Abstract
Impairments in adiponectin multimerization lead to defects in adiponectin secretion and function and are associated with diabetes, yet the underlying mechanisms remain largely unknown. We have identified an adiponectin-interacting protein, previously named GST-kappa, by yeast 2-hybrid screening. The adiponectin-interacting protein contains 2 thioredoxin domains and has very little sequence similarity to other GST isoforms. However, this protein shares high sequence and secondary structure homology to bacterial disulfide-bond A oxidoreductase (DsbA) and is thus renamed DsbA-like protein (DsbA-L). DsbA-L is highly expressed in adipose tissue, and its expression level is negatively correlated with obesity in mice and humans. DsbA-L expression in 3T3-L1 adipocytes is stimulated by the insulin sensitizer rosiglitazone and inhibited by the inflammatory cytokine TNFα. Overexpression of DsbA-L promoted adiponectin multimerization while suppressing DsbA-L expression by RNAi markedly and selectively reduced adiponectin levels and secretion in 3T3-L1 adipocytes. Our results identify DsbA-L as a key regulator for adiponectin biosynthesis and uncover a potential new target for developing therapeutic drugs for the treatment of insulin resistance and its associated metabolic disorders.
Keywords: obesity, yeast 2-hybrid system, adipose tissue, insulin resistance
Adiponectin is an adipocyte-derived hormone that plays an important role in the regulation of lipid and glucose metabolism. Adiponectin stimulates fatty acid oxidation, suppresses hepatic gluconeogenesis, increases insulin sensitivity, and acts to counter the effects of the inflammatory cytokine TNFα. Adiponectin has also been shown to have antiatherogenic effects and to act on the central nervous system to stimulate energy expenditure. Thus, adiponectin is a strong candidate for the development of drugs to treat obesity, insulin resistance, type 2 diabetes, and atherosclerosis (for review, see refs. 1–3).
Adiponectin circulating in serum exists primarily in 3 main species: a low-molecular-mass (LMM) trimer of ≈67 kDa, a hexamer of ≈140 kDa, and a high-molecular-mass (HMM) multimer of >300 kDa (4–6). The interaction between the collagenous domains results in formation of highly ordered trimer, which is further stabilized by an intratrimer disulfide bond mediated by Cys39 (or Cys22, if the N-terminal 17-aa secretory peptide is excluded). The formation of a disulfide bond between 2 trimers mediated by the free Cys39 in each leads to the formation of the hexameric form of adiponectin, serving as the building block for the HMM form, which consists of 12–18 hexamers existing in a bouquet-like structure (7). Adiponectin mutants with impaired multimerization are defective in both secretion and function and are associated with diabetes and hypoadiponectinemia (4, 6). More importantly, it has been shown that adiponectin oligomer distribution, rather than its absolute levels, correlates with thiazolidiedione-mediated increase in insulin sensitivity (8).
A number of studies have shown that adiponectin multimers are stable and do not interconvert from 1 species to another once secreted into the serum (4, 5), indicating that adiponectin multimerization is tightly regulated inside adipocytes. Several factors have been found to affect adiponectin gene expression and secretion. For example, TNFα and IL-6 have been shown to down-regulate adiponectin gene expression and secretion (9). The plasma HMM form of adiponectin has also been shown to be suppressed by insulin and high levels of glucose (4). Conversely, the HMM adiponectin level is up-regulated by moderate weight reduction (10) or by treatment with thiazolidinediones (TZDs), which stimulate peroxisome proliferator activated receptor gamma (PPAR gamma) activity (8, 11). However, the molecules that mediate these metabolic or pharmacological effects on adiponectin disulfide bond formation and multimerization remain unknown. Because multimer complex distribution plays a major role in adiponectin's insulin sensitizing function (8), understanding the mechanisms regulating adiponectin multimerization will provide important information on the development of therapeutic interventions to treat obesity and type 2 diabetes.
Results
Identification of GST-Kappa/Disulfide-Bond A Oxidoreductase-Like Protein (DsbA-L) as an Adiponectin Interactive Protein.
To identify proteins that interact with adiponectin, we screened a yeast 2-hybrid cDNA library derived from human fetal brain, using the full length mouse adiponectin without the N-terminal signal peptide sequence (amino acids 17–247) as bait. We identified 11 independent clones, including the cDNA encoding the C terminus of AdipoR1 (12) and a cDNA encoding the C terminus of a protein previously named GST-kappa (amino acid 188–216) (Fig. 1A). GST-kappa is a 25-kDa protein initially classified as a class theta GST (GST13–13) (13). Analysis of the complete amino acid sequence of the protein reveals that GST-kappa has very little sequence similarity to any other class of GST (14) but shares high sequence and secondary structure homology to Escherichia coli DsbA (15). Crystal structure studies also revealed that GST-kappa has the same general folding as the DsbA (16). Like other eukaryotic protein disulfide isomerases (PDIs), GST-kappa contains 2 thioredoxin-like domains (Fig. 1A). However, the GST-kappa dimer has a very different overall shape when compared with the canonical GST fold (16). For these reasons, GST-kappa has been acknowledged to be misnamed in the protein sequence database and not a member of the GST gene family (15). Based on these findings and our results showing that GST-kappa plays a key role in regulating adiponectin multimerization (see below), we renamed GST-kappa to DsbA-L.
