Abstract
Paroxysmal nocturnal hemoglobinuria is an acquired hematopoietic stem cell (HSC) disorder characterized by the partial or complete deficiency of glycosyl-phosphatidylinositol (GPI)-linked membrane proteins, which leads to intravascular hemolysis. A loss of function mutation in the PIG-A gene, required for GPI biosynthesis, explains how the deficiency of many membrane proteins can result from a single genetic event. However, to date the mechanism of expansion of the GPI− clone has not been fully understood. Two hypotheses have been proposed: A selective advantage of GPI− cells because of a second mutation or a conditional growth advantage of GPI− cells in the presence of an immune attack on normal (GPI+) HSCs. Here, we explore a third possibility, whereby the PNH clone does not have a selective advantage. Simulations in a large virtual population accurately reproduce the known incidence of the disease; and the fit is optimized when the number of stem cells is decreased, reflecting a component of bone marrow failure in PNH. The model also accounts for the occurrence of spontaneous cure in PNH, consequent on clonal extinction. Thus, a clonal advantage may not be always necessary to explain clonal expansion in PNH.
Keywords: mathematical modeling, stem cells, stochastic dynamics, hematopoiesis, mutation
Paroxysmal nocturnal hemoglobinuria (PNH) has been a source of fascination for hematologists for many years ever since it was described by Marchiafava in the 20th century. It is a rare, acquired hematopoietic stem cell (HSC) disorder that can occur at any age and across all human populations (1). It has an estimated prevalence of 1–10 per million population (2). The most characteristic clinical feature is the episodic passage of dark urine because of intravascular hemolysis that gives the disorder its colorful name (2). However, the disease is neither paroxysmal nor restricted to the night, as hemolysis is continuous.
In PNH, intravascular red blood cell destruction is mediated by complement attack, because the cells belonging to the clone express low levels (or completely lack expression) of membrane proteins that protect them from complement mediated lysis (2). The two most important such proteins are: CD55 (decay accelerating factor) and CD59 (membrane inhibitor of reactive lysis) (3). These proteins are attached to the plasma membrane via a glycosyl-phosphatidylinositol lipid anchor (GPI) that is added to the proteins as a posttranslational modification. Glycosyl-phosphatidylinositol biosynthesis involves many steps, an early one of which is catalyzed by N-acetylglucosamine transferase. A subunit of this enzyme is encoded by the PIG-A gene (phosphatidyl inositol glycan-class A gene) (2, 4). Because PIG-A maps to the X chromosome, a single mutation in this gene can eliminate or reduce GPI biosynthesis, leading to deficient expression of the GPI-linked proteins on the cell surface.
Whereas the identification of mutations in the PIG-A gene has provided a direct explanation of the PNH phenotype in red cells and in other blood cells, it does not by itself justify why and how a PIG-A mutant clone expands. To explain this essential component of the pathogenesis of PNH, two models—that are not mutually exclusive—have been put forward. (i) PNH clones expand by virtue of negative selection against normal HSCs (5). (ii) A second mutation (i.e., other than that of PIG-A) confers to a PNH clone a selective advantage. Both models require a relative selective advantage of the PNH clone compared to normal HSCs. The evidence favoring (i) has been reviewed (6). In favor of (ii), a recent paper by Inoue et al. (7) has demonstrated rearrangements involving the HMGA2 gene in two patients with PNH.
In this paper we explore, by mathematical modeling, a sort of null-hypothesis, or limit-case, whereby the PNH clone has no selective advantage. We find, based on the current notion of the stochastic contribution of individual SCs to human hematopoiesis (8–11), that clinical PNH can arise even without a PNH clone having a selective advantage.
The Model.
