]
- 16.Li F-X, Zhang X-G, Armentrout PB. The most reactive third-row transition metal: Guided ion beam and theoretical studies of the activation of methane by Ir+ Int J Mass Spectrom. 2006;255/256:279–300. [Google Scholar]
- 17.Li F-X, Armentrout PB. Activation of methane by gold cations: Guided ion beam and theoretical studies. J Chem Phys. 2006;125 doi: 10.1063/1.2220038. Art. No. 133114. [DOI] [PubMed] [Google Scholar]
- 18.Roth LM, Freiser BS. Gas-phase chemistry and photochemistry of doubly charged transition-metal containing ions. Mass Spectrom Rev. 1991;10:303–328. [Google Scholar]
- 19.Mourges P, Ferhati A, McMahon TB, Ohanessian G. Activation of hydrocarbons by W+ in the gas phase. Organometallics. 1997;16:210–224. [Google Scholar]
- 20.Ranasinghe YA, MacMahon TJ, Freiser BS. Formation of thermodynamically stable dications in the gas-phase by thermal ion molecule reactions—Ta2+ and Zr2+ with small alkanes. J. Phys Chem. 1991;95:7721–7726. [Google Scholar]
- 21.Liyanage R, Zhang XG, Armentrout PB. Activation of methane by size-selected iron cluster cations, Fen+ (n = 2–15): Cluster-CHx (x = 0–3) bond energies and reaction mechanisms. J Chem Phys. 2001;115:9747–9763. [Google Scholar]
- 22.Liu F, Zhang XG, Liyanage R, Armentrout PB. Methane activation by nickel cluster cations, Nin+ (n = 2–16): Reaction mechanisms and thermochemistry of cluster-CHx (x = 0–3) complexes. J Chem Phys. 2004;121:10976–10990. doi: 10.1063/1.1814095. [DOI] [PubMed] [Google Scholar]
- 23.Schröder D, Eller K, Prüsse T, Schwarz H. Ligand-enhanced selectivity in the CH/CC bond activation of ketones by iron(I) ions in the gas phase. Organometallics. 1991;10:2052–2057. [Google Scholar]
- 24.Schröder D, Schwarz H. Ligand effects as a probe for remote C–H bond activation by metal cations in the gas phase. J Organomet Chem. 1995;504:123–138. [Google Scholar]
- 25.Tjelta BL, Armentrout PB. Ligand effects in C–H and C–C bond activation by gas-phase transition metal-ligand complexes. J Am Chem Soc. 1996;118:9652–9660. [Google Scholar]
- 26.Albert G, et al. Methane activation by rhodium cluster argon complexes. Chem Phys Lett. 1997;268:235–244. [Google Scholar]
- 27.Achatz U, et al. The platinum hydride–methyl complex: A frozen reaction intermediate? J Phys Chem A. 1999;103:8200–8206. [Google Scholar]
- 28.Hertwig RH, et al. A comparative computational study of cationic coinage metal–ethylene complexes (C2H4)M+ (M = Cu, Ag, Au) J Phys Chem. 1996;100:12253–12260. [Google Scholar]
- 29.Schlangen M, Schröder D, Schwarz H. Pronounced ligand effects on the nickel-mediated thermal activation of methane. Angew Chem Int Ed. 2007;46:1641–1644. doi: 10.1002/anie.200603266. [DOI] [PubMed] [Google Scholar]
- 30.Schlangen M, Schwarz H, Schröder D. Specific processes and scrambling in the dehydrogenation of ethane and the degenerate hydrogen exchange in the gas-phase ion chemistry of the Ni(C,H3,O)+/C2H6 couple. Helv Chim Acta. 2007;90:847–853. [Google Scholar]
- 31.Schröder D, Fiedler A, Schwarz J, Schwarz H. Generation and stabilities of anionic, neutral, and cationic [Fe,O2] complexes. Inorg Chem. 1994;33:5094–5100. [Google Scholar]