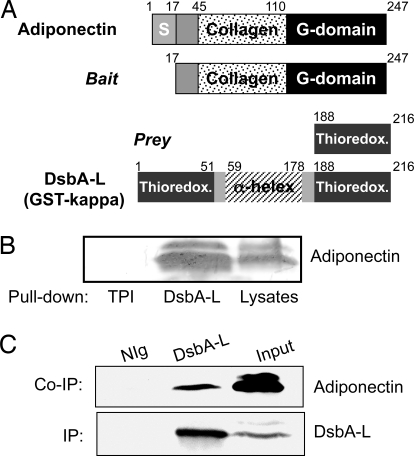
Fig. 1.
Identification of GST-kappa/DsbA-L as an adiponectin interacting protein. (A) Yeast 2-hybrid screening to identify DsbA-L as an adiponectin interactive protein. The signal sequence (S), collagen and globular (G) domains of adiponectin and the thioredoxin domain and alpha-helix region of DsbA-L are indicated. (B) DsbA-L interacts with adiponectin in vitro. 3T3-L1 adipocyte lysates were incubated with the His-tagged control protein triosephosphate isomerase (TPI) or His-tagged DsbA-L bound to Ni-NTA-agarose beads. The bound adiponectin was detected with an anti-adiponectin antibody. (C) Interaction of adiponectin with DsbA-L in 3T3-L1 adipocytes. Endogenous DsbA-L was immunoprecipitated with a homemade antibody to the protein. Coimmunoprecipitated adiponectin was detected by Western blot with an antiadiponectin antibody. Data are representatives of 3 experiments with similar results.
To test whether DsbA-L interacts directly with adiponectin, we expressed DsbA-L in bacterial cells and carried out in vitro binding studies. We found that endogenous adiponectin interacted with His-tagged DsbA-L but not with the nonrelevant control protein (Fig. 1B). Coimmunoprecipitation experiments revealed that endogenous adiponectin interacted with endogenous DsbA-L in 3T3-L1 adipocytes (Fig. 1C).
Metabolic and Pharmacological Regulation of DsbA-L Expression.
DsbA-L transcript has been shown to be ubiquitously expressed in various human (17) and mouse (18) tissues. However, whether this protein is expressed in adipose tissue has not been investigated. Because adiponectin is expressed mainly in adipocytes, we examined the expression of DsbA-L in this tissue. We found that DsbA-L is expressed in a number of mouse tissues such as liver, kidney, pancreas, heart, and lung (Fig. 2A). Interestingly, the highest DsbA-L expression was observed in white adipose tissue (WAT) (Fig. 2A), where adiponectin is synthesized and secreted. We also found that DsbA-L is highly expressed in brown adipose tissue [supporting information (SI) Fig. S1]. This finding is interesting, because adiponectin has been detected in brown adipose tissue and has been implicated to play an important role in glucose and lipid metabolism during the perinatal period (19). Consistent with previous findings (20), we found that adiponectin expression was greatly induced during 3T3-L1 cell differentiation (Fig. 2B Top). 3T3-L1 cell adipogenesis also led to a marked increase in the expression levels of DsbA-L (Fig. 2B Middle). The findings that DsbA-L is highly expressed in adipose tissue and its expression is induced during 3T3-L1 cell differentiation suggest a role of this protein in adipocyte function.
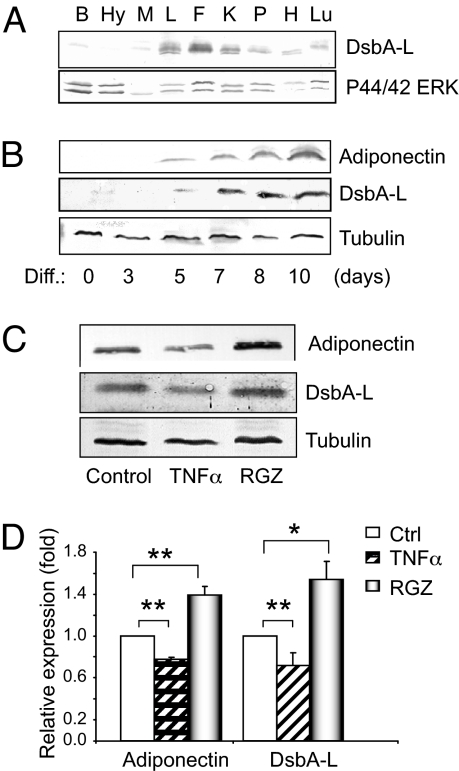
Fig. 2.
Tissue distribution and regulation of DsbA-L protein expression. (A) Tissues from 4-month-old male C57BL/6J mice were homogenized and the expression of DsbA-L and ERK1/2 (as a loading control) was determined by Western blot by using indicated antibodies. B, brain; Hy, hypothalamus; M, skeletal muscle, L, liver; F, fat; K, kidney; P, pancreas; H, heart; Lu, lung. (B) DsbA-L expression is increased during 3T3-L1 cell differentiation. The expression levels of several adipokines and tubulin at different days during 3T3-L1 differentiation was determined by Western blot by using specific antibodies as indicated. (C) DsbA-L expression in 3T3-L1 adipocytes is inhibited by TNFα and stimulated by rosiglitazone (RGZ). Lysates from 3T3-L1 adipocytes treated with 10 μM RGZ or 10 ng/ml TNFα for 24 h were analyzed by Western blot. (D) Quantification of adiponectin and DsbA-L levels from cells treated with TNFα or RGZ. Data are present as means ± SEM from 3 independent experiments. Comparison between groups was made by 1-way ANOVA. **, P < 0.01; *, P < 0.05.
