Abstract
Local thyroid hormone catabolism within the mediobasal hypothalamus (MBH) by thyroid hormone-activating (DIO2) and -inactivating (DIO3) enzymes regulates seasonal reproduction in birds and mammals. Recent functional genomics analysis in birds has shown that long days induce thyroid-stimulating hormone production in the pars tuberalis (PT) of the pituitary gland, which triggers DIO2 expression in the ependymal cells (EC) of the MBH. In mammals, nocturnal melatonin secretion provides an endocrine signal of the photoperiod to the PT that contains melatonin receptors in high density, but the interface between the melatonin signal perceived in the PT and the thyroid hormone levels in the MBH remains unclear. Here we provide evidence in mice that TSH participates in this photoperiodic signal transduction. Although most mouse strains are considered to be nonseasonal, a robust photoperiodic response comprising induced expression of TSHB (TSH β subunit), CGA (TSH α subunit), and DIO2, and reduced expression of DIO3, was observed in melatonin-proficient CBA/N mice. These responses could not be elicited in melatonin-deficient C57BL/6J, but treatment of C57BL/6J mice with exogenous melatonin elicited similar effects on the expression of the above-mentioned genes as observed in CBA/N after transfer to short-day conditions. The EC was found to express TSH receptor (TSHR), and ICV injection of TSH induced DIO2 expression. Finally, we show that melatonin administration did not affect the expression of TSHB, DIO2, and DIO3 in TSHR-null mice. Taken together, our findings suggest that melatonin-dependent regulation of thyroid hormone levels in the MBH appears to involve TSH in mammals.
Keywords: circadian rhythm, melatonin, pars tuberalis, photoperiodism, type 2 and 3 iodothyronine deiodinases
Organisms living outside the tropics detect and predict seasonal changes in day length (photoperiod) to adapt various metabolic and behavioral functions to the environment. This mechanism, called photoperiodism, allows animals to control the timing of reproduction so that they can raise their offspring in spring and summer when food is most abundant. Among vertebrates, birds possess a highly sophisticated photoperiodic mechanism and show robust responses to photoperiodic changes. Taking advantage of the elaborate avian photoperiodic system, we have recently revealed the gene cascade regulating the photoperiodic response of reproduction in Japanese quail (Coturnix japonica) by using a functional genomics approach (1, 2). Exposure to long days induced thyroid-stimulating hormone (TSH), a heterodimer of the TSH β subunit (TSHB), and the common glycoprotein α subunit (CGA, also called TSH α subunit), in the pars tuberalis (PT) of the pituitary gland. TSH triggers the expression of type 2 iodothyronine deiodinase (DIO2) in the ependymal cells (EC) lining the ventrolateral walls of the third ventricle (i.e., the infundibular recess) within the mediobasal hypothalamus (MBH). DIO2 is a thyroid hormone-activating enzyme that converts the prohormone thyroxine (T4) to bioactive triiodothyroine (T3). Induction of DIO2 causes local increases in T3 concentration in the MBH under long-day conditions (3). Furthermore, the expression of thyroid hormone-inactivating enzyme, type 3 deiodinase (DIO3), in the EC has been shown to be induced under short-day conditions and to be suppressed under long-day conditions (4). This reciprocal switching between induction of DIO2 and DIO3 seems to fine-tune the thyroid hormone concentration within the MBH. Photoperiodic changes in DIO2 and DIO3 expression and T3 concentration within the MBH have been confirmed in various vertebrate species, including quail (3, 4), sparrows (5), rats (6), hamsters (5, 7–11), goats (12), and sheep (13), and are understood to play a critical role in the control of seasonal reproduction (2, 14). Although the responses of DIO2 and DIO3 to photoperiodic changes appear conserved among various species, the signal transduction pathway regulating DIO2 and DIO3 appears to differ between birds and mammals. In mammals, light stimuli are perceived by the eye and transmitted to the pineal gland via a multisynaptic pathway including the retinohypothalamic tract, the suprachiasmatic nucleus (SCN), and the sympathetic nervous system. Under the control of this pathway, the pineal organ synthesizes and secretes melatonin during the dark phase (15, 16). The duration of melatonin secretion encodes the length of the dark phase and thus regulates various season-dependent functions and behaviors such as reproduction, body weight, and coat color. Pinealectomy eliminates these melatonin-dependent photoperiodic responses (17, 18). In contrast to mammals, melatonin has little effect on seasonal reproduction in birds (19), which perceive light information for the photoperiodic regulation of reproduction by deep brain photoreceptors. Although melatonin regulates photoperiodic DIO2 and DIO3 expression in the EC of the MBH in mammals (7–11), melatonin receptor expression could not be found in the EC (20, 21). Melatonin receptors are, however, expressed in high density by the thyrotroph cell type in the mammalian PT (22–25), and photoperiodic regulation of TSH in the PT has been known for several decades (26, 27). Nevertheless, the functional significance of TSH derived from the PT remains unclear in mammals. In this study, we first show that laboratory mouse strains may represent valuable animal models for studying photoperiodism when using gene expression as a marker. By investigating TSHR-KO mice (28), we show that melatonin-dependent regulation of DIO2 and DIO3 expression involves TSH signaling.
