Abstract
No adequate data exist on patterns of injection drug use (IDU) prevalence over time within racial/ethnic groups in U.S. geographic areas. The absence of such prevalence data limits our understanding of the causes and consequences of IDU and hampers planning efforts for IDU-related interventions. Here, we (1) describe a method of estimating IDU prevalence among non-Hispanic Black and non-Hispanic White adult residents of 95 large U.S. metropolitan statistical areas (MSAs) annually over an 11-year period (1992–2002); (2) validate the resulting prevalence estimates; and (3) document temporal trends in these prevalence estimates. IDU prevalence estimates for Black adults were calculated in several steps: we (1) created estimates of the proportion of injectors who were Black in each MSA and year by analyzing databases documenting injectors’ encounters with the healthcare system; (2) multiplied the resulting proportions by previously calculated estimates of the total number of injectors in each MSA and year (Brady et al., 2008); (3) divided the result by the number of Black adults living in each MSA each year; and (4) validated the resulting estimates by correlating them cross-sectionally with theoretically related constructs (Black- and White-specific prevalences of drug-related mortality and of mortality from hepatitis C). We used parallel methods to estimate and validate White IDU prevalence. We analyzed trends in the resulting racial/ethnic-specific IDU prevalence estimates using measures of central tendency and hierarchical linear models (HLM). Black IDU prevalence declined from a median of 279 injectors per 10,000 adults in 1992 to 156 injectors per 10,000 adults in 2002. IDU prevalence for White adults remained relatively flat over time (median values ranged between 86 and 97 injectors per 10,000 adults). HLM analyses described similar trends and suggest that declines in Black IDU prevalence decelerated over time. Both sets of IDU estimates correlated cross-sectionally adequately with validators, suggesting that they have acceptable convergent validity (range for Black IDU prevalence validation: 0.27 < r < 0.61; range for White IDU prevalence: 0.38 < r < 0.80). These data give insight, for the first time, into IDU prevalence trends among Black adults and White adults in large U.S. MSAs. The decline seen here for Black adults may partially explain recent reductions in newly reported cases of IDU-related HIV evident in surveillance data on this population. Declining Black IDU prevalence may have been produced by (1) high AIDS-related mortality rates among Black injectors in the 1990s, rates lowered by the advent of HAART; (2) reduced IDU incidence among Black drug users; and/or (3) MSA-level social processes (e.g., diminishing residential segregation). The stability of IDU prevalence among White adults between 1992 and 2002 may be a function of lower AIDS-related mortality rates in this population; relative stability (and perhaps increases in some MSAs) in initiating IDU among White drug users; and social processes. Future research should investigate the extent to which these racial/ethnic-specific IDU prevalence trends (1) explain, and are explained by, recent trends in IDU-related health outcomes, and (2) are determined by MSA-level social processes.
Electronic supplementary material The online version of this article (doi:10.1007/s11524-008-9304-9) contains supplementary material, which is available to authorized users.
Keywords: Injection drug use, Race/ethnicity, Metropolitan statistical areas, Epidemiology
Introduction
No adequate data exist with which to track patterns of injection drug use prevalence over time among members of specific racial/ethnic groups in U.S. geographic areas. Given pronounced racial/ethnic differences in patterns of drug-related health outcomes,1–5 drug treatment needs,6–12 and determinants of illicit substance use,13 the absence of such data hampers public health surveillance and planning efforts, and limits research on the etiology and consequences of injection drug use (IDU). In this paper, we (1) describe a method of estimating IDU prevalence among non-Hispanic Black and non-Hispanic White adult residents of large U.S. metropolitan statistical areas (MSAs) annually over an 11-year period (1992–2002); (2) validate the resulting prevalence estimates; and (3) document temporal trends in these prevalence estimates.
Existing drug-related surveillance methods and estimation techniques are not designed to capture temporal trends in IDU in different racial/ethnic groups and U.S. geographic areas. The National Survey on Drug Use and Health (NSDUH), the primary source of information on illicit drug use in the U.S., is poorly equipped to capture injectors in the general population for four main reasons.14,15 First, IDU is a relatively rare behavior, and thus, its prevalence is difficult to estimate with precision through the household survey methods employed by NSDUH.16,17 Second, NSDUH cautions against using data for longitudinal analyses, given changes in data collection methods over time.18 Third, the NSDUH sampling frame excludes populations with high IDU prevalences (e.g., incarcerated individuals and non-sheltered homeless individuals).14,15,19 Fourth, NSDUH study participants substantially underreport recent injecting.14,15 The latter two threats to validity may render NSDUH estimates of injecting within specific racial/ethnic groups particularly problematic. Populations that NSDUH excludes tend to be disproportionately non-Hispanic Black and Hispanic/Latino,20,21 and the likelihood of underreporting illicit substance use in surveys may vary by race/ethnicity, with non-Hispanic White adults and adolescents most likely to accurately report recent use.22,23
Capture/recapture methods, commonly applied to enumerate IDU populations living in a single city, are ill-suited to estimating IDU prevalence (both in the general population and within particular subgroups) in multiple geographic areas. These methods enumerate “hidden” populations by cross-referencing personal identifiers (e.g., names or social security numbers) in two or more databases.24–26 Because of confidentiality policies and laws in the U.S., gaining access to personally identifying information, particularly when linked to data on illegal behavior, becomes increasingly prohibitive as the number of geographic areas under study rises.
Here, we describe a method of calculating IDU prevalence among non-Hispanic White and non-Hispanic Black (hereafter referred to as White and Black) residents of large U.S. MSAs over time by analyzing three databases documenting injectors’ encounters with the healthcare system between 1992 and 2002. These databases are the Substance Abuse and Mental Health Service Administration’s (SAMHSA’s) Treatment Entry Data System (TEDS);27 the Centers for Disease Control and Prevention’s (CDC’s) AIDS Public Information Data Set (APIDS);28 and the CDC’s HIV Counseling and Testing Services database (CTS).29 As discussed below, we analyzed these three databases because we posited that the biases inhering in each database would counterbalance those in the others. These methods extend methods originally developed to estimate racial/ethnic disparities in IDU in large U.S. MSAs in 1998.30
Developing a method to track racial/ethnic-specific IDU prevalence estimates over time could advance public health research and planning efforts in key ways. The resulting prevalence estimates could support interpretation of surveillance data on racial/ethnic-specific trends in injection-related health problems by providing, for the first time, estimates of trends in the size of the at-risk populations. Recent data on HIV infection illustrate the need for such estimates: between 2001 and 2004, the number of cases of newly diagnosed injection-related HIV in the 35 U.S. areas with name-based reporting declined more steeply for Black adults and adolescents than for their White counterparts, though the number of such cases for Black adults and adolescents substantially exceeded those for Whites throughout this period.5 Presently, it is impossible to determine the extent to which these trends were produced by diverging temporal trends in the size of the at-risk populations (i.e., Black injectors and White injectors). Clarifying the etiology of trends in injection-related HIV infection is vital to evaluating the effectiveness of past interventions and to developing strategies to further reduce HIV incidence.
These estimates may also lay the foundation for advancing research on the structural determinants of injecting. This line of inquiry remains relatively underdeveloped,31 despite resurging public health recognition that social processes shape distributions of health and disease across populations.32–34 The few studies conducted to date on the structural determinants of substance use testify to the promise of this line of inquiry, both for understanding patterns of injecting in the overall population, as well as within and across racial/ethnic groups.13,35–41 The estimates described here are intended to support future analyses of the ways that MSA-level structural factors shape temporal and spatial variations in IDU among Black adults and White adults.
Finally, because drug-related service needs vary by race/ethnicity,6–12 the racial/ethnic-specific IDU prevalence estimates presented here can help inform planning efforts for programs serving large numbers of injectors, such as methadone maintenance programs and syringe exchange programs.
In the following sections, we describe our method of estimating IDU prevalence among Black adults and White adults living in large U.S. MSAs each year between 1992 and 2002, validate these estimates, and report their trends. We close with a discussion of the limitations of this IDU prevalence estimation method, and propose several possible causes and consequences of the observed racial/ethnic-specific IDU prevalence trends that merit investigation.
Materials and methods
Overview
We created our racial/ethnic-specific IDU prevalence estimates in four stages. In stage 1, we estimated the proportion of all injectors in each MSA and year who were Black and who were White by analyzing data documenting injectors’ encounters with the healthcare system. In stage 2, we multiplied these racial/ethnic-specific proportions by our previous estimates of the total number of injectors (regardless of race/ethnicity) living in each MSA each year (Brady et al., 2008) to produce estimates of the number of Black and of White injectors living in each MSA each year of the study period. In stage 3, we divided the resulting counts by the size of their respective “at risk” populations (i.e., Black adults and White adults) to create racial/ethnic-specific IDU prevalence estimates for each MSA and year. In stage 4, we validated the resulting prevalence estimates. We restricted our prevalence estimates to Black and White injectors because the databases analyzed had extensive missing data and small cell sizes, which would yield unstable estimates, for other racial/ethnic groups in many MSAs.