Adiponectin levels are stimulated by PPAR gamma agonists thiazolidinediones. However, adiponectin levels negatively correlate with TNFα release by adipose tissue (21). To obtain further evidence on the potential role of DsbA-L in regulating adiponectin function, we investigated whether DsbA-L expression levels are regulated by these reagents. We found that the expression levels of both adiponectin and DsbA-L were stimulated by rosiglitazone (RGZ) in 3T3-L1 adipocytes. However, TNFα suppressed adiponectin and DsbA-L expression (Fig. 2 C and D).
Overexpression of DsbA-L Promotes Adiponectin Multimerization and Increases Its Cellular Levels.
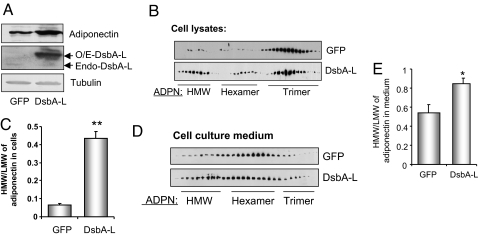
To determine the potential role of DsbA-L, we transiently expressed DsbA-L in differentiated 3T3-L1 CAR cells by adenovirus-mediated infection. Differentiated 3T3-L1 CAR cells stably express the truncated coxsackievirus and adenovirus receptor (CAR) and are ≈100-fold more efficient for adenovirus infection compared with 3T3-L1 adipocytes (22). At differentiation day 9, >90% of the 3T3-L1 CAR cells were differentiated into adipocyte as determined by Oil Red O staining (Fig. S2A Top). In addition, high efficiency of adenovirus-mediated infection (≈80%) was achieved as demonstrated by GFP expression (Fig. S2A Bottom). Overexpressing DsbA-L in 3T3-L1 CAR adipocytes increased the cellular protein but not the mRNA levels of adiponectin (Figs. 3A and S2B). Gel filtration experiments revealed that overexpressing DsbA-L markedly increased the ratio of HMM form to LMM (total hexamer and trimer) of adiponectin in both the cell lysates and the cell culture medium of DsbA-L-overexpressing cells (Fig. 3 B–E).
Fig. 3.
Overexpression of DsbA-L increases cellular adiponectin levels and expression. 3T3- L1 CAR adipocytes were infected with adenoviruses encoding GFP or GFP plus DsbA-L. Thirty-six hours after infection, cells were lysed and proteins in cell lysates were separated by SDS/PAGE and determined by Western blot by using specific antibodies as indicated (A). Adiponectin in cell lysates (B) or cell culture medium (D) were resolved by gel-filtration chromatography and determined by Western blot by using an antibody to adiponectin. Data are representatives of 3 independent experiments with similar results. Quantification of the relative abundance of adiponectin oligomers in cell lysates (C) and culture medium (E) was performed by analyzing Western blots by using the National Institutes of Health Scion Image software. The data were shown as changes in the ratio of HMM/LMM of adiponectin. **, P < 0.01; *, P < 0.05.
Suppressing DsbA-L Expression by RNAi Selectively Inhibits Adiponectin Biosynthesis Levels and Secretion.
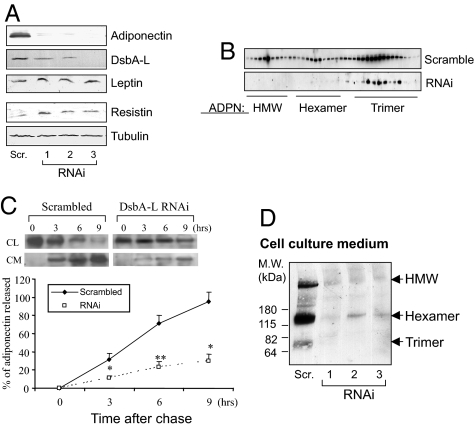
To determine the functional role of endogenous DsbA-L in adiponectin biosynthesis and secretion, we established ST3-L1 cell lines harboring either a scrambled control plasmid or the DsbA-L shRNA plasmid. We selected several independent clones of the DsbA-L shRNA cell lines by limited dilution. Oil red O staining revealed that introducing the DsbA-L shRNA plasmid did not affect 3T3-L1 cell differentiation (Fig. S2C). Suppressing DsbA-L expression led to a dramatic reduction in the cellular protein but not the mRNA levels of adiponectin and had little effect on the expression levels of other adipokines such as leptin and resistin (Figs. 4A and S2D). Gel filtration analysis of adiponectin oligomers revealed that the ratio of HMM to LMM of adiponectin in cell lysates is significantly reduced in the DsbA-L-suppressed 3T3-L1 adipocytes (Fig. 4B). Pulse–chase experiments revealed that suppressing DsbA-L expression greatly inhibited adiponectin secretion (Fig. 4C). In addition, the levels of adiponectin oligomers in the cell culture media of the DsbA-L-suppressed 3T3-L1 adipocyte cell clones were markedly reduced compared with that in the scramble 3T3-L1 adipocytes (Fig. 4D). Suppressing DsbA-L expression also greatly inhibited rosiglitazone-stimulated adiponectin secretion (Fig. S3 A and B) but had little effect on the cellular levels of adiponectin and secretion of several adipokines including resistin, plasminogen activator inhibitor-1 (PAI-1), and lipocalin-2, an inflammatory marker abundantly expressed in adipose tissue (23) (Table S1). These results indicate that DsbA-L plays a selective role in regulating adipokine biosynthesis and secretion.