Results
Photoperiodic Response of Melatonin-Proficient CBA/N and Melatonin-Deficient C57BL/6J Mice.
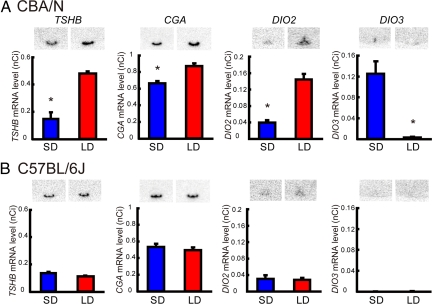
We examined the effect of changing day length on testicular size and TSHB, CGA, DIO2, and DIO3 expression in melatonin-proficient CBA/N and melatonin-deficient C57BL/6J mice. Within the 2 weeks of exposure to changing day length, testicular length did not differ between short-day (8 h light 16 h dark: 8L16D) and long-day (16L8D) conditions in either mouse strain (supporting information (SI) Fig. S1) (Mann-Whitney U test, P > 0.05, n = 5). In contrast, significant induction of TSHB, CGA, and DIO2 expression and reduction of DIO3 expression were observed in CBA/N mice under long day conditions (Mann-Whitney U test, P < 0.05, n = 4–5) (Fig. 1A), whereas the expression of these genes did not change in C57BL/6J mice irrespective of whether they were kept under short- or long-day conditions (Mann-Whitney U test, P > 0.05, n = 4–5; Fig. 1B).
Fig. 1.
Effect of day length on gene expression in melatonin-proficient CBA/N and -deficient C57BL/6J mice. (A) Photoperiodic regulation of TSHB and CGA in the PT of the pituitary gland and of DIO2 and DIO3 in the EC in CBA/N mice. (B) C57BL/6J mice did not respond to changes in the photoperiod. SD, 8L16D; LD, 16L8D. *, P < 0.05 (Mann-Whitney U test, n = 4–5).
Effects of Melatonin Injections in C57BL/6J Mice.
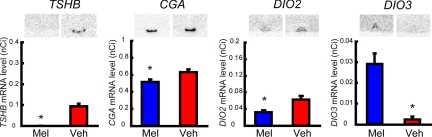
Because C57BL/6J mice are known to express melatonin receptors in the PT (29, 30), we next examined the effect of melatonin injections on gene expression in C57BL/6J mice to test whether C57BL/6J mice are capable of responding to the melatonin signal. As a result, expression of TSHB, CGA, and DIO2 was suppressed, whereas DIO3 expression was induced by melatonin administration (Mann-Whitney U test, P < 0.05, n = 4–5; Fig. 2).
Fig. 2.
Effect of melatonin administration on gene expression in C57BL/6J mice. Vehicle or melatonin injections were given 1 h before lights off to animals kept under 14L10D for 3 weeks. *, P < 0.05 (Mann-Whitney U test, n = 4–5).
Induced Expression of DIO2 by ICV Injection of TSH.