Descriptive statistics and hierarchical linear modeling (HLM) were then used to describe trends in the resulting estimates. Before discussing our estimation methods and their subsequent analysis, we describe our unit of analysis and study sample.
Unit of Analysis and Sample
MSAs are defined by the Office of Management and Budget (OMB) as adjacent counties that collectively form a single cohesive socioeconomic unit and include at least one central city home to 50,000 people or more.42,43 We chose MSAs as our unit of analysis because they are salient epidemiologic units with which to study injecting: drug-related epidemics can migrate from central cities to the surrounding suburbs, and suburban injectors may travel to the central city to receive services and engage in drug-related activity.44,45 OMB altered MSA boundaries slightly during the study period;46 throughout our analyses, we use 1993 MSA boundaries1.
MSAs were included in this sample if they had a population size of 500,000 or more in 1992, the beginning of our study period. Ninety-six MSAs met this criterion; one MSA, San Juan-Bayamon, had to be dropped because of extensive missing data. The remaining 95 MSAs were located in 38 states and Washington, DC, and had a median population in 2000 of 1.26 million (range: 546,061 to 9,545,829). Nearly two thirds of the U.S. population lived in these 95 MSAs in the year 2000.
Estimating IDU Prevalence for Black and for White adults
-
Stage 1:
Estimating the proportion of injectors who were Black and who were White in each MSA and year
To estimate the proportion of injectors in each of these MSAs who were Black and who were White for each year of our study period, we analyzed data from three databases documenting injectors’ encounters with the healthcare system: TEDS, CTS, and APIDS. TEDS documents admissions to all private and public drug treatment programs receiving state funds, certificates, or licenses.47 The extent to which TEDS represents a census of admissions varies across states, depending on interstate differences in reporting practices, availability of publicly funded treatment, and definitions of what constitutes “treatment admission”.47 CTS describes characteristics of individuals tested at each of the HIV counseling and testing sites reporting to the CDC.4 APIDS is a census of individuals diagnosed with AIDS in the U.S.5 Coverage of eligible cases in APIDS and TEDS is high (>83%).48–50 No data are available on coverage in the CTS database.
Cases in these databases were included in the analysis if they: (1) reported injecting drugs and (2) lived or sought services in one of the 95 MSAs studied. Methods of classifying cases as injectors, and of linking them to MSAs, varied across databases. APIDS and CTS classified individuals as injectors if they reported injecting since 1978;51 in TEDS, injectors were individuals who reported injecting any drug at admission.52
Within each of these three databases, we calculated the proportion of injectors who were Black and who were White in each MSA and year as described in Formula 1.
-
Formula 1:Calculating the database-specific proportion of injectors in each MSA and year who are Black and who are White
where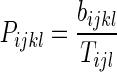
1 - bijkl
- the total number of injectors in study year i, MSA j, racial/ethnic group k, and database l
- Tijl
- the total number of injectors in study year i, MSA j, and database l, regardless of race/ethnicity
Calculating CTS- and TEDS-based Pijkl For TEDS and CTS, we calculated Pijkl directly from the source data in the vast majority of cases (>86.0% of the 1,045 cells for each database and racial/ethnic group2). The total percentages of cells with missing values ranged between 8.4% and 13.5%, depending on the database and racial/ethnic group. Missing values occurred for a variety of reasons. Most arose because the source database obtained by the project lacked data on HIV testing or treatment entry for specific MSAs and years under study. Moreover, we set values of Pijkl to missing if any of the following criteria were met: (1) Tijl < 20, and thus might produce unstable proportion estimates (Pijkl); (2) more than 10.0% of the injectors in the MSA, year, and database were missing data on race/ethnicity, and so the value for bijkl might be artificially low; or (3) Pijkl was unexpectedly high or low (i.e., more than twice, or less than half, the magnitude of the proportion values of both of the adjacent years for that racial/ethnic group and MSA), suggesting a possible error in the source database.When data were missing for any of these reasons, we imputed values of PijkTEDS and PijkCTS using HLM. Where data were available, PijBlackCTS and PijBlackTEDS were highly correlated with each other cross-sectionally (0.68 > r > 0.83), as were values of PijWhiteCTS and PijWhiteTEDS (0.65 > r > 0.81). Using HLM, we therefore imputed missing TEDS-based proportions by regressing PijkTEDS on PijkCTS and on study year. A parallel model was used to impute missing CTS-based proportions. After imputing missing values of PijkTEDS and PijkCTS, we smoothed all CTS- and TEDS-based Pijkl estimates using loess.53
Calculating APIDS-based Pijkl Past research indicates that White illicit drug users have better access to drug treatment services than other illicit drug users54; a similar pattern may be evident for injectors who receive HIV testing and counseling services.55 Basing our estimates of the proportion of injectors who were Black and who were White exclusively on TEDS and CTS data might thus produce IDU prevalence estimates that were biased toward higher values for White adults and toward lower values for Black adults. Incorporating APIDS data into the estimation process is intended to counter this service bias: injectors diagnosed with AIDS are less likely to have participated in nonemergency care services, such as drug treatment and HIV testing, than other injectors56–59; they are also disproportionately likely to be Black or Latino.60,61Using APIDS data to capture the racial/ethnic-specific proportion of the injecting population in a given MSA and year presents several challenges. The racial/ethnic composition of injectors in the APIDS database is produced not only by the racial/ethnic composition of the underlying population of injectors in that MSA and year (our target data), but also by (1) the uneven spread of HIV across racial/ethnic groups of injectors and geographic areas over time;60 and (2) access to therapies delaying the onset of AIDS among HIV-positive injectors, access which varies by race/ethnicity, place, and time.62–65 To use APIDS data to capture the proportion of injectors who were Black and White, we thus first had to adjust APIDS data for HIV seroprevalence and address the advent of highly active antiretroviral therapies (HAART) during the study period. As described in detail in “Appendix”, CTS data were used to adjust APIDS data for HIV seroprevalence, and we determined that the advent of HAART did not significantly impact the percent of injectors in the APIDS database who were Black or White.The resulting CTS-based estimates, TEDS-based estimates, and APIDS-based estimates (adjusted for HIV seroprevalence) of the proportion of injectors who were Black were then averaged across databases to create a single estimate for each year and MSA (we call this single estimate, created by averaging the other three estimates, the “Index”); similar methods were used to estimate the proportion who were White. In the interests of transparency, and to allow us to compare different methods of estimating IDU prevalence by race/ethnicity, we present all results using IDU prevalence estimates based on the Index, and on each of the three databases alone (“database-specific IDU prevalences”). Descriptive data on the Index and database-specific proportions are available in the online appendix (Figs. 1 and 2 in the Web Appendix).
-
Stage 2:
Calculating the number of Black and White injectors in each MSA and year
To calculate the number of Black and White injectors in each MSA and year, we multiplied the proportions calculated in stage 1 by estimates of the total number of injectors (regardless of race/ethnicity) living in each MSA each year of the study period. We applied multiplier/allocation methods to estimate the total number of injectors living in each MSA each year of the study period.66,67 These methods have been described in detail elsewhere68 and are reviewed in “Appendix”. Briefly, we first calculated the number of injectors living in the U.S. during each year of the study period and then allocated these national estimates to each MSA. National estimates were calculated using existing data on the number of injectors living in the U.S. in 1992 and 1998,69,70 and annual data on injectors’ encounters with health services and with the criminal justice system. These national IDU population totals were allocated to each of the 95 MSAs using (1) an estimate derived from published data on the number of injectors living in each MSA in 1992 and in 1998,69,70 and (2) data on injectors’ service use and AIDS diagnoses. Descriptive data on the estimated number of injectors living in these MSAs over time are available in the online appendix (Fig. 3 of the Web appendix). Possible limitations of the resulting IDU estimates are described in the “Discussion” section.