The active stem cell pool, of size NSC, is responsible for maintenance of blood cell formation, and cells in this compartment replicate at a rate of approximately 1/year (9, 12–14). Recent studies suggest that SCs selected for replication continue to contribute toward hematopoiesis for a very long time—in fact, for most of the lifetime of an individual (15, 16) because there is no clear evidence for clonal succession in the human HSC pool (17). However, the requirements for hematopoiesis are not constant during the lifetime of an individual. We have recently determined how the size of the active SC pool changes during human ontogenic growth. Indeed, at birth humans typically require an active pool comprising approximately 20 SCs, whereas a normal adult has an active SC pool of approximately 400 SCs (18). The path that joins these two extreme values (10) follows the classical ontogenic growth curve of humans (19) with the characteristic spurt during the teenage years. Taking this growth into account, we simulate the evolutionary dynamics of the active SC compartment by means of a stochastic birth-export process, in which SCs are selected for replication based on their relative fitness. Because we are assuming neutral evolution, this means that one cell is selected with uniform probability from the active SC pool. Once selected, that cell replicates to generate two daughter cells. Subsequently, one cell from the pool is selected at random for export (it can be viewed as moving down the path of differentiation) in the sense that such a cell cannot be selected again for replication within the HSC pool. This process follows standard Moran dynamics (20) and contrasts with models where HSCs always divide asymmetrically, so that one daughter cell remains as a HSC and the other follows the path of differentiation. However, such models are incompatible with PNH and other HSC-derived disorders, because the mutant cell cannot evolve into a clone. During replication any SC can acquire a mutation in the PIG-A gene at a rate of 5 × 10−7 per division, which is similar to that of normal SCs (21). The probability that a mutant cell will appear, given the constant mutation rate, increases exponentially in time. Once occurring, such a PIG-A mutated cell may have different fates because of the intrinsically stochastic nature of the process (11), as illustrated in Fig. 1. Repeated iteration of the selection-replication-export cycle maps the life-history of the active SC compartment of an individual. When this process is repeated for a large population of individuals (≈109), we can determine the distribution of PIG-A mutated stem cell events as a function of the age of an individual and the number of mutated stem cells. Combining this distribution with the population age distribution of the United States available from the National Bureau of the Census allows us to predict the overall incidence of PNH in the U.S.A. We define a patient as having clinical PNH if the fraction of SCs that belong to the clone is greater than or equal to 20% [individuals with a PIG-A mutated clonal population of <10% do not have clinically significant hemolysis (1)]. Those individuals whose clone is <20% are defined as latent PNH [also called subclinical (1)]. In an adult we have NSC ≈ 400 and each cell replicates once per year under normal conditions (9, 12–14, 18). Thus, when each cell has replicated once, this means that 1 year has passed in the lifetime of an individual.
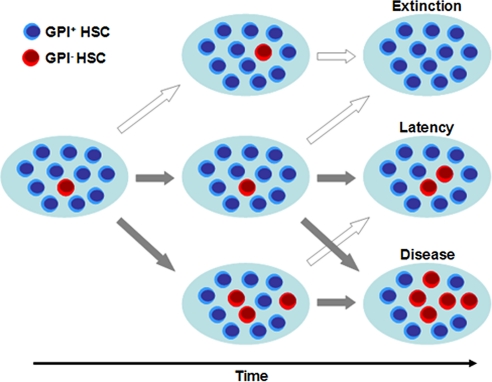
Fig. 1.
Model of stochastic dynamics in the active hematopoietic SC pool (NSC). SCs divide at a rate approximately 1/year and acquire a mutation in the PIG-A gene at a rate of 5 × 10−7 per replication. Cells are selected for replication and export at random. The dynamics of the mutant population (GPI− HSCs, red cells) is followed forward in time. Several scenarios become possible because of the stochastic nature of the problem associated with the neutrality of the PIG-A mutation: (Top) Stochastic extinction of the clone, which may be appreciable given the small size of the active HSC pool. (Middle) Latency, in which the PNH burden in the HSC pool is <20%, a situation which can remain as such for several years (see Fig. 4). (Bottom) Disease, in which case the PNH burden in the HSC pool is >20%. The cross-arrows point out to the fact that the situation of a single patient may change from these different stages during their life history, where dark arrows mean the patient condition worsens, whereas light arrows point to changes where patient condition improves.
Simulations of stem cell dynamics in a hypoplastic bone marrow were carried out assuming that NSC = 100 and NSC = 60. These numbers of SCs, can maintain normal hematopoiesis, because adults with a stem cell pool composed of ≈100 SCs after bone marrow transplantation have normal blood counts (22), and a normal bone marrow can compensate for hemolysis if the average red cell lifespan is reduced to as little as 20 days: To do this, they need to replicate at a rate 4–6 times faster than normal (9).
Finally, our definition of resolution of the disorder required that the size of the clone is <3%, as this constitutes the lower threshold for detection by standard flow cytometry (1). The presence of a single SC with a mutation in PIG-A was used to estimate the prevalence of the mutation in the population.
Results
PIG-A Mutations in the Healthy Population.
The mutation rate in PNH cells is not different from that of normal cells (5 × 10−7/replication) (21, 23). As described in the model, we calculated the incidence of a single mutation in the active SC pool (NSC) taking into account the expansion of the SC pool throughout growth and development, from birth to adulthood. We estimate that, at any time, 400 individuals per million (of any age) in the U.S.A. have at least 1 SC with a mutation in PIG-A.
Mutations in the PIG-A gene were identified in 19 of 19 adult volunteer blood donors, suggesting that the frequency of PIG-A mutation is much higher than our present estimate (24). However, these PIG-A cannot all arise in the most primitive active HSCs for the following reason: If the average of 22:106 polymorphonuclear cells in normal adults with a mutation in PIG-A (24) originated from mutated SCs, then the active SC pool must have a minimum of 45,000 cells because at least 1 cell must be mutated. This is incompatible with the currently accepted size of the total HSC pool in humans, let alone the active SC compartment (9, 25). Moreover, SCs divide at a rate of 1/year (9, 12–14); therefore, a PIG-A mutant clone originating from a SC would not disappear in less than 174 days as reported by Araten et al. (24). These considerations suggest that most of the PIG-A mutations seen in healthy adults occur in common myeloid progenitor cells that have an average life time of 125 days (26, 27). Experimental results reported by Hu et al., have foreshadowed the validity of this conclusion (28). On the other hand, PNH as a clinical entity can only occur if the PIG-A mutation takes place in a cell within the active SC pool that in adults comprises approximately 400 cells (9, 18). Thus, a PIG-A mutation in a SC should be found on average in 0.25% of circulating neutrophils, that is, when tested, in 400 individuals per million.