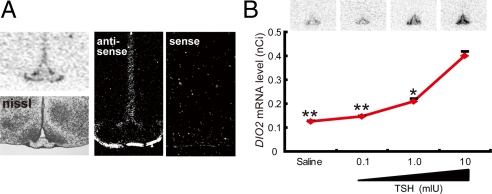
To evaluate whether TSH is involved in the control of DIO2 expression in mice, we first examined the expression site of TSHR. We found expression of TSHR in the EC lining ventrolateral walls of the third ventricle and in the PT (Fig. 3A), as has also been shown in Japanese quail (1). There was no difference in the expression levels of TSHR in the EC and PT between short-day and long-day conditions (unpublished data). We then examined the effect of single ICV injection of TSH into the third ventricle of C57BL/6J mice kept under short-day conditions. Induction of DIO2 expression by TSH was observed in the EC in a dose-dependent manner (one-way ANOVA, F (3, 44) = 8.01, P < 0.01, Scheffé post hoc test, n = 9–13; Fig. 3B). Because the basal expression of DIO3 in the C57BL/6J mice was very low, a suppressive effect of TSH on DIO3 expression could not be shown.
Fig. 3.
Localization of TSHR expression and induction of DIO2 expression by ICV TSH injection. (A) Expression of TSHR in the EC and the PT. Representative autoradiogram (Left Top) and dark-field (Middle and Right) and bright-field photomicrographs (Left Bottom) are shown. (B) Induction of DIO2 expression in the EC by ICV injection of TSH. **, P < 0.01; *, P < 0.05 vs. 10 mIU (one-way ANOVA, F (3, 44) = 8.01, P < 0.01, Scheffé post hoc test, n = 9–13).
Absence of Effects of Melatonin Administration in TSHR-Null Mice.
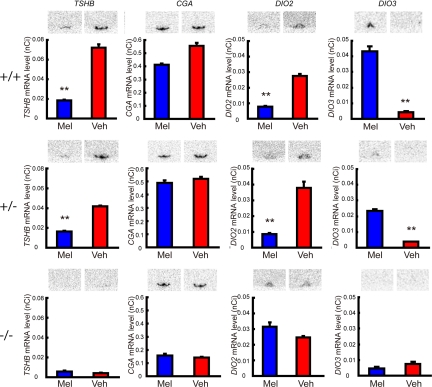
To verify whether TSH mediates the melatonin-dependent regulation of thyroid hormone levels in the MBH, we examined the effect of melatonin administration in TSHR-KO mice. The TSHR-KO mice received thyroid powder replacement therapy to maintain viability. This treatment has been proven to keep serum thyroid hormones within a physiological range (28). Melatonin administration suppressed the expression of TSHB and DIO2 and induced the expression of DIO3 in wild-type and heterozygous mice (Mann-Whitney U test, P < 0.05, n = 6–12; Fig. 4 Top and Middle). In contrast, melatonin treatment did not influence the expression of these genes in homozygous TSHR-KO mice (Mann-Whitney U test, P > 0.05, n = 6–12; (Fig. 4 Bottom). Interestingly, the expression levels of TSHB and DIO3 were very low in the TSHR-KO mice. Melatonin treatment had no significant effect on the expression of CGA in all genotypes (Mann-Whitney U test, P > 0.05, n = 6–12).
Fig. 4.
Effect of melatonin administration on TSHB, CGA, DIO2, and DIO3 expression in TSHR-KO mice. Wild-type (Top), TSHR-KO heterozygotes (Middle), and TSHR-KO homozygotes (Bottom). Vehicle or melatonin injections were given 1 h before lights off to animals kept under 14L10D for 3 weeks. *, P < 0.05; **, P < 0.01 (Mann-Whitney U test, n = 6–12).