-
Stage 3:
Calculating racial/ethnic-specific prevalence estimates of injecting for each MSA and year
To calculate racial/ethnic-specific IDU prevalence estimates, we divided our estimates of numbers of Black and of White injectors living in each MSA each year of the study period (calculated in stage 2) by the total numbers of Black and of White adults aged 15 to 64 years living in that MSA that year (the “at-risk” population). Population data were drawn from the Population Estimates Program (PEP).71 PEP, which is administered by the U.S. Census Bureau, calculates the total number of people living in each U.S. county by race/ethnicity and age each year by analyzing U.S. decennial census data and data on births, deaths, migration, and military deployment.72 PEP data are adjusted in response to successful challenges to the U.S. decennial Census population estimates.73 County-level PEP data were linked to MSAs to calculate the number of MSA residents aged 15–64 years who were Black and who were White for each year of the study period.3
-
Stage 4:
Validating the racial/ethnic-specific IDU prevalence estimates
We validated our estimates by correlating them cross-sectionally with measures of theoretically related constructs: racial/ethnic-specific prevalences (per million) of (1) drug-related mortality and (2) hepatitis C virus (HCV) mortality. HCV was selected as a validator because it is a blood-borne infection most commonly transmitted in the United States via IDU.74The number of people dying from either of these causes in each racial/ethnic group, MSA, and year was extracted from the National Center for Health Statistics’ Multiple Cause of Death database, a census of all deaths in the U.S.75 This database used the ICD-9 coding system to identify causes of death between 1992 and 1998; the ICD-10 coding system was used thereafter.75
Identifying Drug-related Deaths To identify cases of drug-related mortality within the Multiple Cause of Death database, we adapted algorithms proposed by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) which use ICD-9 and ICD-10 codes (depending on the year of interest) to capture “deaths happening shortly after consumption of one or more psychoactive drugs and directly related to this consumption”.764 We adapted these algorithms to capture mortality that was related to the use of drugs that injectors commonly inject (i.e., opioids, cocaine, and psychostimulants/amphetamines). Neither the ICD-9 nor the ICD-10 coding systems identify the mode of drug administration, so we could not limit cases to those that were IDU related.
Identifying HCV Deaths Codes used to identify HCV mortality were based on a review of published literature on acute and chronic HCV.e.g.,77,78 The recent advent of HCV testing (1990 in the U.S.79) has two implications for the validation analysis. First, since dissemination of the HCV test across the U.S. may have taken time, we begin this portion of the validation analysis in 1995. Second, HCV-specific codes appear in the ICD-10 coding system, but not in the ICD-9 system. In the ICD-9 system, HCV was coded as “Other specified viral hepatitis” (i.e., non-A, non-B hepatitis), a category that may have also included hepatitis D, E, and G. Our ICD-9-based measure of HCV mortality is thus less specific than the ICD-10-based measure, though the difference should be slight, given the rarity of mortality from hepatitis D-G in the U.S.80,81
Describing Racial/Ethnic-specific Trajectories of IDU Prevalence over Time
To describe the central tendency of our racial/ethnic-specific IDU prevalence estimates, we calculated median prevalence values for each year of the study period for the Index and for each database-specific estimate (i.e., for each year of the study period and each estimation method, we calculated the median racial/ethnic-specific IDU prevalence value across the 95 MSAs); we remind you that the “Index” refers to the average of the CTS-, TEDS-, and APIDS-based estimates. We complemented these descriptive statistics with the results of HLM analyses. In longitudinal applications, HLM treats time as nested within subjects, characterizing the structure of change by (1) defining an average growth trajectory for all subjects and (2) quantifying the extent to which individual subjects’ growth trajectories vary around this average.82,83 For each of our IDU estimation methods (i.e., the Index-based method and each of the three database-specific methods), we applied HLM to characterize the average growth trajectories of racial/ethnic-specific IDU prevalences across all MSAs between 1992 and 2002, and to quantify the extent of variation around this mean trajectory across these 95 MSAs. In particular, we fit the following statistical model to each set of IDU prevalence estimates:
-
Formula 2:Unconditional Growth Curve Model
where Yij denotes the predicted prevalence of injecting among White (or Black) adults in study year i in MSA j, and Xij represents the number of years that have elapsed since the beginning of the study period, 1992.83 The parenthetical statement (γ00 + γ10Xij) defines the mean trajectory in injecting prevalence for racial/ethnic group k, with γ00, the intercept, denoting the mean prevalence of IDU among racial/ethnic group k across MSAs in 1992 and γ10, the slope, denoting the mean rate of change in IDU prevalence for racial/ethnic group k over time across MSAs.83 The parameters ζ0i + ζ1iXij describe inter-MSA variation around this mean trajectory: the variances of ζ0i + ζ1i capture the extent of variation across MSAs in the intercept and slope, respectively.82 The parameter ɛij is the error term.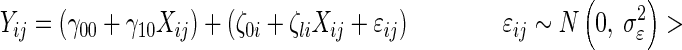
2
Formula 2 assumes that the rate of change in racial/ethnic-specific IDU prevalence is constant over time and that ɛij has constant variance. We tested these assumptions by comparing models that expressed study year in different ways (e.g., linear; linear and quadratic; logged), and by comparing nested error structures. Likelihood ratio tests (LRT) were used to select the optimal model when models were nested; otherwise, we selected the model with the optimal AIC. As a final model-building step, we tested HLM assumptions about the distribution of residuals and the extent to which the model-based trajectories fitted the empirical data.835 All analyses were conducted using SAS version 9.1.84
Results
Describing Growth Trajectories in IDU Prevalence
Growth Trajectories in Black IDU Prevalence Estimates Regardless of the estimation method used, measures of central tendency and the HLM-based results indicate that the prevalence of IDU among Black adults declined between 1992 and 2002, with declines steepest in the early years of the study period. For brevity’s sake, we focus our discussion on the Index-based results (as noted above, the Index is the average of the TEDS-, CTS-, and (adjusted) APIDS-based estimates), noting where database-specific trajectories in Black IDU prevalence diverged from them.6
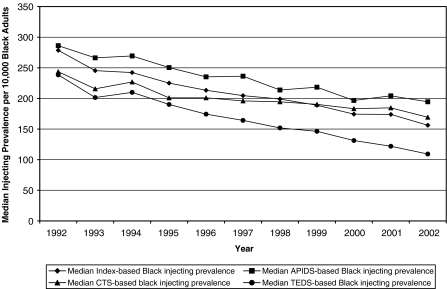
Descriptive Statistics As indicated in Fig. 1, in 1992, the median Index-based IDU prevalence estimate for Black adults was 279 injectors per 10,000 Black adults; by 2002, this prevalence had declined by almost 45% to 156 injectors per 10,000 Black adults. Database-specific IDU prevalence estimates followed an essentially similar trajectory, though the decline in TEDS-based IDU prevalence medians was steeper than those of CTS- and APIDS-based IDU prevalence medians.
FIGURE 1.
Median prevalence of injection drug use per 10,000 Black adult residents of 95 large U.S. metropolitan statistical areas (1992–2002), as estimated using four methods (the “Index” is the average of the CTS-, TEDS-, and APIDS-based estimates).
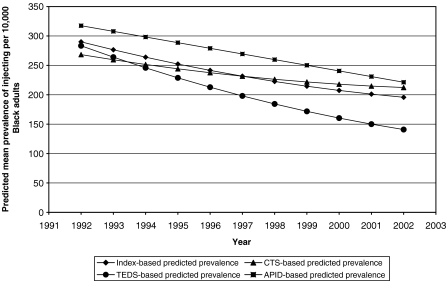
Model-based Statistics The optimal model for the Index-based estimates of Black IDU prevalence included both linear and quadratic expressions of time; the average growth trajectory in Black IDU prevalence across all 95 MSAs is thus a curve defined by three parameters: γ00 (the average initial Black IDU prevalence value), γ10 (the average instantaneous rate of change), and γ20 (the average deceleration/acceleration of this change, modeled as study year squared). This model suggests that, on average, there were 290 injectors for every 10,000 Black adults living in the 95 MSAs studied at the beginning of the study period (Table 1 and Fig. 2). On average, across the 95 MSAs, the number of injectors per 10,000 Black adults declined by approximately 14 people in 1992, from 290 to 276. This decline did not, however, persist throughout the study period (γ20 = 0.45). The formula ([−γ10]/ [2*γ20]) calculates the inflection point of that curve.83 By applying this formula to our parameters, we find that the decline did not reverse during the study period, though it did slow. There was substantial variation across MSAs around this growth trajectory: the model indicates substantial inter-MSAs variation in the initial Black IDU prevalence value (γ00), in the instantaneous rate of change (γ10), and in whether and how much this rate changed over time (γ20; see Table 1). This variation had a particular structure: MSAs with lower IDU prevalence values in 1992 had more modest instantaneous declines (r: −0.48) than other MSAs; MSAs with steeper instantaneous declines saw these declines decay more rapidly than MSAs with more gradual instantaneous declines (r: −0.88). Declines also decelerated more rapidly in MSAs with higher prevalence values in 1992 (r: 0.29).The model-based average trajectories of the CTS- and TEDS-based estimates followed essentially similar trajectories to the Index-based trajectory: 1992 values were 268 and 283 injectors per 10,000 residents, respectively, and declined thereafter, with declines decelerating over time. In contrast, the model-based average trajectory for APIDS-based estimates was higher initially (γ00 = 318) than CTS- and TEDS-based trajectories, and the APIDS-based estimates declined steadily over time. As with the Index-based trajectories, there was considerable variation around each of three mean database-specific IDU prevalence trajectories. In the main, this variation was structured similarly to that around the mean Index-based trajectory.
Table 1.