Prevalence of Clinical PNH.
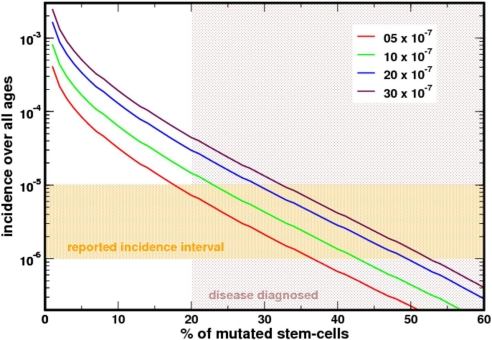
In an attempt to estimate the prevalence of PNH, we followed the stochastic evolution of the active SC pool in a population of 109 virtual people who each lived for 100 years. Individuals in whom the PIG-A mutant clone appeared and expanded to occupy more than 20% of NSC were defined as having clinical PNH. The population distribution function of this large theoretical population was matched with the age distribution structure of the United States based on the census for the year 2000. As can be seen from Fig. 2, the prevalence of the disease is ≈8/million population in excellent agreement with prior estimates (2). The average age at diagnosis of PNH in this virtual population was 58 years, with a range from 29–100 years: Most patients in previously reported series (29, 30) are within this range.
Fig. 2.
Prevalence of PNH based on the US population census. The reported prevalence is 1–10/million population (orange shaded area). In our model, diagnosis requires that ≥20% of the active SCs have a mutation in PIG-A. In an adult this will be ≈80 SCs (and less in a growing child). Our estimate falls well within the expected prevalence of the disease. Different curves were obtained for different mutation rates. The average mutation rate (red curve) is compatible with the prevalence of the disease in the U.S.
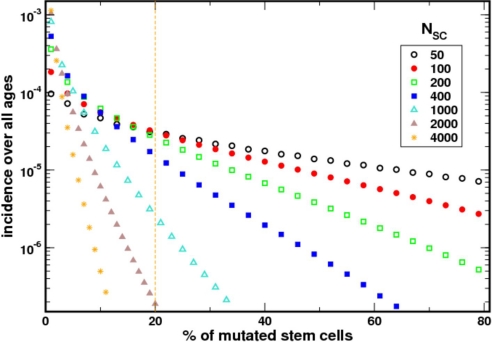
We evaluated the impact of the size of NSC on the incidence of PNH. Apart from the allometric estimate of 400 HSCs each replicating on average once per year, there are at present no other estimates for the size of the active HSC pool and replication rates. In our model, although keeping the replication rate at once per year, we varied NSC. As expected, the predicted incidence of the disease decreases as NSC gets larger (Fig. 3). The intuition for this result is as follows: (i) the time required for the appearance of a PIG-A mutated HSC decreases as NSC increases, but (ii) quantitatively, the mutant clone has to expand significantly more to reach the minimum threshold of 20% necessary for clinically relevant PNH. The time required for expansion of the mutant clone grows faster than the time for the appearance of the first mutation. As a result, the incidence of the disease falls as the population of active HSCs increases. However, even for NSC = 1,000, the predicted incidence is still within the observed range (2).
Fig. 3.
The incidence of the disease depends on the number of HSCs that are actively contributing to hematopoiesis. The incidence predicted by the model matches the epidemiological data optimally when NSC ≈ 400. As the size of the HSCs increases, the incidence of the disease decreases. In these simulations, the rate of HSC replication was kept constant at ≈1/year because there is no experimental data relating how the rate of replication of HSCs changes as their population increases.
Mutant Clone Size and Evolution.
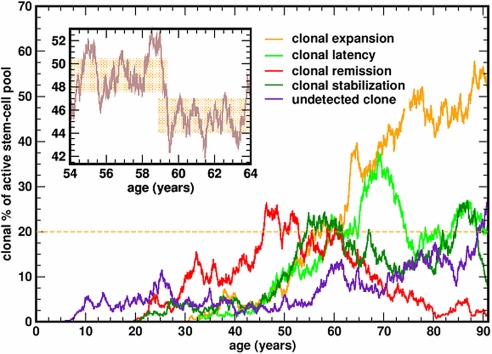
The average size of the mutant clone in our virtual cohort was 29.25% with a median of 27.8% of the cells in NSC (range 20–99%). However, individual life histories vary and the size of the clone can increase to become the dominant contributor to hematopoiesis (>50%); it may also remain fairly stable for several years or it may undergo extinction (Fig. 4). Despite stochastic fluctuations, the clone may appear to be fairly constant in size for many years [as reported by (30)] given the 3% limit on the resolution of flow cytometry as can be seen from the inset in Fig. 3. We also evaluated how clone size varies as a function of the mutation rate, which may be as high as 3 × 10−6/ replication (21, 23). Interestingly, in our model the median clone size does not change appreciably as we varied the mutation rate from 5 × 10−7 to 3 × 10−6 per replication.