Discussion
Of the various available mammalian species, hamsters and sheep are frequently used for studies on photoperiodism because they show robust responses to photoperiodic changes. Unfortunately, however, transgenic and gene-targeting techniques remain unavailable in these species to date. These techniques are available in laboratory mice, but mice are generally considered inappropriate experimental animals for investigations of photoperiodism because they fail to show photoperiodic responses. We found no significant difference in testicular size between mice kept under short-day and long-day conditions for 2 weeks (Fig. S1). This study succeeded, however, in showing robust photoperiodic responses of TSHB and CGA expression in the PT of the pituitary gland and of DIO2 and DIO3 expression in the EC of the MBH in melatonin-proficient CBA/N mice (Fig. 1). The effects of changing day length on the expression of these genes were consistent with those found in Japanese quail (1), suggesting molecular similarities in the photoperiodic mechanisms of birds and mammals. It is also of note that melatonin secretion profiles of CBA/N mice are different between different photoperiods (Daiichiro Nakahara, personal communication). Thus, certain laboratory mouse strains are potentially capable of transforming photoperiodic information into neuroendocrine responses at the level of the MBH. These observations suggest that, depending on the strain, mice may represent an appropriate model to study the molecular mechanism of photoperiodism in the hypothalamus of mammals.
In mammals, the pineal hormone melatonin plays an essential role in the control of multiple season-dependent functions and behaviors (15, 16). It is well established that most laboratory mouse strains (e.g., C57BL/6J mice) produce little or no melatonin and represent a natural melatonin “knockdown” or “knockout” because of a truncation in arylalkylamine N-acetyltransferase (AA-NAT), the enzyme that controls melatonin production (31–33). It is therefore not surprising that the expression of the genes encoding for TSHB, CGA, DIO2, and DIO3 did not respond to changing day length in melatonin-deficient C57BL/6J mice (Fig. 1). Because most of the KO mice are hybrids of the C57BL/6J and 129S1/Sv strains, which also lack melatonin (32), we first examined whether the melatonin-deficient C57BL/6J strain was able to respond to administration of exogenous melatonin. We found that melatonin administration affected TSHB, CGA, DIO2, and DIO3 expression in C57BL/6J mice (Fig. 2) in a way similar to the transfer of CBA/N mice to short-day conditions.
It is also well established that melatonin receptors are expressed in high density by cells located in the PT, and that they express TSHB and CGA, whereas the EC, which expresses DIO2 and DIO3, lacks melatonin receptors (20, 21). It was therefore tempting to evaluate whether melatonin regulates the expression of TSHB and CGA in the PT and whether TSH may transmit the long-day signal to the EC as shown for the photoperiodic mechanisms in quail. To address these questions, we first showed the expression of TSHR in the EC and PT (Fig. 3A). We also performed ICV injection of TSH on animals kept under short-day conditions and showed that this treatment induced expression of DIO2 in a dose-dependent manner (Fig. 3B). Because the basal expression level of DIO3 was extremely low in C57BL/6J mice, it was impossible to reveal a potentially suppressive effect of TSH treatment on DIO3 expression. These findings are consistent with recent reports on quail and sheep (1, 13) and strongly suggest that melatonin-dependent TSH production in the PT controls the expression of DIO2 (and probably DIO3) in the EC of the MBH. To further verify this hypothesis, we examined the effect of melatonin administration on DIO2 and DIO3 expression in TSHR-KO mice. As expected, homozygous TSHR-KO mice were unresponsive to melatonin administration (Fig. 4), although melatonin administration elicited similar effects on DIO2 and DIO3 expression in wild-type and heterozygous mice as short-day conditions did in CBA/N mice. These findings conform to the hypothesis that melatonin-dependent regulation of DIO2 and DIO3 expression is mediated by TSH that derives from the PT and acts upon TSHR in the EC of the MBH.
Notably, we found very low expression levels of TSHB in the PT of the TSHR-KO mice as compared with wild-type animals (Fig. 4). Because TSHR-KO mice die within 1 week of weaning unless fed a diet supplemented with thyroid powder, knockout mice were supplemented with thyroid powder in this study as previously reported (28). Therefore, it may be assumed that downregulation of TSHB in the PT of TSHR-KO mice is caused by a thyroid hormone diet. However, this is unlikely because expression of TSHB in the PT thyrotroph is not regulated via the classical thyrotroph receptors and their intracellular pathways; that is, (i) PT thyrotroph lacks receptors for TRH (TSH releasing hormone) and thyroid hormones, and (ii) TRH or thyroid hormone treatment does not alter the expression of TSHB in the PT, though it significantly affects TSHB expression in the pars distalis thyrotroph (34). However, this downregulation of TSHB suggests that TSHR may activate the expression of the TSHB in the PT via a positive feedback loop. Such a positive feedback loop has also been inferred from findings in Soay sheep, which showed that TSH increased cAMP levels and DIO2 expression in the PT and median eminence (13).