Model-predicted trajectories in injecting prevalence per 10,000 Black adults in 95 large US metropolitan statistical areas (MSAs) over time (1992–2002), as calculated using four estimation methods
| Model estimates | Index-based prevalencea | CTS-based prevalence | TEDS-based prevalence | APIDS-based prevalence |
|---|---|---|---|---|
| Intercept (γ00) | 290.47*** | 268.33*** | 283.31*** | 317.50*** |
| Time (γ10) | −13.93*** | −9.10** | −19.85*** | −9.62*** |
| Study year, squared (g20) | 0.45* | 0.35* | 0.56** | –b |
| Variance of intercept | 29,501.00*** | 25,074.00*** | 43,989.00*** | 33,185.00*** |
| Variance of study year | 656.63*** | 600.52*** | 562.37*** | 372.96*** |
| Variance of study year, squared | 2.78*** | 2.71*** | 2.34*** | N/A |
| Correlation of γ00 and γ10 (r12) | −0.48*** | −0.39** | −0.50*** | −0.51** |
| Correlation of γ00 and γ20 (r13) | 0.29** | 0.16 | 0.12 | –b |
| Correlation of γ10 and γ20 (r23) | −0.88*** | −0.81*** | −0.78*** | –b |
*p < 0.05
**p < 0.001
***p < 0.0001
aThe Index is the average of the TEDS-, CTS-, and APIDS-based estimates.
bThe variable “study year squared” was not included in this model.
FIGURE 2.
Model-predicted mean prevalence of injection drug use per 10,000 Black adult residents of 95 large U.S. metropolitan statistical areas (1992–2002), as calculated using four methods (the “Index” is the average of the CTS, TEDS, and APID Estimates).
Growth Trajectories in White IDU Prevalence Estimates
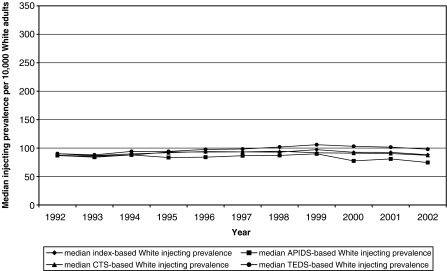
Descriptive Statistics Median IDU prevalence values for White adults were essentially flat throughout the study period (see Fig. 3). The median Index-based IDU prevalence ranged from 86 to 97 IDUs per 10,000 White adults over time; this variation is negligible, given the error inhering in these estimates. Median CTS-, APIDS-, and TEDS-based prevalence estimates were likewise relatively constant over time: CTS-based estimates ranged from 87 to 95 IDUs per 10,000 adults; TEDS-based estimate ranged from 88 to 106 IDUs per 10,000 adults; APIDS-based estimates ranged from 75 to 90.
FIGURE 3.
Median prevalence of injection drug use per 10,000 White adult residents of 95 large U.S. metropolitan areas (1992–2002), as calculated using four estimation methods (the “Index” is the average of the CTS-, APIDS, and TEDS-based estimates).
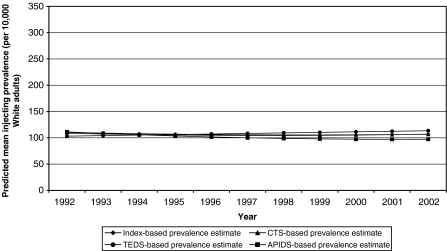
Model-Based Statistics HLM-based trajectories indicate that the mean Index-based IDU prevalence estimates for White adults were constant over the study period (see Table 2 and Fig. 4). While the parameters for time were statistically significant or close to statistically significant (p = 0.06 for γ10), the changes in prevalence they describe are not substantively significant. We therefore conclude that the model-based mean Index trajectory held steady at approximately 109 IDUs per 10,000 White adults throughout the study period. There was, however, variation around this average trajectory. Inter-MSA variation followed the same structure as that found for Index-based Black IDU prevalence, except that there was no association between the magnitude of the intercept and the deceleration/acceleration parameter. Likewise, we conclude that the CTS-, TEDS-, and APIDS-based mean trajectories were also flat (CTS-based mean prevalence: approximately 110 IDUs per 10,000 adults; TEDS-based mean prevalence: approximately 103 IDUs per 10,000 adults; APIDS-based mean prevalence: approximately 111 IDU per 10,000 adults). Inter-MSA variation around each of the database-specific mean trajectories was structured similarly to that around the Index-based trajectory.
Table 2.
Model-predicted trajectories in injecting prevalence per 10,000 White adults in 95 large U.S. metropolitan statistical areas (MSAs) over time (1992–2002), as calculated using four estimation methods
| Model estimates | Index-based prevalencea | CTS-based prevalence | TEDS-based PREVALENCE | APIDS-based prevalence |
|---|---|---|---|---|
| Intercept (γ00) | 108.61*** | 110.36*** | 103.22*** | 111.31*** |
| Time (γ10) | –1.46 | –1.46 | 1.03* | −3.10* |
| Study year, squared (γ20) | 0.13* | 0.11* | –b | 0.17* |
| Variance of intercept | 4,823.59*** | 4,806.36*** | 4,313.80*** | 6,089.98*** |
| Variance of study year | 51.63*** | 52.71*** | 23.18*** | 80.33*** |
| Variance of study year, squared | 0.24*** | 0.26*** | –b | 0.30*** |
| Correlation of γ00 and γ10 (r12) | −0.32* | −0.26* | −0.34* | −0.46*** |
| Correlation of γ00 and γ20 (r13) | 0.05 | 0.06 | –b | 0.14 |
| Correlation of γ10 and γ20 (r23) | −0.76*** | −0.79*** | –b | −0.73*** |
*p < 0.05
**p < 0.001
***p < 0.0001
aThe Index is the average of the TEDS-, CTS-, and APIDS-based estimates.
bThe variable “study year squared” was not included in this model.
FIGURE 4.
Model-predicted mean prevalence of injection drug use among White adults in 95 large metropolitan statistical areas over time (1992–2002), as calculated using four estimation methods (The “Index” is the mean of the CTS, TEDS, and APIDS estimates).
Validation
Validating Black IDU Prevalence Estimates For Black adults, the cross-sectional correlations between the Index-based IDU prevalence and HCV mortality ranged from 0.45 to 0.61 (see Table 3). The correlations between Index-based Black IDU prevalence estimates and the second validator, drug-related mortality prevalence, were lower (0.27 to 0.54). Correlations between the two validators and the database-specific measures of Black IDU prevalence were roughly equivalent to correlations between the Index-based IDU prevalence measure and the validators. Overall, correlations between drug-related mortality prevalence and IDU prevalence were lower in the initial years of the study period.
Table 3.
Validation analysis results for estimates of Black injection drug use (IDU) prevalence in 95 large U.S. metropolitan areas (1992–2002): cross-sectional Pearson correlations of four sets of project estimates of IDU prevalence per 10,000 Black adults with two validators: prevalences per million Black adults of (a) drug-related mortality and (b) hepatitis C virus (HCV) mortalitya,b
| Year | Correlations of index-based IDU prevalencec estimates with | Correlations of CTS-based IDU prevalence estimates with | Correlations of TEDS-based IDU prevalence estimates with | Correlations of APIDS-based IDU prevalence estimates with | ||||
|---|---|---|---|---|---|---|---|---|
| Drug-related mortality | HCV mortality | Drug-related mortality | HCV mortality | Drug-related mortality | HCV mortality | Drug-related mortality | HCV mortality | |
| 1992 | 0.27* | — | 0.31* | — | 0.22* | — | 0.24* | — |
| 1993 | 0.41*** | — | 0.43*** | — | 0.34** | — | 0.37** | — |
| 1994 | 0.40*** | — | 0.40*** | — | 0.35** | — | 0.34** | — |
| 1995 | 0.49*** | 0.45*** | 0.48*** | 0.44*** | 0.44*** | 0.38*** | 0.45*** | 0.43*** |
| 1996 | 0.51*** | 0.49*** | 0.48*** | 0.46*** | 0.46*** | 0.43*** | 0.44*** | 0.43*** |
| 1997 | 0.52*** | 0.51*** | 0.48*** | 0.47*** | 0.48*** | 0.47*** | 0.46*** | 0.46*** |
| 1998 | 0.54*** | 0.55*** | 0.49*** | 0.51*** | 0.53*** | 0.50*** | 0.45*** | 0.49*** |
| Initiation of ICD-10 coding system | ||||||||
| 1999 | 0.49*** | 0.49*** | 0.43*** | 0.45*** | 0.52*** | 0.47*** | 0.34* | 0.41** |
| 2000 | 0.53*** | 0.59*** | 0.48*** | 0.57*** | 0.55*** | 0.51*** | 0.42*** | 0.54*** |
| 2001 | 0.48*** | 0.55*** | 0.43*** | 0.53*** | 0.51*** | 0.47*** | 0.36** | 0.50*** |
| 2002 | 0.50*** | 0.61*** | 0.46*** | 0.61*** | 0.50*** | 0.50*** | 0.39** | 0.60*** |
*p < 0.05
**p < 0.001
***p < 0.0001
aAs noted in the text, correlations of IDU prevalence and HCV mortality were only calculated for 1995–2002 because the test for HCV was first licensed in the USA in 1990; we allowed 5 years for the dissemination of this technology.
bThe database analyzed to calculated drug-related mortality and HCV mortality used the ICD-9 coding system for 1992–1997; the ICD-10 coding system was used thereafter.
cThe Index is the average of the TEDS-, CTS-, and APIDS-based estimates.