Fig. 4.
Representative life histories of the PIG-A mutant clone. Once the mutation appears in a HSC, the mutated SC may stochastically expand to become the dominant contributor to hematopoiesis (and PNH will be diagnosed), undergo stochastic extinction, or “stabilize” for many years. Given the limited resolution of standard flow cytometry, the size of the clone may appear “stable” for years (Inset).
PNH with Multiple Clones.
Some patients with PNH have more than one clone with distinct mutations in the PIG-A gene (1, 31–33). These mutations result in cells with partial (PNH type II) or complete (PNH type III) deficiency of GPI-linked proteins. We tracked the incidence of different mutations in our virtual cohort of patients and recorded all individuals who had at least 2 concomitant clones that together represented more than 20% of the age-adjusted NSC. According to our simulations, only 0.4% of patients with PNH are expected to have ≥2 distinct clones that arise at the level of the HSC. This is compatible with what has been reported elsewhere based on a combination of simulations and analytic approximations (34). In many cases, clones additional to the dominant PNH clone may be explained by independent mutations in PIG-A within progenitor cells as discussed above and supported by experimental observations (28, 35, 36); however, we acknowledge that in more than 0.4% of PNH patients there is more than 1 clone.
Stochastic Clonal Extinction.
One of the most fascinating characteristics of PNH is that, rarely, patients may have resolution of the disorder despite the fact that they have they received only supportive treatment. We studied the expected frequency of this eventuality by using two possible criteria: (i) the clone disappears completely (no PIG-A mutant SCs present) and (ii) the size of the clone is <3% when it is not detected by standard flow cytometry. Criterion (i) is more stringent and the model predicts that it will occur in only 2.5% of patients. By using criterion (ii), we predict that in almost 12% of virtual patients with PNH the clone will be stochastically eliminated, with consequent, “cure” based on flow cytometry. This is similar to what Hillmen et al. reported for patients based on the Ham's test as well as flow cytometric detection of the absence of CD55 and CD58 deficient erythrocytes and neutrophils (29). More recently, Boschetti et al. (37) also provided proof of clonal disappearance by flow cytometry.
PIG-A Mutations and Marrow Failure.
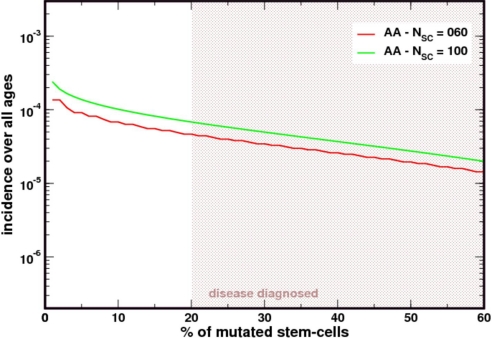
PNH has been proposed as a “blessing in disguise” because the clone maintains hematopoiesis in the presence of marrow failure (2). We have seen that with NSC = 400, and with each HSC replicating on average 1/year, our simulations provide an estimate of the incidence of the disease in the US population. However, in most cases of PNH there is an element of bone marrow failure (BMF). To incorporate this effect in our model, we reduced the size of the active HSC pool; at the same time, we increased the rate of HSC replication up to a level that would still provide normal blood cell production by the bone marrow (i.e., compensated BMF). To this end, we carried out simulations for a pool size NSC = 100 with the cells replicating 4 times/year; and for a pool size of NSC = 60, with a replication rate of 6 times/year. As can be seen from Fig. 5, the prevalence of PIG-A mutations is at least 10-fold higher than what would be expected in the normal population. The difference becomes more pronounced as the size of NSC decreases even further and the median size of the clone increases exponentially with decreasing NSC (P < 0.0022). The clone size also increases to a mean of 47.8% (median 42%, range 20–100%) and the median age of the cohort goes down to 41 years and compatible with epidemiological data (30). This is in keeping with the observation that PNH is (often) associated with bone marrow failure and supported by the reduced number of normal BFU-E observed in patients with PNH (38). In reality, bone marrow failure is a time dependent process, and hence the scenarios depicted in Figs. 2 and 5 portray extreme limits of what one expects whenever PNH progresses to aplastic anemia. In keeping with this analysis, the occurrence of bone marrow failure will produce an increase in clone size and also in population incidence rates; conversely, mean age at diagnosis will decrease.