Responses of CGA to photoperiodic changes or melatonin were less robust than those of TSHB. In quail, expression of both CGA and TSHB was upregulated under long-term exposure to long-day conditions, but the temporal dynamics of the expression profiles of TSHB and CGA differed slightly (1). Under short-day conditions, TSHB expression was suppressed to very low levels, and CGA was rhythmically expressed. Expression of TSHB was rather acutely induced in the first long day, and induction of CGA was not observed during this period. Thus, the mechanisms controlling the expression of these two genes may be slightly different, and this difference may relate to the difference in robustness in CGA expression.
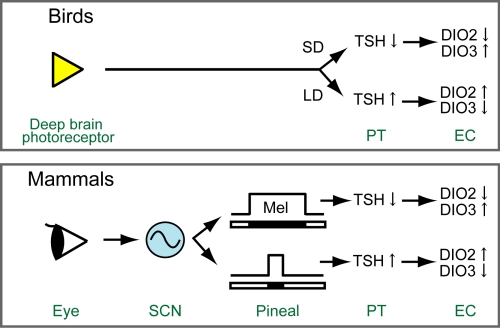
Despite the conservation of seasonal reproduction among various classes of vertebrates, there are some remarkable differences in the regulatory mechanism of seasonal reproduction between birds and mammals. Thus, melatonin is an essential photoperiod messenger in mammals, but it has little effect in birds. Moreover, in mammals, information about the photoperiod is solely perceived in the eye by classical and novel photoreceptors, whereas in birds, deep brain photoreceptors play an essential role in perceiving and transmitting these environmental cues. Irrespective of these species differences, some molecular mechanisms that participate in the control of seasonal functions appear to be conserved in birds and mammals. One of these conserved pathways may employ TSH as a “retrograde” messenger, which is involved in the transmission of photoperiodic signals from the PT to the EC in quail (1) and may serve a similar function in Soay sheep (13) and mice (Fig. 5). Notably, nearly 30 years ago, such retrograde signaling from the hypophysis to the hypothalamus was shown for other hormones derived from the pituitary (35, 36).
Fig. 5.
Schematic diagram of the evolving model of the cellular and molecular mechanisms involved in the control of photoperiodism in birds and mammals. In birds, long-day stimulus is perceived by deep brain photoreceptors and regulates the expression of TSH in the PT. In mammals, light information is solely perceived by the retina and transmitted to the pineal gland through a multisynaptic pathway that includes the retinohypothalamic tract, the SCN, and the sympathetic noradrenergic system. This pathway controls the production and secretion of melatonin in the pineal. The duration of the melatonin signal encodes the duration of night (darkness). Melatonin-dependent control of DIO2 and DIO3 expression in the EC may employ TSH as a retrograde transmitter that is released from the PT and acts upon the EC.
In the present study, we found that certain mouse strains are capable of transforming photoperiodic information, but their reproductive activity appeared to be unaffected at least within 2 weeks of exposure to short- or long-day conditions. Comparison of downstream genes of thyroid hormone action between mice and photoperiodic animals such as quail and sheep may reveal the reason why mice are insensitive (or less sensitive) to the thyroid signals. Seasonal variation in litter size, bodyweight, and sexual maturation in mice has been known for a long time (37). Future studies are needed to clarify whether mice show photoperiodic responses in physiology and behavior when exposed to different photoperiod for a much longer period.
Methods
Animals and Treatment.