Fig. 4.
Suppression of DsbA-L expression by RNAi reduces the protein levels of adiponectin in 3T3-L1 adipocytes. (A) Western blot analysis of adiponectin and DsbA-L expression in the scramble and 3 independent shRNA-containing 3T3-L1 cell lines at differentiation day 8. Data are representatives of 3 independent experiments with similar results. (B) Adiponectin oligomers were separated by FPLC system by using a Superdex 200 column and analyzed by Western blot with an antibody to adiponectin. Because of very low expression levels of the adiponectin multimers in the DsbA-L-suppressed cells, a longer exposure time was used to visualize the gel filtration profile of the adiponectin oligomers in the DsbA-L-suppressed cells. (C) The effect of DsbA-L suppression on adiponectin secretion as determined by pulse–chase experiments. At different time intervals after chase, scramble and DsbA-L-shRNA cells were lysed. Cell lysates and culture media were collected and subjected to immunoprecipitation using rabbit anti-mouse adiponectin IgG. The precipitated immunocomplexes were separated by 15% SDS/PAGE and visualized by phosphoimaging (Upper). The graphs (Lower) represent the percentage of adiponectin released into the culture media at different time points after chase. P < 0.05 (*) and P < 0.01 (**). (D) Suppression of DsbA-L reduced the HMM and hexamer forms of adiponectin in the cell culture medium. Culture media of scramble and DsbA-L-suppressed 3T3-L1 adipocytes at differentiation day 8 were collected and proteins in the media were analyzed by nonreducing gel electrophoresis. Data are representative of 3 independent experiments with similar results.
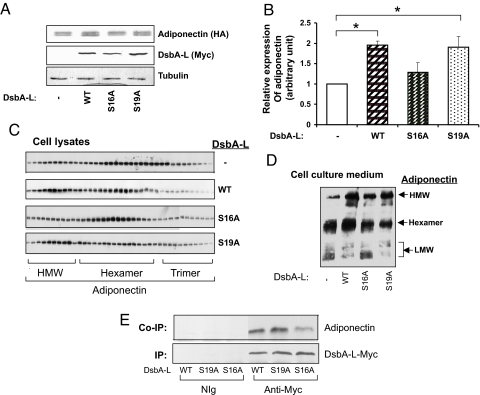
Ser16 in DsbA-L Plays a Critical Role in Regulating Adiponectin Multimerization.
DsbA-L does not have the presumed active site (Cys-Gly-His-Cys) involved in disulfide bond formation, but instead contains a conserved sequence in which the 2 cysteine residues are replaced with serines (16Ser-Pro-Tyr-19Ser), which is also found in the testis-specific protein disulfide isomerase-like protein (PDILT) (24) and bacterial HCCA (2-hydroxychromene-2-carboxylate) isomerase, an enzyme involved in bacterial metabolism of naphthalene to salicylate (25) (Fig. S4A). Interestingly, the Ser-Pro-Tyr-Ser motif and the surrounding of DsbA-L sequences are extremely conserved in all species ranging from Caenorhabditis elegans to humans (Fig. S4B), suggesting an important functional role for this region. To determine the role of Ser16 and Ser19 in DsbA-L function, we transiently expressed myc-tagged DsbA-L and HA or Flag-tagged adiponectin in CHO/IR cells. Similar to the results obtained in 3T3-L1 adipocytes, overexpressing wild-type DsbA-L notably increased the protein levels of adiponectin (Fig. 5 A and B) and the ratio of HMM to LMM of intracellular adiponectin (Fig. 5C) and adiponectin in the cell culture medium (Fig. 5D). The ability of DsbA-L to increase the relative ratio of adiponectin HMM to LMM or hexamer to trimer was diminished when Ser16 but not Ser19 of DsbA-L was replaced with alanine (Fig. 5 C and D). These findings suggest that the first serine residue in the SXXS motif plays a major role in DsbA-L activity. Coimmunoprecipitation experiments showed that mutation at Ser16, but not Ser19, markedly inhibited DsbA-L binding to adiponectin (Fig. 5E). In addition, overexpression of DsbA-L neither enhanced the cellular levels of the C39S mutant of adiponectin, which is defective in forming disulfide bond (Fig. S4C) nor had any effect on the mutant adiponectin multimerization (Fig. S4D). This indicates that disulfide bond formation and multimerization is essential for DsbA-L-mediated increase in cellular levels of adiponectin.
Fig. 5.
Ser16 of DsbA-L plays a critical role in regulating adiponectin stability and multimerization. A mammalian expression vector encoding HA-tagged adiponectin was transfected into CHO/IR cells together with a plasmid encoding myc-tagged wild-type, S16A, or S19A mutant of DsbA-L. (A) The expression of these proteins in cell lysates was determined by Western blot by using antibodies as indicated. (B) Semiquantitation of adiponectin levels by analyzing the Western blot data from 3 independent experiments as described in A by using the National Institutes of Health IMAGE program. All data were expressed as means ± SEM. **, P < 0.01; *, P < 0.05. (C) Adiponectin oligomers in cell lysates were separated by gel filtration and determined by Western blot by using an anti-adiponectin antibody. (D) Adiponectin oligomers in cell culture medium were separated by nonreducing gel electrophoresis and determined by Western blot with an antiadiponectin antibody. Data are representatives of 3 independent experiments with similar results. (E) HA-adiponectin and myc-tagged wild-type or mutants of DsbA-L were transiently expressed in CHO/IR cells. HA-tagged adiponectin coimmunoprecipitated with myc-tagged DsbA-L was detected by Western blot with an antibody to the HA-tag.