- 32.Schröder D, Fiedler A, Herrmann WA, Schwarz H. Coordination of dioxygen in three representative transition-metal cations FeO2+, CrO2+, and CH3Re(O2)2O+ Angew Chem Int Ed Engl. 1995;34:2517–2520. [Google Scholar]
- 33.Fiedler A, Kretzschmar I, Schröder D, Schwarz H. Chromium dioxide cation CrO2+ in the gas phase: Structure, electronic states, and the reactivity with hydrogen and hydrocarbons. J Am Chem Soc. 1996;118:9941–9952. [Google Scholar]
- 34.Beyer MK, et al. Corroding the chromium cation. Mol Phys Chem. 2001;99:699–702. [Google Scholar]
- 35.Schröder D, Diefenbach M, Klapötke TM, Schwarz H. UF3+—A thermochemically stable diatomic trication with a covalent bond. Angew Chem Int Ed Engl. 1999;38:137–140. [Google Scholar]
- 36.Mandich ML, Steigerwald ML, Reents WD. The effects of chloro substitution on the electronic-structure of ClCr+, ClMn+, and ClFe+ and their reactivity with small alkanes. J Am Chem Soc. 1986;108:6197–6202. [Google Scholar]
- 37.Mazurek U, Schröder D, Schwarz H. Generation and reactivity of chromium fluoride cations (CrFn+, n = 0–4) in the gas phase. Coll Czech Chem Comm. 1998;63:1498–1512. [Google Scholar]
- 38.Roithová J, Schröder D. Bimolecular reactions of molecular dications: New reactivity paradigms and bond-forming processes. Phys Chem Chem Phys. 2007;9:2341–2349. doi: 10.1039/b617681j. [DOI] [PubMed] [Google Scholar]
- 39.Schröder D, Hrušák J, Schwarz H. Ligand effects on the reactivity of iron (II) cations FeX+ in the gas phase. Ber Bunsenges Phys Chem. 1993;97:1085–1090. [Google Scholar]
- 40.Schlangen M, Schwarz H, Schröder D. Ligand- and substrate effects in gas-phase reactions of NiX+/RH Couples (X = F, Cl, Br, I; R = CH3, C2H5, n-C3H7, n-C4H9) Chem Eur J. 2007;13:6810–6816. doi: 10.1002/chem.200700506. [DOI] [PubMed] [Google Scholar]
- 41.Schröder D, Schwarz H. Activation of methane by gaseous platinum(II) ions PtX+ (X = H, Cl, Br, CHO) Can J Chem. 2005;83:1936–1940. [Google Scholar]
- 42.Carlin TJ, Sallans L, Cassady CJ, Jacobson DB, Freiser BS. Gas-phase reactions of group-8 metal hydride ions (FeD+, CoD+, and NiD+) with hydrocarbons. J Am Chem Soc. 1983;105:6320–6321. [Google Scholar]
- 43.Halle LF, Klein FS, Beauchamp JL. Properties and reactions of organometallic fragments in the gas phase—Ion-beam studies of FeH+ J Am Chem Soc. 1984;106:2543–2549. 1984. [Google Scholar]
- 44.Zhang Q, Bowers MT. Activation of methane by MH+ (M = Fe, Co, and Ni): A combined mass spectrometric and DFT study. J Phys Chem A. 2004;108:9755–9761. [Google Scholar]
- 45.Schlangen M, Schwarz H. Thermal activation of methane by group 10 metal hydrides MH+: The same and not the same. Angew Chem Int Ed Engl. 2007;46:5614–5617. doi: 10.1002/anie.200605145. [DOI] [PubMed] [Google Scholar]
- 46.Schröder D, Schwarz H. C–H and C–C bond activation by ionic transition-metal oxides in the gas phase. Angew Chem Int Ed Engl. 1995;34:1973–1995. [Google Scholar]