Male, 4-week-old C57BL/6J mice and CBA/N mice were purchased from a local dealer and kept under short-day conditions (8L16D) for 3 weeks in light-tight boxes placed in a room at a temperature of 24 °C ± 1 °C. Food and water were provided to the animals ad libitum. Thereafter, at the age of 7 weeks, the mice were divided into two groups; one group was transferred to long-day conditions (16L8D), and the other group was maintained under short-day conditions (8L16D) for 2 weeks. Brains of the 9-week-old mice were collected at midday—that is, 8 h or 4 h after dawn under 16L8D or 8L16D, respectively. TSHR-KO (B6;129S1-Tshr<tm1Rmar>/J) (28) heterozygotes were obtained from the Jackson laboratory, and male and female F2 progenies were used in this study. TSHR-KO homozygotes were placed on a hormone-replacement therapy diet supplemented with 100 ppm desiccated thyroid powder (Sigma) as suggested by Marians et al. (28). Normal serum T4 and T3 levels were found under this modest thyroid supplement.
For the melatonin administration, mice were transferred to 14L10D conditions at 4 weeks of age. Melatonin injections were performed as described by Yasuo et al. (6). At 6 weeks of age, the animals received a daily s.c. injection of melatonin (Sigma) (6 μg in 0.1 ml 5% ethanol/0.9% NaCl) or vehicle (0.1 ml 5% ethanol/0.9% NaCl) 1 h before lights off for 3 weeks, and brains were collected at midday (7 h after lights on).
Bovine TSH (T8931; Sigma) (0.1, 1.0, 10 mIU/10 μl) was injected at 4 h after dawn through a guide cannula (24 gauge, 7 mm) implanted into the third ventricle of 7-week-old C57BL/6J mice that were kept under short-day (8L16D) conditions. Brains were collected 4 h after the injection—that is, at time point when the induction of DIO2 is predicted to occur. Animals were treated in accordance with the guidelines of Nagoya University.
In Situ Hybridization.
In situ hybridization was carried out as previously described (38). Sense and antisense 45-mer oligonucleotide probes for each specific gene were labeled with [33P]dATP (NEN Life Science Products) using terminal deoxyribonucleotidyl transferase (Invitrogen Life Technologies, Inc.). Coronal sections (20 μm thick) of the MBH were prepared using a cryostat (Leica Microsystems, Inc.). Hybridization was carried out overnight at 42 °C. Two high-stringency posthybridization washes were performed at 55 °C. The sections were air dried and apposed to BioMax MR film (Eastman Kodak) for 2 weeks. After exposure to film, each slide was dipped in type NTB2 autoradiography emulsion (Eastman Kodak), diluted twice with sterile distilled water, and exposed for 1.5 months at 4 °C. Thereafter, sections were developed and counterstained with cresyl violet (Merck). Gene-specific probes: TSHB, antisense 5′-gccattgatatcccgtgtcatacaatacccagcacagatggtggtg-3′, CGA, antisense 5′-ccagcattgtcttcttggacctggcgggagtgggatatgccctgg-3′, TSHR, antisense 5′-cagcagtggctcgggtaagagaggtcagcccgagtgaggtggagg-3′, DIO2, antisense 5′-tgcttgagcagaatgaccgagtcatagagcgccaggaagaggcag-3′, DIO3, antisense 5′-ctggtaaccgtcggggccacggcctccctggtacatgatggtgcc-3′.
Supplementary Material
Acknowledgments.
We thank Takashi Yamamura, Tsuyoshi Watanabe, and Shigeru Tomida for technical assistance, and Hiroki R. Ueda for helpful discussion and Nagoya University Radioisotope Center for use of facilities. This work was supported by Grant-in-Aid for Young Scientists (S) from the Japanese Society for the Promotion of Science and the Lipid Signaling Forschungszentrum Frankfurt.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808952105/DCSupplemental.