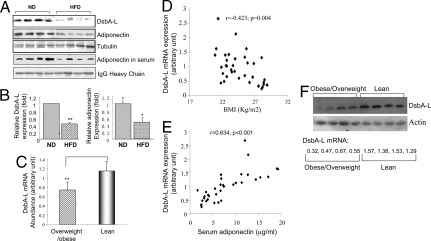
Expression Level of DsbA-L in Adipose Tissue Negatively Correlates with Obesity in Mice and Human Subjects.
Adiponectin expression is reduced in both human obesity and mouse models of genetic obesity (26). To determine whether obesity affects DsbA-L expression, we examined the protein levels of adiponectin and DsbA-L in the epididymal WAT of normal diet (ND)-fed or high-fat-diet (HFD)-fed C57BL/6 mice (Table S4). We found that HFD feeding significantly reduced the expression levels of both adiponectin and DsbA-L (Fig. 6 A and B). To confirm the clinical relevance of the above findings, we examined the expression levels of DsbA-L in the s.c. fat of 23 lean and 12 obese human subjects (Table S2). Real-time PCR experiments revealed that the mRNA levels of DsbA-L in overweight/obese subjects were significantly lower than that in lean controls (P < 0.01, Fig. 6C). There is significant inverse correlation between the adipose tissue DsbA-L mRNA levels and body mass index (BMI) (Fig. 6D). Furthermore, there is a strong positive correlation between DsbA-L mRNA levels in s.c. fat and serum levels of adiponectin in these individuals (Fig. 6E). We also examined the protein expression levels of DsbA-L in the s.c. fat of 4 randomly selected lean and 4 obese/overweight human subjects by Western blot analysis. Consistent with reduced DsbA-L mRNA levels, this preliminary study showed that protein levels of DsbA-L are also greatly reduced in fat tissues of overweight/obese human subjects compared with lean human subjects (Fig. 6F).
Fig. 6.
DsbA-L expression levels are reduced in obese mice and humans. (A) Twenty micrograms of proteins isolated from adipose tissues of ND- or HFD-fed male C57BL/6 mice were separated by SDS/PAGE and detected by Western blot with indicated antibodies. (B) Quantitation of the relative levels of DsbA-L or adiponectin expression in adipose tissues from normal chow diet and HFD-fed mice. The fold change was determined by analyzing Western blots by using the National Institutes of Health IMAGE program and was normalized for the amount of tubulin expression. (C) Comparison of DsbA-L mRNA expression in the s.c. fat between overweight/obese (BMI >23 kg/m2, n = 12) and lean individuals (BMI <23 kg/m2, n = 23) (Table S2). Correlation between DsbA-L mRNA expression and BMI (D) or serum levels of adiponectin E in 35 human subjects. (F) The protein expression of DsbA-L in adipose tissues of lean (BMI 19 ± 2; n = 4) and overweight/obese (BMI 29.5 ± 2.5; n = 4) human subjects (Table S3) were examined by Western blot with an antibody to DsbA-L. Equal amounts of proteins (40 μg) were loaded per lane. The relative mRNA levels of DsbA-L in each individual are shown.
Discussion
Data accumulated over the past several years provide strong evidence that defects in the regulation of adiponectin multimerization and secretion play important roles in obesity, insulin resistance, and type 2 diabetes. Several proteins have recently been shown to regulate adiponectin trafficking and secretion (27–29). However, the mechanisms regulating adiponectin multimerization and secretion remain largely unknown.
In the present study, we present evidence showing that DsbA-L plays a critical and selective role in regulating adiponectin biosynthesis. Consistent with previous findings, we found that the secretion and expression levels of adiponectin were stimulated by rosiglitazone and inhibited by TNFα; the latter has been shown to reduce insulin sensitivity in cells (30, 31). Furthermore, our present study suggests that DsbA-L plays a role in mediating the stimulatory effect of rosiglitazone on adiponectin biosynthesis and secretion. One possible mechanism by which suppressing DsbA-L affects rosiglitazone-stimulated adiponectin secretion is that reducing the expression levels of DsbA-L inhibits adiponectin multimerization, which is critical for secretion. Consistent with this view, adiponectin mutants unable to form HMM multimers have been shown to be defective in secretion from cells (6).
In addition to impaired secretion, suppressing DsbA-L cellular levels by RNAi also greatly reduced the cellular levels of adiponectin. Interestingly, suppressing DsbA-L had no significant effect on the mRNA levels of adiponectin (Fig. S2D), suggesting that reduced biosynthesis and/or accelerated degradation may play a major role in reducing cellular levels of adiponectin. One possibility is that a deficiency in DsbA-L expression increases the levels of unfolded or misfolded adiponectin, leading to unfolded protein response (UPR). UPR has been shown to attenuate general translation and up-regulate ER-associated protein degradation via activation of the PKR-like eukaryotic initiation factor 2α kinase (PERK), the activating transcription factor-6 (ATF6), and the inositol requiring enzyme-1 (IRE-1) signaling pathways. UPR has also been shown to increase disposal of misfolded proteins via the ubiquitin proteasome-dependent ER-associated degradation (ERAD) pathway (32, 33). Suppression of DsbA-L expression in cells may thus reduce adiponectin levels by activating the UPR-stimulated pathways. We are currently investigating this possibility.