- 47.Schröder D, Shaik S, Schwarz H. Characterization, orbital description, and reactivity patterns of transition-metal oxo species in the gas phase. Struct Bond. 2000;97:91–123. [Google Scholar]
- 48.Schröder D, Schwarz H. Intrinsic mechanisms of oxidation reactions as revealed by gas-phase experiments. Top Organomet Chem. 2007;22:1–15. [Google Scholar]
- 49.Schröder D, Shaik S, Schwarz H. Intrinsic mechanisms of oxidation reactions as revealed by gas-phase experiments. Acc Chem Res. 2000;33:139–145. doi: 10.1021/ar990028j. [DOI] [PubMed] [Google Scholar]
- 50.Shaik S, Hirao H, Kumar D. Reactivity of high-valent iron-oxo species in enzymes and synthetic reagents: A tale of many states. Acc Chem Res. 2007;40:532–542. doi: 10.1021/ar600042c. [DOI] [PubMed] [Google Scholar]
- 51.Ryan MF, Fiedler A, Schröder D, Schwarz H. Radical-like behavior of manganese oxide cation in its gas-phase reactions with dihydrogen and alkanes. J Am Chem Soc. 1995;117:2033–2040. [Google Scholar]
- 52.Schröder D, Schwarz H. FeO+ activates methane. Angew Chem Int Ed Engl. 1990;29:1433–1434. [Google Scholar]
- 53.Schröder D, et al. Activation of hydrogen and methane by thermalized FeO+ in the gas phase as studied by multiple mass-spectrometric techniques. Int J Mass Spectrom Ion Processes. 1997;161:175–191. [Google Scholar]
- 54.Schröder D, Fiedler A, Ryan MF, Schwarz H. Surprisingly low reactivity of bare FeO+ in its spin-allowed, highly exothermic reaction with molecular hydrogen to generate Fe+ and water. J Phys Chem. 1994;98:68–70. [Google Scholar]
- 55.Irikura KK, Beauchamp JL. Osmium-tetroxide and its fragment ions in the gas phase—Reactivity with hydrocarbons and small molecules. J Am Chem Soc. 1989;111:75–85. [Google Scholar]
- 56.Zhang GB, Li SH, Jiang YS. Density functional study on the mechanisms of the reactions of gas-phase OsOn+ (n = 1–4) with methane. Organometallics. 2004;23:3656–3667. [Google Scholar]
- 57.Schwarz H. Relativistic effects in gas-phase ion chemistry: An experimentalist's view. Angew Chem Int Ed Engl. 2003;42:4442–4454. doi: 10.1002/anie.200300572. [DOI] [PubMed] [Google Scholar]
- 58.Rivalta I, Russo N, Sicilia E. Methane activation by chromium oxide cations in the gas phase: A theoretical study. J Comp Chem. 2006;27:174–187. doi: 10.1002/jcc.20335. [DOI] [PubMed] [Google Scholar]
- 59.Schröder D, Schwarz H. Oxidations of alkanes by [Fe(O)OH]+ in the gas phase—the role of iron oxidation state in C–H activations. Angew Chem Int Ed Engl. 1991;30:991–993. [Google Scholar]
- 60.Kretzschmar I, Fiedler A, Harvey JN, Schröder D, Schwarz H. Effects of sequential ligation of molybdenum cation by chalcogenides on electronic structures and gas-phase reactivity. J Phys Chem A. 1997;101:6252–6264. [Google Scholar]
- 61.Brönstrup M, Schröder D, Kretzschmar I, Schwarz H, Harvey JN. Platinum dioxide cation: Easy to generate experimentally but difficult to describe theoretically. J Am Chem Soc. 2001;123:142–147. doi: 10.1021/ja003138q. [DOI] [PubMed] [Google Scholar]
- 62.Schröder D, Roithová J. Thermal activation of methane by MgO+ cations: It also works without a transition metal! Angew Chem Int Ed. 2006;45:5705–5708. doi: 10.1002/anie.200601273. [DOI] [PubMed] [Google Scholar]