References
- 1.Nakao N, et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452:317–322. doi: 10.1038/nature06738. [DOI] [PubMed] [Google Scholar]
- 2.Nakao N, Ono H, Yoshimura T. Thyroid hormones and seasonal reproductive neuroendocrine interactions. Reproduction. 2008;136:1–8. doi: 10.1530/REP-08-0041. [DOI] [PubMed] [Google Scholar]
- 3.Yoshimura T, et al. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. [DOI] [PubMed] [Google Scholar]
- 4.Yasuo S, et al. The reciprocal switching of two thyroid hormone-activating and -inactivating enzyme genes is involved in the photoperiodic gonadal response of Japanese quail. Endocrinology. 2005;146:2551–2554. doi: 10.1210/en.2005-0057. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe T, et al. Hypothalamic expression of thyroid hormone-activating and -inactivating enzyme genes in relation to photorefractoriness in birds and mammals. Am J Physiol Regul Integr Comp Physiol. 2007;292:R568–R572. doi: 10.1152/ajpregu.00521.2006. [DOI] [PubMed] [Google Scholar]
- 6.Yasuo S, et al. Differential response of type 2 deiodinase gene expression to photoperiod between photoperiodic Fischer 344 and nonphotoperiodic Wistar rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:1315–1319. doi: 10.1152/ajpregu.00396.2006. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe M, et al. Photoperiodic regulation of type 2 deiodinase gene in Djungarian hamster: Possible homologies between avian and mammalian photoperiodic regulation of reproduction. Endocrinology. 2004;145:1546–1549. doi: 10.1210/en.2003-1593. [DOI] [PubMed] [Google Scholar]
- 8.Revel FG, Saboureau M, Pévet P, Mikkelsen JD, Simonneaux V. Melatonin regulates type 2 deiodinase gene expression in the Syrian hamster. Endocrinology. 2006;147:4680–4687. doi: 10.1210/en.2006-0606. [DOI] [PubMed] [Google Scholar]
- 9.Barrett P, et al. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology. 2007;148:3608–3617. doi: 10.1210/en.2007-0316. [DOI] [PubMed] [Google Scholar]
- 10.Freeman DA, Teubner BJ, Smith CD, Prendergast BJ. Exogenous T3 mimics long day lengths in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2368–R2372. doi: 10.1152/ajpregu.00713.2006. [DOI] [PubMed] [Google Scholar]
- 11.Yasuo S, Yoshimura T, Ebihara S, Korf HW. Temporal dynamics of type 2 deiodinase expression after melatonin injections in Syrian hamsters. Endocrinology. 2007;148:3608–3617. doi: 10.1210/en.2007-0497. [DOI] [PubMed] [Google Scholar]
- 12.Yasuo S, et al. Long-day suppressed expression of type 2 deiodinase gene in the mediobasal hypothalamus of the Saanen goat, a short-day breeder: Implication for seasonal window of thyroid hormone action on reproductive neuroendocrine axis. Endocrinology. 2006;147:432–440. doi: 10.1210/en.2005-0507. [DOI] [PubMed] [Google Scholar]
- 13.Hanon EA, et al. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol. 2008;18:1147–1152. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- 14.Hazlerigg D, Loudon A. New insights into ancient seasonal life timers. Curr Biol. 2008;18:R795–R804. doi: 10.1016/j.cub.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 15.Reiter RJ. The pineal and its hormones in the control of reproduction in mammals. Endocr Rev. 1980;1:109–131. doi: 10.1210/edrv-1-2-109. [DOI] [PubMed] [Google Scholar]
- 16.Arendt J. Melatonin and the Mammalian Pineal Gland. London: Chapman Hall; 1995. [Google Scholar]
- 17.Hoffman RA, Reiter RJ. Pineal gland: Influence on gonads of male hamsters. Science. 1965;148:1609–1611. doi: 10.1126/science.148.3677.1609. [DOI] [PubMed] [Google Scholar]
- 18.Bittman EL, Karsch FJ, Hopkins JW. Role of the pineal gland in ovine photoperiodism: Regulation of seasonal breeding and negative feedback effects of estradiol upon luteinizing hormone secretion. Endocrinology. 1983;113:329–336. doi: 10.1210/endo-113-1-329. [DOI] [PubMed] [Google Scholar]
- 19.Kumar V, Juss T, Follett B. Melatonin and the pineal gland: From basic science to clinical application. In: Touitou Y, Arendt J, Pâevet P, editors. Proceedings of the International Symposium on Melatonin and the Pineal Gland; Amsterdam: Excerpta Medica; 1993. pp. 163–168. [Google Scholar]