How DsbA-L regulates adiponectin multimerization remains to be elucidated. One possibility is that DsbA-L functions as a PDI to regulate adiponectin disulfide bond formation, which is essential for multimerization. First, DsbA-L shares high sequence and secondary structure homology to bacterial DsbA (15, 16), which catalyze disulfide bond formation during the folding of secreted proteins in bacteria (34). Like other eukaryotic PDIs, DsbA-L contains 2 thioredoxin-like domains (Fig. 1). Interestingly, DsbA-L contains a SXXS sequence (Ser16 and Ser19), which is localized in place of the classic “CXXC” redox active motif found in DsbA and TcpG, a DsbA homolog in the pathogenic bacterium Vibrio cholerae responsible for the folding, maturation, and secretion of virulence factors (35) (Fig. S4A). It becomes evident that not all PDIs contains the classic contain classical CXXS motifs. For example, the testis-specific protein disulfide isomerase-like protein (PDILT) contains an SXXS and an SXXC motif (24). The 2 monocysteinic thioredoxins in Arabidopsis thaliana contain the CXXS motif and are very efficient as disulfide isomerases (36). Ser16 and Ser19 of DsbA-L are extremely conserved among all species examined (Fig. S4B), suggesting an important function for these residues. Consistent with this, X-ray crystal structure studies revealed that DsbA-L has the same general fold as DsbA and promotes formation of the thiolate E·GS− through interaction of the hydroxyl group of Ser16 with the sulfur of reduced glutathione (GSH) (16). Furthermore, a mutant human PDI-related protein (hPDIr) with all of its active-site cysteines mutated to serine retained 57% of the oxidative refolding activity of wild-type enzyme in the reactivation of α1-antitrypsin (37). In agreement with these findings, we found that replacing Ser16 with alanine disrupted the ability of DsbA-L in promoting adiponectin trimer to higher order multimers in cells (Fig. 5). Alternatively, DsbA-L may play an indirect role in facilitating adiponectin disulfide bond formation. Consistent with this view, we found that incubation with DsbA-L alone was insufficient to promote adiponectin multimerization in vitro (data not shown), suggesting that additional factors may be necessary for adiponectin multimerization in intact cells. It has been shown that ≈29% of adenovirus-mediated overexpressed adiponectin are in HMM form in the liver, suggesting that hepatocytes express the intracellular machinery necessary for producing the HMM species (38). Our results showed that DsbA-L is highly expressed in the liver (Fig. 2A), which is consistent with a role of DsbA-L in regulating adiponectin multimerization. These findings suggest the regulation of adiponectin multimerization is cell type-specific, probably because of the presence or absence of specific cofactors. Further studies will be needed to test these possibilities.
It is well established that different forms of the adiponectin multimers, whose cellular levels vary in response to changes in metabolic and disease states, have distinct functions in metabolism (21, 39). In fact, the ratio of HMM forms of adiponectin to the total adiponectin level, rather than just total adiponectin level itself, has been shown to correlate more significantly with key features of central obesity and insulin resistance status (8). Our study identifies DsbA-L as a critical regulator of adiponectin multimerization and uncovers a potential therapeutic target for the diagnosis and treatment of insulin resistance and its associated diseases.
Materials and Methods
Cell Culture, Transfection, in Vitro Binding, Immunoprecipitation, and Western Blot.
The maintenance and/or differentiation of CHO/IR and 3T3-L1 cells were performed as described in our previous studies (40). Cell transfection was performed by using Lipofectamine Plus (Invitrogen). Cell lysates and tissue homogenates were prepared in ice-cold lysis buffer, and the general procedures used for coimmunoprecipitation, in vitro binding and Western blot have been described (41). Additional information is provided in the SI Methods.
Yeast 2-Hybrid cDNA Library Screening.
The adiponectin bait plasmid was constructed by subcloning a cDNA fragment encoding mouse adiponectin without the signal peptide (amino acids 13–244) into the pLexA 2-hybrid vector (Clontech). Screening of a yeast 2-hybrid cDNA library derived from human fetal brain was performed as described (12).
RNA Interference and Generation of DsbA-L-Suppressed Cells.
The sense and anti-sense sequences of siRNA were chemically synthesized and ligated into the pSIREN-RetroQ (BDKnowckout RNAi system; BD Biosciences). The sequence for siRNA and scrambled control are 5′-GCATGGAGCAACCAGAGAT-3′ and 5′-GCCGTAGCACTGGAAGAAA-3′, respectively. 3T3-L1 stable cell lines containing the DsbA-L siRNA construct or a scrambled control were generated by transfection of cells with the respected plasmids and selection with 3 μg/ml of puromycin as described (42). Independent clones were selected by limited dilution.
Adenovirus Generation and Adenoviral Infection.
Adenoviruses encoding GFP and mouse DsbA-L were generated by using the pAdEasy system as described (40). On differentiation day 9, 3T3-L1 CAR adipocytes were infected with adenoviruses at an multiplicity of infection of 30. The efficiency of adenovirus infection was assessed by fluorescent GFP expression with a fluorescence microscope 24 h after infection. To measure adiponectin in cell culture medium by Western blot, cells were incubated in serum-free DMEM containing 0.05% BSA for 24 h and the conditioned media were collected and concentrated with iCON concentrators (Pierce).
Size Exclusion Chromatography.