Fig. 5.
Patients with aplastic anemia (AA) have a higher prevalence of PIG-A mutated SCs. As the size of the stem cell pool decreases as in AA, the prevalence of PIG-A mutants increases. The figure compares the results from Fig. 2 with those obtained by considering a population of 100 or 60 SCs that must replicate at a rate 4 and 6 times faster than normal to maintain hematopoiesis. Under stochastic dynamics and neutral evolution, the clone is more likely to increase in size and appear “more often” in patients with AA.
Discussion
The etiology of PNH has been studied for many years and the discovery of PIG-A (39) was a landmark advance, because a specific, acquired mutation in HSCs explained the deficiency of a multitude of GPI-linked proteins on the surface of circulating blood cells (2). However, to explain the mechanism of expansion of the mutant hematopoietic clone has proven more difficult, especially because PNH progenitor cells do not have a proliferative advantage when compared with normal SCs (40, 41), although they might be more resistant to apoptosis, at least in vitro (42, 43). In principle, it is possible to envisage at least two not mutually exclusive mechanisms. On one hand (i), expansion may result from an acquired somatic mutation, other than the PIG-A mutation, that confers to the clone a growth advantage. On the other hand (ii), expansion may be the consequence of a selective immune attack against normal (GPI+) HSCs to which PNH (GPI−) HSCs are invulnerable.
Mutation-Driven Growth Advantage.
The first mechanism has been recently exemplified by two PNH patients in whom an acquired rearrangement of chromosome 12 produces ectopic expression of the HMGA gene, which might favor growth (7). This gene is deregulated in a number of benign mesenchymal tumors and it is over-expressed at the mRNA level in the PNH clone; hence, the authors suggest that aberrant expression of HMGA2 might explain the expansion of a clone arising originally from the PIG-A mutant stem cell. However, as recently argued elsewhere, it is unlikely that a second mutation in the same HSC explains clonal expansion of GPI− cells in the majority of patients with PNH (34, 44). Indeed, a recent study of a relatively large group of patients with PNH failed to detect mutations in HMGA2 (45).
Conditional Growth Advantage.
The second mechanism is based on a large body of evidence that links PNH to idiopathic aplastic anemia (IAA) (46, 47). This close relationship has suggested that autoreactive T cells against HSC—believed to be responsible for IAA (47)—may be at work also in PNH. Thus, the expansion of GPI− cells characteristic of PNH may be the consequence of a selective immune attack against normal (GPI+) HSCs to which PNH (GPI) HSCs are invulnerable (5). In recent years considerable evidence has accumulated in favor of such an autoimmune mechanism (48–53). Perhaps the most specific evidence is that in PNH patients CD8+ CD57+ T cells are oligoclonal; and in more than two-thirds of patients, there are T cells bearing a set of highly homologous TCR molecules (53). The presence of T cell clones with recurrent clonotypes in most patients with PNH but not in healthy controls is consistent with an immune process driven by the same (or similar) antigen(s). However, a selective auto immune attack against GPI+ HSCs has not been proven as yet; and to date there have been few PNH patients reported in whom immunosuppressive therapy has ameliorated hematopoiesis (54).
In this paper we have explored a third possibility: namely, that PNH clones have neither an absolute nor a conditional growth advantage. The model we have outlined is based on: (i) the stochastic nature of hematopoiesis (8, 55); (ii) the best estimate of the size of the active SC pool (9, 18); (iii) the rate of SC replication (12–14); and (iv) the gene specific mutation rate for PIG-A (21). The basic idea is that, because PIG-A mutations in HSCs occur spontaneously (although very rarely), and given the stochastic nature of hematopoiesis, even if we assume neutral co-evolution of PIG-A mutated HSCs and normal HSCs, PNH would develop whenever the clone of PIG-A mutated HSCs becomes sufficiently large. On the other hand, our model does not address what could happen to the PNH cells downstream of the HSC pool. The fact that a significant fraction of patients with PNH have the majority of their marrow and circulating blood cells with the PNH phenotype may suggest that additional mechanisms downstream of the HSC pool could increase production of such cells.
The most remarkable feature of our model is that it predicts quite accurately the population frequency of PNH. Moreover, although we have focused on the US, the frequency of PNH is estimated to be similar in different parts of the world: this fact in itself would be consistent with a pathogenetic mechanism intrinsic to the body, not requiring specific environmental factors. It must be noted that the age distribution derived from our simulations is shifted upwards when compared to epidemiological data; whereas the distribution of size of the PNH cell population is shifted downwards compared to existing data (this could be due at least in part to referral bias, because patients with larger PNH cell populations will have, on average, more severe disease and therefore may be more likely to come to the attention of referral centers). Interestingly, when the number of SCs was reduced in our simulation (in keeping with the fact that some degree of bone marrow failure is often or always present in PNH patients), the fit of our model improved: in fact, it matched very well the actual data from of a large group of patients in West Yorkshire, England (A. Hill, personal communication) and the recently reported data by Peffault de Latour et al. on a cohort of 460 patients with this disease (56). In our simulations we have assumed that the HSCs reduced in number divide at a faster rate (as they do after BMT): however, the model can be adapted to the actual rate of HSC division once relevant quantitative data became available.