Validating White IDU Prevalence Estimates As evident in Table 4, cross-sectional correlations between the Index-based measure of White IDU prevalence and HCV mortality were high (range: 0.61 to 0.65) and consistent over time. The correlations between the Index-based measure of White IDU prevalence and drug-related mortality were moderate to high (range: 0.38 to 0.80) and declined toward the end of the study period. Compared to correlations with the Index-based IDU prevalence measure, correlations between the validators and the database-specific measures of White IDU prevalence were of roughly similar magnitude and followed an essentially similar pattern. Overall, correlations were higher for White IDU prevalence estimates than for Black IDU prevalence estimates. We raise possible reasons for this—and for temporal trends in some of the correlations—below.
Table 4.
Validation analysis results for White injection drug use (IDU) prevalence estimates in 95 large U.S. metropolitan areas (1992–2002): cross-sectional Pearson correlations of four sets of project estimates of IDU prevalence per 10,000 White adults with two validators: prevalences per million White adults of (a) drug-related mortality and (b) hepatitis C virus (HCV) mortalitya,b
| Year | Correlations of index-based IDU prevalencec estimates with | Correlations of CTS-based IDU prevalence estimates with | Correlations of TEDS-based IDU prevalence estimates with | Correlations of APIDS-based IDU prevalence estimates with | ||||
|---|---|---|---|---|---|---|---|---|
| Drug-related mortality | HCV mortality | Drug-related mortality | HCV mortality | Drug-related mortality | HCV mortality | Drug-related mortality | HCV mortality | |
| 1992 | 0.68*** | — | 0.69*** | — | 0.63*** | — | 0.66*** | — |
| 1993 | 0.80*** | — | 0.80*** | — | 0.77*** | — | 0.79*** | — |
| 1994 | 0.72*** | — | 0.73*** | — | 0.67*** | — | 0.73*** | — |
| 1995 | 0.77*** | 0.61*** | 0.76*** | 0.61*** | 0.74*** | 0.61*** | 0.77*** | 0.58*** |
| 1996 | 0.73*** | 0.62*** | 0.71*** | 0.62*** | 0.70*** | 0.62*** | 0.73*** | 0.60*** |
| 1997 | 0.69*** | 0.64*** | 0.68*** | 0.63*** | 0.68*** | 0.64*** | 0.69*** | 0.61*** |
| 1998 | 0.65*** | 0.65*** | 0.63*** | 0.64*** | 0.64*** | 0.65*** | 0.65*** | 0.63*** |
| Initiation of ICD-10 coding system | ||||||||
| 1999 | 0.59*** | 0.64*** | 0.56*** | 0.62*** | 0.57*** | 0.63*** | 0.59*** | 0.62*** |
| 2000 | 0.53*** | 0.64*** | 0.51*** | 0.62*** | 0.53*** | 0.64*** | 0.53*** | 0.60*** |
| 2001 | 0.46*** | 0.62*** | 0.44*** | 0.60*** | 0.46*** | 0.61*** | 0.44*** | 0.60*** |
| 2002 | 0.38*** | 0.61*** | 0.37** | 0.59*** | 0.40*** | 0.61*** | 0.33** | 0.57*** |
*p < 0.05
**p < 0.001
***p < 0.0001
aAs noted in the text, correlations of IDU prevalence and HCV mortality were only calculated for 1995–2002 because the test for HCV was first licensed in the USA in 1990; we allowed 5 years for the dissemination of this technology.
bThe database analyzed to calculate drug-related mortality and HCV mortality used the ICD-9 coding system for 1992–1997; the ICD-10 coding system was used thereafter.
cThe Index is the average of the TEDS-, CTS-, and APIDS-based estimates.
Discussion
To our knowledge, this analysis represents the first attempt to create racial/ethnic-specific IDU prevalence estimates over time in U.S. MSAs, or in any set of geographic areas. We found substantial racial/ethnic and temporal variation in IDU prevalence in 95 large U.S. MSAs, MSAs that collectively are home to almost two thirds of the U.S. population. Regardless of the estimation method used, our data indicate that the prevalence of IDU among Black adults declined between 1992 and 2002; Index-, TEDS-, and CTS-based estimates indicate that these declines decelerated as time passed. According to the Index, in 1992, the model-based mean IDU prevalence among Black adults was 290 injectors per 10,000 adults; by 2002, this mean prevalence had declined by almost one third, to 196 injectors per 10,000 adults. In contrast, the model-based mean White IDU prevalence (as calculated by the Index) remained constant during the study period, at approximately 109 injectors per 10,000 adults.
There was substantial variation across MSAs around these mean trajectories: MSAs with lower IDU prevalence values in 1992 experienced more gradual instantaneous declines than other MSAs, and MSAs with steep instantaneous declines saw those declines decay more rapidly than declines elsewhere. This variation is consonant with both regression to the mean and with a floor effect (i.e., IDU prevalence cannot drop below zero). Inter-MSA variation in the structure of change may also be a product of inter-MSA epidemiologic and social processes, as we discuss below. Before discussing possible determinants and implications of this spatial and temporal variation, we first review the limitations of our IDU prevalence estimates.
Limitations and Validation Limitations inhering in TEDS, CTS, and APIDS may have biased database-specific estimates of the proportion of injectors in each MSA and year who were Black and who were White as calculated in stage 1. Admissions, rather than individuals, are the units of analysis in the TEDS database; an individual who enters drug treatment twice or more in a particular year is thus counted as two or more independent cases. TEDS-based estimates of the proportion of injectors in each racial/ethnic group may be systematically biased in MSAs where this limitation is coupled with racial/ethnic differences in patterns of re-admission to treatment in a single year. CTS-based estimates may be affected by the extent to which the history of the local HIV epidemic varies across racial/ethnic groups of injectors.85,86 For instance, in an MSA where HIV entered the local communities of Black injectors before it entered communities of White injectors, CTS-based estimates of Black IDU prevalence may be artificially low, given that HIV-positive individuals are unlikely to be retested. This bias, however, might be countered by frequent retests among HIV-negative Black injectors, who might be particularly concerned about HIV transmission. Moreover, the racial/ethnic composition of injectors seeking drug treatment or HIV counseling and testing may depend on the geographic location of service sites, given high levels of residential segregation in many U.S. MSAs.87 Given that predominately Black neighborhoods tend to be medically underserved,88 CTS and TEDS might underestimate the proportions of injectors who were Black throughout the study period in segregated MSAs.
As noted in the “Materials and methods” section, we found that the advent of the HAART era had no significant effect on the APIDS-based estimates of the proportion of injectors who were White or who were Black. Even in the pre-HAART era, however, HIV-positive White injectors had better access to existing AIDS-prevention therapies than other seropositive injectors.89–93 Our APIDS-based IDU prevalence estimates thus likely overestimate the prevalence of IDU among Black adults and underestimate its prevalence for White adults throughout the study period, a pattern that may be evident in Figs. 1, 2, 3 and 4. Moreover, our capacity to adjust APIDS-based estimates for local HIV seroprevalence was limited given (1) our use of CTS data to calculate HIV seroprevalence,70,85 and (2) spatial, temporal, and racial/ethnic variations in the time between HIV diagnosis and AIDS diagnosis,62–65 variations that we could not adjust for. In addition, CDC reporting guidelines for APIDS and for CTS define injectors as anyone who has injected since 1978.49,51 These two databases will thus be less sensitive to change over time than the TEDS database (which captures current IDU27), though the effect of this limitation may be diminished by underreporting of a distant history of discontinued IDU.
Despite these limitations, the CTS, APIDS, and TEDS databases produced remarkably similar estimates of the proportions of injectors who were Black and who were White (Pijkl). Before any missing data were imputed (a step that made the database-specific proportions interdependent), values of Pijkl were moderately to highly correlated across databases (for Black prevalence: r ≥ 0.64; for White prevalence: r ≥ 0.44). While not calculated as a validation step, these concordances suggest that each database was capturing the same underlying construct, a construct that can reasonably be interpreted as the proportion of injectors in each racial/ethnic group.