- 20.Schuster C, et al. Photic regulation of mt1 melatonin receptors in the Siberian hamster pars tuberalis and suprachiasmatic nuclei: Involvement of the circadian clock and intergeniculate leaflet. J Neuroendocrinol. 2000;12:207–216. doi: 10.1046/j.1365-2826.2000.00039.x. [DOI] [PubMed] [Google Scholar]
- 21.Song CK, Bartness TJ. CNS sympathetic outflow neurons to white fat that express MEL receptors may mediate seasonal adiposity. Am J Physiol Reg Int Comp Physiol. 2001;281:R666–R672. doi: 10.1152/ajpregu.2001.281.2.R666. [DOI] [PubMed] [Google Scholar]
- 22.Williams LM, Morgan PJ. Demonstration of melatonin-binding sites on the pars tuberalis of the rat. J Endocrinol. 1988;119:R1–R3. doi: 10.1677/joe.0.119r001. [DOI] [PubMed] [Google Scholar]
- 23.Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13:1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 24.Wittkowski W, Bockmann J, Kreutz MR, Böckers TM. Cell and molecular biology of the pars tuberalis of the pituitary. Int Rev Cytol. 1999;185:157–194. doi: 10.1016/s0074-7696(08)60151-5. [DOI] [PubMed] [Google Scholar]
- 25.Klosen P, et al. The mt1 melatonin receptor and RORbeta receptor are co-localized in specific TSH-immunoreactive cells in the pars tuberalis of the rat pituitary. J Histochem Cytochem. 2002;50:1647–1657. doi: 10.1177/002215540205001209. [DOI] [PubMed] [Google Scholar]
- 26.Wittkowski W, Bergmann M, Hoffmann K, Pera F. Photoperiod-dependent changes in TSH-like immunoreactivity of cells in the hypophysial pars tuberalis of the Djungarian hamster, Phodopus sungorus. Cell Tissue Res. 1988;251:183–187. doi: 10.1007/BF00215463. [DOI] [PubMed] [Google Scholar]
- 27.Bockmann J, et al. Short photoperiod-dependent down-regulation of thyrotropin-alpha and -beta in hamster pars tuberalis-specific cells is prevented by pinealectomy. Endocrinology. 1996;137:1804–1813. doi: 10.1210/endo.137.5.8612518. [DOI] [PubMed] [Google Scholar]
- 28.Marians RC, et al. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci USA. 2002;99:1576–1581. doi: 10.1073/pnas.242322099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siuciak JA, Fang JM, Dubocovich ML. Autoradiographic localization of 2-[125I]iodomelatonin binding sites in the brains of C3H/HeN and C57BL/6J strains of mice. Eur J Pharmacol. 1990;180:387–390. doi: 10.1016/0014-2999(90)90328-4. [DOI] [PubMed] [Google Scholar]
- 30.Roca AL, Godson C, Weaver DR, Reppert SM. Structure, characterization, and expression of the gene encoding the mouse Mel1a melatonin receptor. Endocrinology. 1996;137:3469–3477. doi: 10.1210/endo.137.8.8754776. [DOI] [PubMed] [Google Scholar]
- 31.Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986;231:491–493. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- 32.Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 33.Roseboom PH, et al. Natural melatonin ‘knockdown’ in C57BL/6J mice: Rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63:189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 34.Bockmann J, et al. Thyrotropin expression in hypophyseal pars tuberalis-specific cells is 3,5,3′-triiodothyronine, thyrotropin-releasing hormone, and Pit-1 independent. Endocrinology. 1997;138:1019–1028. doi: 10.1210/endo.138.3.5007. [DOI] [PubMed] [Google Scholar]
- 35.Krieger DT, Liotta AS. Pituitary hormones in brain: Where, how, and why? Science. 1979;205:366–372. doi: 10.1126/science.221983. [DOI] [PubMed] [Google Scholar]
- 36.Mezey E, Kivovics P, Palkovits M. Pituitary-brain retrograde transport. Trends Neurosci. 1979;2:57–60. [Google Scholar]
- 37.Drickamer LC. Seasonal variation in litter size, bodyweight and sexual maturation in juvenile female house mice (Mus musculus) Lab Anim. 1977;11:159–162. doi: 10.1258/002367777780936639. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimura T, et al. Molecular analysis of avian circadian clock genes. Brain Res Mol Brain Res. 2000;78:207–215. doi: 10.1016/s0169-328x(00)00091-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.