The current models for the expansion of PNH clones require, in addition to a PIG-A mutation, a second pathogenetic event (another somatic mutation) or factor (selection). In addition, the selective damage to GPI+ cells postulated by the conditional growth advantage model implies a paradox: why are (GPI+) nonhematopoietic cells not damaged? It is not impossible that in some rare cases (44), there is a second mutation in the PNH clone; in some cases, there is an environment that gives the PNH clone a selective advantage; and in some cases, there is neither. By comparison, “the pure neutral stochastic drift proposed in this paper provides the simplest explanation for clonal expansion in PNH”; it has the attraction of complying with the wisdom of William of Ockham (1295–1349): “Entia non sunt multiplicanda praeter necessitatem” (“entities should not be multiplied beyond necessity”). On the other hand, a component of bone marrow failure is demonstrable in a significant proportion of patients with PNH, and may be present in most (not just in those who are classified as having PNH/AA); this fact is not predicted by the stochastic model developed here. In the future, with additional data, we may be able to choose rationally what model best reflects the reality of this unique disorder.
Acknowledgments.
This work was supported by Mayo Foundation (D.D.) and FCT-Portugal (J.M.P.). We thank Professor Martin A. Nowak and the Program for Evolutionary Dynamics at Harvard University for hospitality during the initial stages of this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Parker C, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699–3709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luzzatto L, Bessler M, Rotoli B. Somatic mutations in paroxysmal nocturnal hemoglobinuria: A blessing in disguise? Cell. 1997;88:1–4. doi: 10.1016/s0092-8674(00)81850-4. [DOI] [PubMed] [Google Scholar]
- 3.Hillmen P, Bessler M, Mason PJ, Watkins WM, Luzzatto L. Specific defect in N-acetylglucosamine incorporation in the biosynthesis of the glycosylphosphatidylinositol anchor in cloned cell lines from patients with paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci USA. 1993;90:5272–5276. doi: 10.1073/pnas.90.11.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyata T, et al. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1994;330:249–255. doi: 10.1056/NEJM199401273300404. [DOI] [PubMed] [Google Scholar]
- 5.Rotoli B, Luzzatto L. Paroxysmal nocturnal hemoglobinuria. Semin Hematol. 1989;26:201–207. [PubMed] [Google Scholar]
- 6.Karadimitris A, Luzzatto L. The cellular pathogenesis of paroxysmal nocturnal haemoglobinuria. Leukemia. 2001;15:1148–1152. doi: 10.1038/sj.leu.2402180. [DOI] [PubMed] [Google Scholar]
- 7.Inoue N, et al. Molecular basis of clonal expansion of hematopoiesis in 2 patients with paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2006;108:4232–4236. doi: 10.1182/blood-2006-05-025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon MY, Blackett NM. Routes to repopulation—a unification of the stochastic model and separation of stem-cell subpopulations. Leukemia. 1994;8:1068–1073. [PubMed] [Google Scholar]
- 9.Dingli D, Pacheco JM. Allometric scaling of the active hematopoietic stem cell pool across mammals. PLoS ONE. 2006;1:e2. doi: 10.1371/journal.pone.0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingli D, Pacheco JM. Ontogenic growth of the haemopoietic stem cell pool in humans. Proc Biol Sci. 2007;274:2497–2501. doi: 10.1098/rspb.2007.0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dingli D, Traulsen A, Pacheco JM. Stochastic dynamics of hematopoietic tumor stem cells. Cell Cycle. 2007;6:461–466. doi: 10.4161/cc.6.4.3853. [DOI] [PubMed] [Google Scholar]
- 12.Rufer N, et al. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaziri H, et al. Evidence for a mitotic clock in human hematopoietic stem cells: Loss of telomeric DNA with age. Proc Natl Acad Sci USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shepherd BE, Guttorp P, Lansdorp PM, Abkowitz JL. Estimating human hematopoietic stem cell kinetics using granulocyte telomere lengths. Exp Hematol. 2004;32:1040–1050. doi: 10.1016/j.exphem.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Gale RE, Fielding AK, Harrison CN, Linch DC. Acquired skewing of X-chromosome inactivation patterns in myeloid cells of the elderly suggests stochastic clonal loss with age. Br J Haematol. 1997;98:512–519. doi: 10.1046/j.1365-2141.1997.2573078.x. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie JL, Gan OI, Doedens M, Wang JC, Dick JE. Individual stem cells with highly variable proliferation and self-renewal properties comprise the human hematopoietic stem cell compartment. Nat Immunol. 2006;7:1225–1233. doi: 10.1038/ni1393. [DOI] [PubMed] [Google Scholar]