By combining TEDS-, CTS-, and APIDS-based estimates of the proportion of injectors who were Black and who were White in the Index, we intended to diminish the impact of some of these database-specific limitations. In particular, in some cases, the bias introduced by one database should be tempered by the bias introduced by another database. For example, an MSA where Black injectors have poor access to drug treatment and/or HIV counseling and testing services might have an elevated rate of IDU-related AIDS diagnoses among Black adults, given that treatment and early HIV diagnosis protect against AIDS.9,56–59,94–96 Moreover, the Index-based IDU prevalence estimates should capture a broader spectrum of the underlying injecting population than any single database alone. To illustrate, TEDS may capture a segment of the underlying population of injectors who can access relatively high threshold health services (i.e., inpatient or outpatient drug treatment), while APIDS may capture injectors who have less contact with drug-related health services.9,56–59,94–96 Given that Black drug users tend to have poorer access to services than their White counterparts,9,97 these inferences would produce precisely the pattern in database-specific IDU prevalence estimates evident in Figs. 1, 2, 3 and 4: APIDS-based IDU estimates are higher for Black adults than White adults, while TEDS-based estimates show the reverse trend. Theoretically, then, the Index-based estimate may be the “best estimate” of the proportions of injectors who are Black and who are White compared to the database-specific estimates, a hypothesis supported in the validation analysis (see below).
Stage 2 might have introduced additional bias and/or imprecision. The complexities and limitations of estimating the total number of injectors in each MSA and year are discussed in detail in Brady et al.’s 2008 paper. A principle limitation of these IDU totals is their partial reliance on service-based data to allocate injectors to MSAs each year of the study period. The project will underestimate the total number of injectors in MSAs (and years) with fewer CTS or drug treatment sites and, comparatively, will overestimate the number of injectors in MSAs and years with more CTS or treatment sites. As discussed above, however, these biases should be minimized by the inclusion of AIDS case data, which should introduce the opposite bias to the estimates. Moreover, Brady et al’s validation analysis indicated moderate to high construct and convergent validity for the project’s IDU estimates.
Despite the limitations inhering in our racial/ethnic-specific IDU prevalence estimates, both sets of estimates (regardless of calculation method) correlated adequately with the two validators (HCV mortality prevalence and drug-related mortality prevalence), suggesting they had acceptable convergent validity. Our inability to limit deaths from drug-related causes to those that were IDU-related may explain the consistently lower magnitude of correlations found between Black IDU prevalence and drug-related mortality compared to White IDU prevalence and this validator: Black drug users (regardless of IDU status) tend to have higher mortality rates than their White counterparts.98,99 Our inability to exclude (or adequately adjust for) non-IDU fatalities may also explain the decline in correlations between drug-related mortality and White IDU prevalence in the latter half of the study period in most databases. The timing of these declines (1998–2002) roughly coincides with an increase in the nonmedical use of prescription opiates in this population,100 a class of drugs that are typically not injected.100 Likewise, the lower correlations found between drug-related mortality and Black IDU prevalence (as measured by the index, CTS, and TEDS) in the early years of the study period may reflect the ongoing decline of non-injection crack/cocaine use among Black residents of some MSAs in the sample during these years.101
For Black and White adults, correlations between the Index-based estimate of IDU prevalence and both validators were roughly equivalent to validation correlations for the database-specific estimates. In light of this pattern, and because we posit that the Index captures a broader spectrum of injectors than any single database, we propose that the Index-based estimates are the “best estimates” of IDU prevalence among Black and White adults compared to database-specific estimates.
Notably, even these “best estimates” provide an incomplete picture of IDU trends among Black and White adults: small sample sizes in many databases, MSAs, and years prevented us from decomposing these racial/ethnic-specific estimates by injector social class, gender, age, sexuality, or other salient characteristics. Interpretations and applications of these data should recognize that our estimates might conceal considerable variation within racial/ethnic groups in IDU prevalence.
As a post hoc validation effort, we identified MSAs with unusually high IDU prevalence estimates (>5%) and contacted local community experts to gain their insights into the estimates’ accuracy. Community experts included local researchers and department of health staff specializing in users’ health, and staff members at local drug treatment programs and syringe exchange programs (SEPs). While no MSAs had high White IDU prevalence values during the study period, six MSAs were classified as having high Black IDU prevalence values. These MSAs were Allentown, Baltimore, Portland-Vancouver, Stockton-Lodi, San Francisco, and Tucson. An expert in San Francisco reported that the project’s estimates were likely in the high end of the correct range and noted that the rising IDU prevalence among Black adults in this MSA may be produced by the exodus of middle-class, non-injecting Black residents during the internet boom. An expert in Baltimore suggested that the elevated prevalence was produced by adverse social conditions, easy access to drugs, and a poor (though recently improving) treatment system. Experts in Stockton-Lodi and in Allentown reported that atypical service patterns (unusually high numbers of drug treatment slots, or of referrals from prison/jail to treatment) might have inflated project estimates. Experts in two MSAs could not speak to the validity of the data, though one of these experts shared data indicating that project estimates of the percent of injectors who are Black were similar to local data on the percent of SEP participants and HIV outreach clients who are Black. We note that excluding these six MSAs from our HLM analyses does not alter the values of the instantaneous rate of change parameter or of the acceleration/deceleration parameter, though as expected, omitting these MSAs reduces the intercept’s magnitude (from 290.47 to 259.06). Collectively, then, we conclude that (1) when studying a specific MSA, project estimates should be interpreted in the context of additional information on local patterns of racial/ethnic-specific IDU prevalence (we note that these additional data may not be available, and so new data collection efforts may need to be undertaken); and (2) when studying trends in racial/ethnic-specific IDU prevalence across MSAs, inferential statistics appear to be relatively robust to local data limitations.
Interpretation and Possible Applications of Findings
Prevalence is a function of case incidence and duration,102 and the temporal trends in IDU prevalence observed here may have been generated by changes in both the initiation of IDU and the length of time people inject. Existing research suggests that the combination of several epidemiologic and social processes might have helped to produce the course of Black IDU prevalence observed here (i.e., declining IDU prevalence over time, with declines particularly steep in the early years). First, Black injectors suffered a heavy burden of AIDS-related mortality during the study period,103 mortality that would reduce IDU prevalence by reducing case duration. In “Appendix”, we attempt to quantify the magnitude of AIDS-related mortality in this population, and estimate that, at a minimum, 2.5% of Black injectors were newly diagnosed with AIDS in 1992 and died before 2000 (see “Appendix” for details).
Temporal patterns in AIDS-related mortality may help explain the steeper declines in IDU prevalence seen in the early years of our study period. The burden of AIDS-related mortality borne by Black injectors was particularly high in the early- to mid-1990s, an era of less effective AIDS therapies104 and, in some MSAs, high HIV seroprevalence and poor access to AIDS therapies among Black injectors.89–93,105,106 The advent of HAART and the spread of harm reduction actions, services, and policies during the 1990s reduced the burden of AIDS-related mortality among Black injectors,104,107–109 and may have contributed to decelerating declines in Black IDU prevalence in the latter half of the study period. Inter-MSA differences in the extent of harm reduction actions, services, and policies,110,111 and in HAART coverage,64,112 might have contributed to the inter-MSA variation in Black IDU prevalence trajectories found in our analysis.
Second, a large and increasing “forced migration” of tens of thousands of Black drug users into prison may have further reduced Black IDU prevalence during the study period (see “Appendix” for details).113–115 Prisons tend to be located outside of MSA boundaries,116 and so, CTS, TEDS, and APIDS will rarely capture imprisoned injectors. Inter-MSA variation in drug-related laws, enforcement activities, and sentencing practices would have contributed to variations in IDU prevalence trajectories across MSAs. Notably, while this mass exodus would have reduced Black IDU prevalence within MSAs, ample evidence indicates that this migration harms both incarcerated individuals and the communities in which they are embedded and that people may resume injecting post-release.117–122 Together, AIDS-related deaths and prison admissions may, in epidemiologic terms, have reduced the “case duration” of injecting among Black adults during the study period.
Data suggest that Black injectors who died or entered prison during the study period were not necessarily replaced by new injectors. The incidence of IDU among Black drug users appears to be waning in some areas. In MSAs where relevant research has been conducted, Black drug users had lower rates of initiating IDU (and, for individuals who had once injected and then ceased, of re-initiating IDU) compared to their White counterparts.123,124
The relative stasis in IDU prevalence among Whites throughout the study period may likewise reflect processes affecting both case duration and incidence. White injectors have experienced less AIDS-related mortality and incarceration than their Black counterparts (see “Appendix” for details).113,125–128 For example, we estimate that, at a minimum, 0.7% of White injectors (compared to a minimum of 2.5% of Black injectors) were newly diagnosed with AIDS in 1992 and died before 2000.