A Superdex 200 10/30 column (GE Healthcare Bio-Sciences) was equilibrated with buffer containing 50 mM phosphate buffer, pH 7.0, 150 mM NaCl, and 2 mM KCl and calibrated with standards: thyroglobulin, 669 kDa; ferritin, 440 kDa; catalase, 232 kDa; aldolase, 158 kDa; and ovalbumin, 44 kDa. Cell lysates were clarified by centrifugation at 12,000 × g for 15 min at 4 °C. The clarified supernatant (≈0.5 mL) was applied to the column and eluted with the same buffer at a flow rate of 0.5 mL/min by FPLC. Fractions (0.1 mL of for cell lysates and 0.2 mL of for concentrated cell culture media) were collected and separated by SDS/PAGE, and the position of adiponectin in the elution profile was determined by Western blot by using an antiadiponectin or anti-HA tag antibody.
Pulse–Chase Experiments.
Scramble or DsbA-L-suppressed 3T3-L1 cells were induced for differentiation for 8 days, starved in methionine- and cysteine-free DMEM for 1 h, and then replaced with the same fresh medium plus 50 μCi/ml of [35S]methionine and cysteine (Redivue Pro-mix L-[35S], GE Healthcare) for 2 h. The labeling medium was then replaced with cold DMEM plus a 20-fold excess of methionine and cysteine for different time period. Both cell culture medium and cells were harvested for immunoprecipitation as we described (43).
DsbA-L Expression in Human Subjects.
Thirty-five healthy premenopausal Chinese women (23 lean and 12 overweight/obese individuals, age: 43 ± 7 years) undergoing abdominal surgery for benign gynecological conditions, such as uterine fibroids or ovarian cysts, at the Department of Obstetrics and Gynecology, University of Hong Kong, Queen Mary Hospital, were recruited. All subjects were given informed consent, and the protocol was approved by the Ethics Committee of the University of Hong Kong. Preoperative serum, BMI, waist circumference, waist–hip ratio, blood pressure, fat percentage, and glucose levels at 30-min intervals for 2 h during a 75 gram Oral Glucose Tolerance Test (OGTT) were determined. Serum adiponectin levels were measured as described (43, 44) (Table S2). During the operation, abdominal s.c. adipose tissues (≈2 cc each) were collected, snap-frozen, and stored at −70 °C before RNA extractions using the Qiagen mini-RNA purification kits. One microgram of total RNA from each sample was reverse-transcribed, and the relative mRNA abundance of DsbA-L was quantified by quantitative real-time PCR using the fluorescent TaqMan 5′-nuclease assay on an Applied Biosystems Prism 7000 sequence detection system. The TaqMan real-time PCR was performed by using 2′ TaqMan Master Mix and 20× assay-on-demand TaqMan primers and probes (Assay ID: CF02642588-81, Applied Biosystems). Analysis was performed with ABI Prism 7000 SDS Software.
Data Analysis.
Statistical analysis of DsbA-L and adipokine mRNA levels in human was performed with the SPSS 11.5 statistical software package (SPSS). The data for mRNA, protein of human or mouse WAT and clinical characteristics were analyzed by using an unpaired t test, unless otherwise indicated. Data are presented as means ± SEM, and P value of <0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments.
We thank Dr. Wenhong Cao (The Hamner Institute for Health Science, Research) for providing WAT from ND- or HFD-fed mice. This work was supported by grants from the National Institutes of Health (DK76902, to F.L.; and DK69930, to L.Q.D.) and Hong Kong Research Grant Council (CRF 2/07C, to A.X.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 18077.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806341105/DCSupplemental.
References
- 1.Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74–80. doi: 10.1016/j.febslet.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 2.Bouskila M, Pajvani UB, Scherer PE. Adiponectin: A relevant player in PPARgamma-agonist-mediated improvements in hepatic insulin sensitivity? Int J Obes (Lond) 2005;29(Suppl 1):S17–S23. doi: 10.1038/sj.ijo.0802908. [DOI] [PubMed] [Google Scholar]
- 3.Ahima RS, Qi Y, Singhal NS. Adipokines that link obesity and diabetes to the hypothalamus. Prog Brain Res. 2006;153:155–174. doi: 10.1016/S0079-6123(06)53009-2. [DOI] [PubMed] [Google Scholar]
- 4.Pajvani UB, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 5.Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J Biol Chem. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- 6.Waki H, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, et al. Post-translational modifications of the 4 conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. 2006;281:16391–16400. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- 8.Pajvani UB, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 9.Fasshauer M, et al. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3–L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 10.Bobbert T, et al. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54:2712–2719. doi: 10.2337/diabetes.54.9.2712. [DOI] [PubMed] [Google Scholar]