- 17.Abkowitz JL, et al. Evidence for the maintenance of hematopoiesis in a large animal by the sequential activation of stem-cell clones. Proc Natl Acad Sci USA. 1990;87:9062–9066. doi: 10.1073/pnas.87.22.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buescher ES, Alling DW, Gallin JI. Use of an X-linked human neutrophil marker to estimate timing of lyonization and size of the dividing stem cell pool. J Clin Invest. 1985;76:1581–1584. doi: 10.1172/JCI112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brody S. Bioenergetics and Growth. Darien, CT: Hafner Press; 1964. [Google Scholar]
- 20.Ewens WJ. Mathematical Population Genetics. NY: Springer; 2004. [Google Scholar]
- 21.Araten DJ, Luzzatto L. The mutation rate in PIG-A is normal in patients with paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2006;108:734–736. doi: 10.1182/blood-2006-01-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nash R, Storb R, Neiman P. Polyclonal reconstitution of human marrow after allogeneic bone marrow transplantation. Blood. 1988;72:2031–2037. [PubMed] [Google Scholar]
- 23.Araten DJ, et al. A quantitative measurement of the human somatic mutation rate. Cancer Res. 2005;65:8111–8117. doi: 10.1158/0008-5472.CAN-04-1198. [DOI] [PubMed] [Google Scholar]
- 24.Araten DJ, Nafa K, Pakdeesuwan K, Luzzatto L. Clonal populations of hematopoietic cells with paroxysmal nocturnal hemoglobinuria genotype and phenotype are present in normal individuals. Proc Natl Acad Sci USA. 1999;96:5209–5214. doi: 10.1073/pnas.96.9.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abkowitz JL, Catlin SN, McCallie MT, Guttorp P. Evidence that the number of hematopoietic stem cells per animal is conserved in mammals. Blood. 2002;100:2665–2667. doi: 10.1182/blood-2002-03-0822. [DOI] [PubMed] [Google Scholar]
- 26.Michor F, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–1270. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- 27.Dingli D, Traulsen A, Pacheco JM. Compartmental architecture and dynamics of hematopoiesis. PLoS ONE. 2007;2:e345. doi: 10.1371/journal.pone.0000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu R, et al. PIG-A mutations in normal hematopoiesis. Blood. 2005;105:3848–3854. doi: 10.1182/blood-2004-04-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253–1258. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura J, et al. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine (Baltimore) 2004;83:193–207. doi: 10.1097/01.md.0000126763.68170.46. [DOI] [PubMed] [Google Scholar]
- 31.Bessler M, Mason PJ, Hillmen P, Luzzatto L. Mutations in the PIG-A gene causing partial deficiency of GPI-linked surface proteins (PNH II) in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1994;87:863–866. doi: 10.1111/j.1365-2141.1994.tb06754.x. [DOI] [PubMed] [Google Scholar]
- 32.Bessler M, Mason P, Hillmen P, Luzzatto L. Somatic mutations and cellular selection in paroxysmal nocturnal haemoglobinuria. Lancet. 1994;343:951–953. doi: 10.1016/s0140-6736(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 33.Mortazavi Y, et al. The spectrum of PIG-A gene mutations in aplastic anemia/paroxysmal nocturnal hemoglobinuria (AA/PNH): A high incidence of multiple mutations and evidence of a mutational hot spot. Blood. 2003;101:2833–2841. doi: 10.1182/blood-2002-07-2095. [DOI] [PubMed] [Google Scholar]
- 34.Traulsen A, Pacheco JM, Dingli D. On the origin of multiple mutant clones in paroxysmal nocturnal hemoglobinuria. Stem Cells. 2007;25:3081–3084. doi: 10.1634/stemcells.2007-0427. [DOI] [PubMed] [Google Scholar]
- 35.Noji H, et al. The distribution of PIG-A gene abnormalities in paroxysmal nocturnal hemoglobinuria granulocytes and cultured erythroblasts. Exp Hematol. 2001;29:391–400. doi: 10.1016/s0301-472x(00)00684-6. [DOI] [PubMed] [Google Scholar]
- 36.Kai T, et al. Phenotypes and phosphatidylinositol glycan-class A gene abnormalities during cell differentiation and maturation from precursor cells to mature granulocytes in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2002;100:3812–3818. doi: 10.1182/blood.V100.10.3812. [DOI] [PubMed] [Google Scholar]