Moreover, White injectors who died of AIDS or entered prison may have been replaced by new injectors. National data suggest increases in heroin use among young Whites during the study period.129,130 One research report found increasing IDU prevalence among young affluent Whites in one MSA in our sample.131 A recent analysis of the racial/ethnic composition of young injectors enrolling in research studies in five MSAs in our sample suggests that the proportion of young injectors who are White is rising in these areas.132 While we did not interpret the statistically significant rise in the mean trajectory of TEDS-based White IDU prevalence as substantively meaningful, perhaps this database was indeed sensitive to increasing IDU prevalence among White adults in some MSAs. The relative stasis in IDU prevalence among Whites may thus conceal a dynamic process in which White injectors who died or entered prison were replaced by new injectors. Spatial variations in injection initiation, AIDS-related mortality, and incarceration may have contributed to inter-MSA variation in White IDU prevalence trajectories.
The possible roles that AIDS-related mortality, incarceration, and injection initiation may have played in shaping trends in injection prevalence among Black adults and White adults can be empirically investigated. The impact of changing racial/ethnic inequality on trajectories in Black injecting prevalence also merits empirical investigation, given documented links between racial/ethnic discrimination and substance use.133–138 For instance, past cross-sectional research has concluded that MSAs with higher levels of Black residential isolation had higher Black IDU prevalence in 1998.41 Possibly, the documented declines in Black isolation in the 1990s may have contributed to declining IDU prevalence in this population during the study period.140 The estimates described here provide the foundation needed to explore this possibility, as well as to investigate whether other social processes (e.g., organized resistance to racism, absolute poverty, policies limiting drug users’ access to welfare and public housing) shape temporal and spatial variations in racial/ethnic-specific IDU prevalence.
In addition to raising new etiologic questions, project estimates of temporal trends in IDU prevalence among Black adults and White adults strengthen our capacity to interpret surveillance data on the consequences of IDU by providing data on trends in the sizes of the at-risk populations. For example, our estimates suggest that the greater declines in the numbers of newly diagnosed cases of IDU-related HIV found among Black adults compared to White adults may be produced, in part, by the substantial declines in the at-risk populations of Black adults.
These racial/ethnic-specific IDU-prevalence estimates also add a layer of complexity to our understanding of the causes of the recent nationwide increase in overdoses. Population-level data indicate that the prevalence of heroin and cocaine overdose morbidity and mortality rose among both Black and White adults during the study period.2,3,141–143 Given the close links between IDU and overdose,144–147 these trends are striking in a context of declines or stability in IDU prevalence among Black and White adults, and suggest that (1) factors creating vulnerability to overdoses, or to mortality from overdoses, among active injectors may be at play, and/or (2) non-injection drug users may bear a heavy burden of this increase.
As noted earlier, these IDU prevalence estimates can help plan local drug-related interventions and policies, when accompanied by local data sources. For example, where local data suggest that declines in Black IDU prevalence are at least partially produced by interventions that reduce IDU incidence (e.g., drug treatment on demand), public funding for these interventions should be expanded to ensure their continued success. Likewise, where both our estimates and local data suggest that White IDU prevalence is steady or rising, syringe exchange programs and methadone maintenance programs might intensify outreach efforts targeting this population (or some subset of this population, as identified by local data sources).
Conclusion
In closing, these estimates provide insight into temporal trends in IDU prevalence among Black and among White adult residents of 95 large U.S. MSAs. These data suggest that, on average across these MSAs, IDU prevalence declined substantially for Black adults and was static for White adults, though there was considerable inter-MSA variation around these average trajectories. With appropriate recognition of their limitations, these estimates can (1) provide the foundation with which to explore the social determinants of IDU prevalence within racial/ethnic groups; (2) strengthen interpretations of the causes of population-level patterns of drug-related health problems; and (3) inform planning efforts for IDU-related health services.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Median Percent of Injectors who are Black in 95 Large US Metropolitan Statistical Areas Over Time (1992-2002) as Estimated Using Each of Four Methods (The "Index" is the mean of the CTS-, TEDS-, & APIDS-based estimates). (PDF 560 KB)
Median Percent of Injectors Who Are White in 95 Large US Metropolitan Statistical Areas Over Time (1992-2002) As Estimated Using Four Methods (The "Index" is the average of the CTS-, TEDS-, & APIDS-based Estimates). (PDF 88 KB)
Median Number of Injection Drug Users Living in 95 Large US Metropolitan Statistical Areas Over Time (1992-2002), as published in Brady et al, 2008 (see article bibliography for full citation). (PDF 72 KB)
Estimated Prevalence of Injection Drug Use per 10,000 Black Adult Residents of 95 Large US Metropolitan Statistical Areas (1992-2002) as Estimated Using the Index. (PDF 560 KB)
Estimated Prevalence of Injection Drug Use per 10,000 White Adult Residents of 95 Large US Metropolitan Statistical Areas (1992-2002) as Estimated Using the Index. (PDF 120 KB)
Appendix
Adjusting Values of PijkAPIDS for HIV Seroprevalence and Determining whether the Advent of HAART Altered the Racial/Ethnic Composition of Injectors in the APIDS Database
We calculated values of PijkAPIDS that were adjusted for HIV seroprevalence as described in Formula 3. HIV seroprevalence values for each racial/ethnic group of injectors, MSA, and year were estimated using CTS data. The resulting values of PijkAPIDS (adjusted for HIV seroprevalence) were smoothed using loess.53
Formula 3: Calculating values of PijkAPIDS that are adjusted for HIV seroprevalence
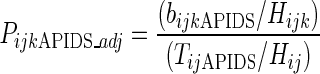 |
3 |
where
- Hijk
the proportion injectors testing positive for HIV in year i, MSA j, racial/ethnic group k
- Hij
the proportion injectors testing positive for HIV in year i and MSA j, regardless of racial/ethnic group
- bijkAPIDS and TijAPIDS
as defined in Formula 1.
Calculating the HIV seroprevalence values used in Formula 3 was accomplished as follows: The number of cases in the CTS database testing positive for injection-related HIV reported for each racial/ethnic group, year, and MSA was divided by the corresponding number of injectors tested in the CTS database. The CTS database released to the project suppressed cell values of <5. We classified missing data on the number of seropositive tests as “suppressed” if CTS data indicated that at least one injector was tested in that racial/ethnic group, MSA, and year. This classification system indicated that positive test results were suppressed for 17.42% of cells for Black injectors, 16.46% of cells for White injectors, and 23.44% of cells for all injectors between 1992 and 2002. Where observations were suppressed, we used regression imputation to estimate the number of observations in each racial/ethnic group (and for all injectors, regardless of racial/ethnic group) who tested positive for injection-related HIV in each year and MSA. We imputed suppressed test results for White injectors for each MSA and year as a function of (1) the total number of White injectors tested in that year and MSA; (2) the percent of all injectors (regardless of race/ethnicity) testing positive in that MSA between 1992 and 2002; and (3) the percent of all White MSA residents tested that year who were injectors. Because the outcome was a count and overdispersed, a negative binomial distribution was assumed; the intercept was set to zero to allow predicted values to range between zero and four. Similar methods were used to impute missing suppressed serostatus values for Black injectors and for all injectors in each year and MSA.
HIV seroprevalence values for Black and White injectors, and for all injectors regardless of race/ethnicity, were then calculated for each year and MSA as described above; seroprevalence values were set to missing where the number of injectors tested was <20 because of concern about the stability of these estimates (approximately 26.9% of cells for Black injectors, 32.8% for White injectors, and 25.2% for all injectors between 1992 and 2002).
Because HAART prolongs time to AIDS diagnoses among HIV-positive individuals if the therapy is initiated sufficiently early,148–150 and because access to HAART varies across MSAs and racial/ethnic groups,62–65 we explored whether the advent of the HAART era in our study altered the racial/ethnic composition of injectors in the APIDS database. Specifically, we tested whether the relationship between study year and the APIDS-based estimates of the proportion of injectors who were White (or Black) varied according to whether the study year predated or postdated the advent of HAART (circa 1997 for injectors). The interaction was not statistically significant, and its magnitude was low. We thus concluded that, while HAART reduced the number of injectors diagnosed with AIDS, it had a negligible effect on the proportion of injectors in each racial/ethnic group. No adjustments were made to the APIDS-based estimates to address the onset of the HAART era.
Calculating the Number of Injectors (Regardless of Race/Ethnicity) Living in each MSA each Year of the Study Period
We calculated the number of injectors living in each MSA during each year of the study period in a two-stage process: stage 1 consisted of estimating the total number of injectors living in the US each year; stage 2 consisted of allocating this national estimate to each MSA.
Stage 1: Calculating Nationwide IDU Estimates Using CTS data, we first calculated a set of “scores” that describe annual changes in the size of the injecting population in the US by dividing the number of injectors seeking HIV counseling and testing services nationwide each year by the average annual number of injectors seeking such services nationwide across all years of the study period. Through a parallel process, two additional sets of scores were calculated, one based on drug treatment data and another based on data on arrests for heroin or cocaine possession (adjusted for the percent of heroin or cocaine users who inject). These three database-specific sets of scores were averaged to create a single score for each year of the study period.