- 63.Brönstrup M, Kretzschmar I, Schröder D, Schwarz H. Iron-mediated amination of hydrocarbons in the gas phase. Helv Chim Acta. 1998;81:2348–2369. [Google Scholar]
- 64.Schröder D, Hrušák J, Schwarz H. Generation of bare FeF+ by C–F activation in the gas phase and evaluation of thermochemical data. Helv Chim Acta. 1992;75:2215–2218. [Google Scholar]
- 65.Schröder D, Schwarz H. Benzene oxidation by “bare” FeO+ in the gas phase. Helv Chim Acta. 1992;75:1281–1286. [Google Scholar]
- 66.Becker H, Schröder D, Zummack W, Schwarz H. Generation, fragmentation and interconversion processes of [Fe,C6,H6,O]+ isomers relevant for the oxygenation of aromatic hydrocarbons. J Am Chem Soc. 1994;116:1096–1100. [Google Scholar]
- 67.Stöckigt D, Schwarz H. Catalytic gas-phase oxidation of olefins mediated by Fe(C6H6)+ and a comparison of Fe(L)+ complexes (L = benzene, pyridine, naphthalene) Liebigs Ann. 1995:429–431. [Google Scholar]
- 68.Schröder D, Holthausen MC, Schwarz H. Radical-like activation of alkanes by the ligated copper-oxide cation (phen)CuO+ J Phys Chem B. 2004;108:14407–14416. [Google Scholar]
- 69.Butschke B, Schlangen M, Schwarz H, Schröder D. C–H bond activation of methane with gaseous [(CH3)Pt(L)]+ complexes (L = pyridine, bipyridine, and phenanthroline) Z Naturf B. 2007;62b:309–313. [Google Scholar]
- 70.Schröder D, Schwarz H. Fe+-catalyzed gas-phase oxidation of ethane by N2O. Angew Chem Int Ed Engl. 1990;29:1431–1433. 1990. [Google Scholar]
- 71.Schröder D, Brown R, Schwerdtfeger P, Schwarz H. Kinetics of radiative/termolecular associations in the low pressure regime: Reactions of bare Au+ with benzene. Int J Mass Spectrom. 2000;203:155–163. [Google Scholar]
- 72.Schröder D, Schwarz H, Aliaga-Alcalde N, Neese F. Fragmentation of the (cyclam-acetato) iron-azide cation in the gas phase. Eur J Inorg Chem. 2007;6:816–821. [Google Scholar]
- 73.Aliaga-Alcalde N, et al. The geometric and electronic structure of [(cyclam-acetato)Fe(N)+]: A genuine iron(V) species with a ground-state spin S = 1/2. Angew Chem Int Ed. 2005;44:2908–2912. doi: 10.1002/anie.200462368. [DOI] [PubMed] [Google Scholar]
-
74.Schlangen M, et al. Gas phase C–H and N–H bond activation by a high valent nitrido-iron dication and
 NH
NH -transfer to activated olefins. J Am Chem Soc. 2008;130:4285–4294. doi: 10.1021/ja075617w. [DOI] [PubMed] [Google Scholar]
-transfer to activated olefins. J Am Chem Soc. 2008;130:4285–4294. doi: 10.1021/ja075617w. [DOI] [PubMed] [Google Scholar] - 75.Feyel S, Döbler J, Schröder D, Sauer J, Schwarz H. Thermal methane activation by tetranuclear V4O10+: From a “Holy Grail” to an achievable goal? Angew Chem Int Ed. 2006;45:4681–4685. doi: 10.1002/anie.200600188. [DOI] [PubMed] [Google Scholar]
- 76.Feyel S, et al. Activation of methane by oligomeric (Al2O3)x+ (x = 3, 4, 5): The role of oxygen-centered radicals in thermal hydrogen-atom abstraction. Angew Chem Int Ed. 2008;47:1946–1950. doi: 10.1002/anie.200704791. [DOI] [PubMed] [Google Scholar]