- 37.Boschetti C, et al. Clinical and molecular aspects of 23 patients affected by paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2004;77:36–44. doi: 10.1002/ajh.20144. [DOI] [PubMed] [Google Scholar]
- 38.Rotoli B, Robledo R, Luzzatto L. Decreased number of circulating BFU-Es in paroxysmal nocturnal hemoglobinuria. Blood. 1982;60:157–159. [PubMed] [Google Scholar]
- 39.Takeda J, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73:703–711. doi: 10.1016/0092-8674(93)90250-t. [DOI] [PubMed] [Google Scholar]
- 40.Keller P, et al. FES-Cre targets phosphatidylinositol glycan class A (PIGA) inactivation to hematopoietic stem cells in the bone marrow. J Exp Med. 2001;194:581–589. doi: 10.1084/jem.194.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Araten DJ, et al. Dynamics of hematopoiesis in paroxysmal nocturnal hemoglobinuria (PNH): No evidence for intrinsic growth advantage of PNH clones. Leukemia. 2002;16:2243–2248. doi: 10.1038/sj.leu.2402694. [DOI] [PubMed] [Google Scholar]
- 42.Brodsky RA, et al. Resistance of paroxysmal nocturnal hemoglobinuria cells to the glycosylphosphatidylinositol-binding toxin aerolysin. Blood. 1999;93:1749–1756. [PubMed] [Google Scholar]
- 43.Horikawa K, et al. Apoptosis resistance of blood cells from patients with paroxysmal nocturnal hemoglobinuria, aplastic anemia, and myelodysplastic syndrome. Blood. 1997;90:2716–2722. [PubMed] [Google Scholar]
- 44.Dingli D, Pacheco JM, Traulsen A. Multiple mutant clones in blood rarely coexist. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;77 doi: 10.1103/PhysRevE.77.021915. 021915. [DOI] [PubMed] [Google Scholar]
- 45.Kelly RJ, Tooze RM, Doogy GM, Richards SJ, Hillmen P. The investigation of HMGA2 dysregulation and promoter mutations in PIG-M in the molecular pathogenesis of paroxysmal nocturnal haemoglobinuria (PNH) Blood. 2007;110:3671. [Google Scholar]
- 46.Dacie JV, Lewis SM. Paroxysmal nocturnal haemoglobinuria: Clinical manifestations, haematology, and nature of the disease. Ser Haematol. 1972;5:3–23. [PubMed] [Google Scholar]
- 47.Young NS. Acquired aplastic anemia. Ann Intern Med. 2002;136:534–546. doi: 10.7326/0003-4819-136-7-200204020-00011. [DOI] [PubMed] [Google Scholar]
- 48.Karadimitris A, et al. Abnormal T-cell repertoire is consistent with immune process underlying the pathogenesis of paroxysmal nocturnal hemoglobinuria. Blood. 2000;96:2613–2620. [PubMed] [Google Scholar]
- 49.Karadimitris A, et al. Association of clonal T-cell large granular lymphocyte disease and paroxysmal nocturnal haemoglobinuria (PNH): Further evidence for a pathogenetic link between T cells, aplastic anaemia, and PNH. Br J Haematol. 2001;115:1010–1014. doi: 10.1046/j.1365-2141.2001.03172.x. [DOI] [PubMed] [Google Scholar]
- 50.Risitano AM, et al. Oligoclonal and polyclonal CD4 and CD8 lymphocytes in aplastic anemia and paroxysmal nocturnal hemoglobinuria measured by V beta CDR3 spectratyping and flow cytometry. Blood. 2002;100:178–183. doi: 10.1182/blood-2002-01-0236. [DOI] [PubMed] [Google Scholar]
- 51.Risitano AM, et al. Large granular lymphocyte (LGL)-like clonal expansions in paroxysmal nocturnal hemoglobinuria (PNH) patients. Leukemia. 2005;19:217–222. doi: 10.1038/sj.leu.2403617. [DOI] [PubMed] [Google Scholar]
- 52.Poggi A, et al. Patients with paroxysmal nocturnal hemoglobinuria have a high frequency of peripheral-blood T cells expressing activating isoforms of inhibiting superfamily receptors. Blood. 2005;106:2399–2408. doi: 10.1182/blood-2004-11-4315. [DOI] [PubMed] [Google Scholar]
- 53.Gargiulo L, et al. Highly homologous T-cell receptor beta sequences support a common target for autoreactive T cells in most patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;109:5036–5042. doi: 10.1182/blood-2006-10-052381. [DOI] [PubMed] [Google Scholar]
- 54.Paquette RL, et al. Clinical characteristics predict response to antithymocyte globulin in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1997;96:92–97. doi: 10.1046/j.1365-2141.1997.d01-1984.x. [DOI] [PubMed] [Google Scholar]
- 55.Abkowitz JL, Catlin SN, Guttorp P. Evidence that hematopoiesis may be a stochastic process in vivo. Nat Med. 1996;2:190–197. doi: 10.1038/nm0296-190. [DOI] [PubMed] [Google Scholar]
- 56.Peffault de Latour R, et al. Paroxysmal nocturnal hemoglobinuria: Natural history of disease subcategories. Blood. 2008;112:3099–3106. doi: 10.1182/blood-2008-01-133918. [DOI] [PubMed] [Google Scholar]