- 11.Combs TP, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: A potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 12.Mao X, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 13.Harris JM, Meyer DJ, Coles B, Ketterer B. A novel glutathione transferase (13-13) isolated from the matrix of rat liver mitochondria having structural similarity to class theta enzymes. Biochem J. 1991;278:137–141. doi: 10.1042/bj2780137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pemble SE, Wardle AF, Taylor JB. Glutathione S-transferase class Kappa: Characterization by the cloning of rat mitochondrial GST and identification of a human homologue. Biochem J. 1996;319:749–754. doi: 10.1042/bj3190749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nebert DW, Vasiliou V. Analysis of the glutathione S-transferase (GST) gene family. Hum Genomics. 2004;1:460–464. doi: 10.1186/1479-7364-1-6-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladner JE, Parsons JF, Rife CL, Gilliland GL, Armstrong RN. Parallel evolutionary pathways for glutathione transferases: Structure and mechanism of the mitochondrial class kappa enzyme rGSTK1-1. Biochemistry. 2004;43:352–361. doi: 10.1021/bi035832z. [DOI] [PubMed] [Google Scholar]
- 17.Morel F, et al. Gene and protein characterization of the human glutathione S-transferase kappa and evidence for a peroxisomal localization. J Biol Chem. 2004;279:16246–16253. doi: 10.1074/jbc.M313357200. [DOI] [PubMed] [Google Scholar]
- 18.Jowsey IR, Thomson RE, Orton TC, Elcombe CR, Hayes JD. Biochemical and genetic characterization of a murine class Kappa glutathione S-transferase. Biochem J. 2003;373:559–569. doi: 10.1042/BJ20030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimoto N, et al. Adiponectin is expressed in the brown adipose tissue and surrounding immature tissues in mouse embryos. Biochim Biophys Acta. 2005;1731:1–12. doi: 10.1016/j.bbaexp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 21.Kadowaki T, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross SA, Song X, Burney MW, Kasai Y, Orlicky DJ. Efficient adenovirus transduction of 3T3–L1 adipocytes stably expressing coxsackie-adenovirus receptor. Biochem Biophys Res Commun. 2003;302:354–358. doi: 10.1016/s0006-291x(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007;53:34–41. doi: 10.1373/clinchem.2006.075614. [DOI] [PubMed] [Google Scholar]
- 24.van Lith M, Hartigan N, Hatch J, Benham AM. PDILT, a divergent testis-specific protein disulfide isomerase with a nonclassical SXXC motif that engages in disulfide-dependent interactions in the endoplasmic reticulum. J Biol Chem. 2005;280:1376–1383. doi: 10.1074/jbc.M408651200. [DOI] [PubMed] [Google Scholar]
- 25.Denome SA, Stanley DC, Olson ES, Young KD. Metabolism of dibenzothiophene and naphthalene in Pseudomonas strains: Complete DNA sequence of an upper naphthalene catabolic pathway. J Bacteriol. 1993;175:6890–6901. doi: 10.1128/jb.175.21.6890-6901.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 27.Qiang L, Wang H, Farmer SR. Adiponectin Secretion is Regulated by SIRT1 and the ER oxidoreductase Ero1-L{alpha} Mol Cell Biol. 2007;27:4698–4707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang ZV, et al. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie L, et al. Intracellular trafficking and secretion of adiponectin is dependent on GGA-coated vesicles. J Biol Chem. 2006;281:7253–7259. doi: 10.1074/jbc.M511313200. [DOI] [PubMed] [Google Scholar]
- 30.Rangwala SM, Lazar MA. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol Sci. 2004;25:331–336. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447–455. doi: 10.1016/s1359-6101(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 32.Gregor MF, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: The endoplasmic reticulum and metabolic disease. J Lipid Res. 2007;48:1905–1914. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 34.Kadokura H, Katzen F, Beckwith J. Protein disulfide bond formation in prokaryotes. Annu Rev Biochem. 2003;72:111–135. doi: 10.1146/annurev.biochem.72.121801.161459. [DOI] [PubMed] [Google Scholar]
- 35.Hu SH, Peek JA, Rattigan E, Taylor RK, Martin JL. Structure of TcpG, the DsbA protein folding catalyst from Vibrio cholerae. J Mol Biol. 1997;268:137–146. doi: 10.1006/jmbi.1997.0940. [DOI] [PubMed] [Google Scholar]
- 36.Serrato AJ, Guilleminot J, Meyer Y, Vignols F. AtCXXS: Atypical members of the Arabidopsis thaliana thioredoxin h family with a remarkably high disulfide isomerase activity. Physiol Plant. 2008;133:611–622. doi: 10.1111/j.1399-3054.2008.01093.x. [DOI] [PubMed] [Google Scholar]
- 37.Horibe T, et al. Different contributions of the 3 CXXC motifs of human protein-disulfide isomerase-related protein to isomerase activity and oxidative refolding. J Biol Chem. 2004;279:4604–4611. doi: 10.1074/jbc.M310922200. [DOI] [PubMed] [Google Scholar]
- 38.Satoh H, et al. Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes. 2005;54:1304–1313. doi: 10.2337/diabetes.54.5.1304. [DOI] [PubMed] [Google Scholar]
- 39.Scherer PE. Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 40.Wick KR, et al. Grb10 inhibits insulin-stimulated IRS/PI 3-Kinase/Akt Signaling pathway by disrupting the association of IRS-1/IRS-2 with the insulin receptor. J Biol Chem. 2003;278:8460–8467. doi: 10.1074/jbc.M208518200. [DOI] [PubMed] [Google Scholar]
- 41.Dong LQ, et al. Phosphorylation of PKN by PDK1 mediates insulin signals to the actin cytoskeleton. Proc Natl Acad Sci USA. 2000;97:5089–5094. doi: 10.1073/pnas.090491897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langlais P, et al. Negative regulation of insulin-stimulated MAP kinase signaling by Grb10. Mol Endocrinol. 2004;18:350–358. doi: 10.1210/me.2003-0117. [DOI] [PubMed] [Google Scholar]
- 43.Xu A, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–18080. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 44.Xu A, Yin S, Wong L, Chan KW, Lam KS. Adiponectin ameliorates dyslipidemia induced by the human immunodeficiency virus protease inhibitor ritonavir in mice. Endocrinology. 2004;145:487–494. doi: 10.1210/en.2003-1140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.