- 77.Schröder D, et al. Equilibrium isotope effects in cationic transition-metal(I) ethene complexes M(C2X4)+ with M = Cu, Ag, Au and X = H, D. Organometallics. 2000;19:2608–2615. [Google Scholar]
- 78.Derrick PJ. Isotope Effects in Fragmentation. Mass Spectrom Rev. 1985;2:285–298. [Google Scholar]
- 79.Heinemann C, Wesendrup R, Schwarz H. Pt+-mediated activation of methane—Theory and experiment. Chem Phys Lett. 1995;239:75–83. 1995. [Google Scholar]
- 80.Saueressig G, et al. Carbon 13 and D kinetic isotope effects in the reactions of CH4 with O(D-1) and OH: New laboratory measurements and their implications for the isotopic composition of stratospheric methane. J Geophys Res Atmos. 2001;106:23127–23138. [Google Scholar]
- 81.Shiota Y, Yoshizawa K. Methane-to-methanol conversion by first-row transition-metal oxide ions: ScO+, TiO+, VO+, CrO+, MnO+, FeO+, CoO+, NiO+, and CuO+ J Am Chem Soc. 2000;122:12317–12326. [Google Scholar]
- 82.Schröder D, Fiedler A, Hrušák J, Schwarz H. Experimental and theoretical studies towards a characterization of conceivable intermediates involved in the gas-phase oxid methane by bare FeO+. Generation of four distinguishable [Fe,C,H4,O]+ isomers. J Am Chem Soc. 1992;114:1215–1222. [Google Scholar]
- 83.Aguirre F, Husband J, Thompson CJ, Stringer KL, Metz RB. Electronic spectroscopy of intermediates involved in the conversion of methane to methanol by FeO+ J Chem Phys. 2002;116:4071–4078. [Google Scholar]
- 84.Shiota Y, Yoshizawa K. A spin-orbit coupling study on the spin inversion processes in the direct methane-to-methanol conversion by FeO+ J Chem Phys. 2003;118:5872–5879. [Google Scholar]
- 85.Schwarz H. On the spin-forbiddeness of gas-phase ion–molecule reactions: A fruitful intersection of experimental and computational studies. Int J Mass Spectrom. 2004;237:75–105. [Google Scholar]
- 86.Harvey JN, Poli R, Smith KM. Understanding the reactivity of transition metal complexes involving multiple spin states. Coord Chem Rev. 2003;238:347–361. [Google Scholar]
- 87.Böhme DK, Schwarz H. Gas-phase catalysis by atomic and cluster metal ions: The ultimate single-site catalysts. Angew Chem Int Ed. 2005;44:2336–2354. doi: 10.1002/anie.200461698. [DOI] [PubMed] [Google Scholar]
- 88.Shilov AE. Activation of Saturated Hydrocarbons by Transition Metal Complexes. Dordrecht, The Netherlands: Reidel; 1984. [Google Scholar]
- 89.Periana RA, et al. Platinum catalysts for the high-yield oxidation of methane to a methanol derivative. Science. 1998;280:560–564. doi: 10.1126/science.280.5363.560. [DOI] [PubMed] [Google Scholar]
- 90.Labinger JA, Bercaw JE. Understanding and exploiting C–H bond activation. Nature. 2002;417:507–514. doi: 10.1038/417507a. [DOI] [PubMed] [Google Scholar]
- 91.Labinger JA. Selective alkane oxidation: Hot and cold approaches to a hot problem. J Mol Catal. 2004;220:27–35. [Google Scholar]
- 92.Lersch M, Tilset M. Mechanistic aspects of C–H activation by Pt complexes. Chem Rev. 2005;105:2471–2526. doi: 10.1021/cr030710y. [DOI] [PubMed] [Google Scholar]