The total number of injectors living in the US had been calculated previously for 1992 and 1998.69,70 We viewed these two data points as anchors. The proportion of the final annual score to the score in 1992 was then multiplied by the 1992 IDU estimate anchor point. This process was repeated with the 1998 IDU estimate anchor point, creating two strands of nationwide annual IDU estimates (one anchored with 1992 data and the other with 1998 data). The results of these two strands were then averaged to estimate the total number of injectors living in the US during each year of the study period.
Allocating Nationwide IDU Estimates to MSAs The resulting annual national IDU estimates were then allocated to each MSA using ratio methods.66,67 For each of four data series (described below), we calculated the proportion of injectors nationwide who lived in MSA i in year j. We then multiplied these database-specific proportions by our estimate of the number of injectors living in the US for each year of the study period, thereby generating four sets of estimates of the number of injectors living in each MSA each year. These four sets of estimates were smoothed using loess, and then averaged to produce a single estimate for each MSA and year. The four data series analyzed captured information on (1) IDU-related AIDS diagnoses; (2) injectors’ participation in HIV counseling and testing services; (3) drug treatment utilization among injectors; and (4) previously calculated estimates of the number of injectors living in the MSAs studied in 1992 and 1998 (interpolated and extrapolated to cover the remaining years of the study period).
Quantifying the Magnitude of AIDS-Related Mortality and of Incarceration among Black Injectors and White Injectors During the Study Period
The combination of AIDS-related mortality and incarceration might have reduced IDU prevalence among Black adults during the study period. Black injectors suffered a heavy burden of AIDS-related mortality during the study period.103 According to CDC surveillance records, 67,314 Black injectors living in the MSAs in our sample died of AIDS-related causes between the date AIDS was first diagnosed in the US and 1999.1517 To begin to capture the toll that AIDS took on the population of Black injectors, we note that, according to our estimates, there were 349,867 Black injectors living in the 95 MSAs in 1992; CDC data indicates that 9,187 (or 2.5%) of these injectors were diagnosed with AIDS in 1992 and died before the year 2000.151 This is a considerable underestimate of the total number of Black injectors alive in 1992 who died of AIDS during our study period because it ignores (1) individuals diagnosed with AIDS in 1992 who died after 1999; and (2) injectors alive in 1992 who were diagnosed with AIDS before or after 1992 and subsequently died. AIDS-related mortality may thus have had powerful effects on IDU prevalence among Black injectors.
Incarceration for drug-related offenses may have further reduced the number of Black injectors living in the MSAs under study between 1992 and 2002. A recent Human Rights Watch report indicates that in the 34 US states for which data are available, 59,535 Black men and women entered prison in 2003 alone to serve time for a drug-related offense; this figure represents a 400% increase since 1986 and 0.26% of the total Black adult population in these states (a percentage that would be substantially higher for the population of Black adult drug users).113 Prisons tend to be located outside of MSA boundaries,116 and so CTS, TEDS, and APIDS will rarely capture imprisoned injectors.
While significant, AIDS-related mortality and incarceration may have had less of an impact on White injectors compared to their Black counterparts. Fewer White injectors than Black injectors died of AIDS during the study period.125–128 According to CDC records, 33,760 White injecting residents of the MSAs in our sample died of AIDS-related causes between the date that AIDS was first diagnosed in the US and 1999.151 Of the 736,100 White injectors residing in the MSAs under study in 1992, 4,791 (0.7%) were diagnosed with AIDS in 1992 and died before 2000.151 Compared to Black adults, White adults have also been less affected by rising incarceration rates for drug-related offenses. In 2003, 37,003 White men and women (or 0.03% of all White adults) entered prison to serve time for a drug-related offense in the 34 states for which data are available.112 The absolute number of White injectors removed from the MSAs during the study period was thus smaller than the absolute number of Black injectors removed; moreover, this removal would have had a smaller impact on White IDU prevalence because there were substantially more White injectors than Black injectors in these MSAs (e.g., in 1992, we estimate that there were 736,100 White injectors and 349,867 Black injectors living in these MSAs).
Footnotes
While MSAs are constructed using counties in almost all U.S. regions, in New England, MSAs are based on cities and towns. New England County Metropolitan Areas (NECMAs), however, are county-based areas. To ensure comparability across the sample, we used NECMAs in New England. For brevity’s sake, we refer to NECMAs as MSAs henceforth.
For each database and race/ethnicity, cells were defined by year and MSA (11 years × 95 MSAs = 1,045 cells).
Notably, between 2000 and 2002, PEP counted individuals who identified as belonging to multiple racial/ethnic groups as multiple people (e.g., a single individual who self-identifies as non-Hispanic Black and non-Hispanic White will appear in the PEP database during these years as two distinct people, one of each racial/ethnic group). In contrast, multiracial individuals appear only once in 1992–1999 PEP data, either in the single racial/ethnic category they identify with most closely or in a “more than one race/other race” group. TEDS, APIDS, and CTS all used the latter classification method throughout the time period. Given that only about 1% of residents of the MSAs in our sample identified themselves as belonging to more than one racial/ethnic group in the 2000 Census, this shift should have a negligible impact on our estimates.
These fatalities include those arising from harmful drug use, dependence, poisonings (accidental, intentional, and of undetermined intent), and from drug-related mental and behavioral disorders.
Visual inspection of quantile–quantile plots for each racial/ethnic-specific IDU estimation method indicated deviations from normality for some MSAs. Removing these MSAs did not affect our substantive findings, and so we report results calculated with the full dataset.
Tables reporting index-based estimates of IDU prevalence for Black adults and for White adults for each MSA and year of the study period are available in this paper’s online “Appendix”.
The CDC’s AIDS-related database does not report the date of death. Instead, it records the date of AIDS diagnosis for each case and an indicator of whether the individual was alive in 1999. Deaths occurring after 1999 are not recorded.
Electronic supplementary material The online version of this article (doi:10.1007/s11524-008-9304-9) contains supplementary material, which is available to authorized users.
An erratum to this article can be found at http://dx.doi.org/10.1007/s11524-008-9324-5
References
- 1.Yang JC, Huang D, Hser Y-I. Long-term morbidity and mortality among a sample of cocaine-dependent black and white veterans. J Urban Health. 2006;83(5):926–940. [DOI] [PMC free article] [PubMed]
- 2.Galea S, Ahern J, Tardiff K, et al. Racial/ethnic disparities in overdose mortality trends in New York City, 1990–1998. J Urban Health. 2003;80(2):201–211. [DOI] [PMC free article] [PubMed]
- 3.Fernandez W, Hackman H, Mckeown L, Anderson T, Hume B. Trends in opioid-related fatal overdoses in Massachusetts, 1990–2003. J Subst Abuse Treatment. 2006;31:151–156. [DOI] [PubMed]
- 4.Centers for Disease Control and Prevention. HIV counseling and testing at CDC-supported sites—United States, 1999–2004. Atlanta, GA: CDC; 2006.
- 5.Centers for Disease Control and Prevention. Cases of HIV infection and AIDS in the United States, by race/ethnicity, 2000–2004. HIV/AIDS surveillance supplemental report. Atlanta, GA: CDC; 2006.
- 6.Kendall J, Sherman M, Bigelow G. Psychiatric symptoms in polysubstance abusers: relationship to race, sex, and age. Addictive Behav. 1995;20:685–690. [DOI] [PubMed]
- 7.Amaro H, Larson MJ, Gampel J, Richardson E, Savage A, Wagler D. Racial/ethnic differences in social vulnerability among women with co-occurring mental heath and substance abuse disorders: implications for treatment services. J Community Psychol. 2005;33(4):495–511.
- 8.Petry NM. A comparison of African American and non-Hispanic Caucasian cocaine-abusing outpatients. Drug and Alcohol Dependence. 2003;69(1):43–49. [DOI] [PubMed]
- 9.Walton MA, Blow FC, Booth BA. Diversity in relapse prevention needs: gender and race comparisons among substance abuse treatment patients. Am J Drug Alcohol Abuse. 2001;27(2):225–240. [DOI] [PubMed]
- 10.Roberts A. Psychiatric comorbidity in white and African-American illicit substance abusers: evidence for differential etiology. Clin Psychol Rev. 2000;20:667–677. [DOI] [PubMed]
- 11.Dembo R, Williams L, Schmeidler J. Psychosocial, alcohol/other drug use, and delinquency differences between urban black and white male high risk youth. Int J Addict. 1994;29:461–483. [DOI] [PubMed]
- 12.Ziedonis D, Rayford B, Bryant K, Rounsaville B. Psychiatric comorbidity in White and African-American cocaine addicts seeking substance abuse treatment. Hosp Community Psychiatry. 1994;45:43–49. [DOI] [PubMed]
- 13.Fuller CM, Borrell L, Latkin CA, et al. Effects of race, neighborhood, and social network on age at initiation of injection drug use. Am J Public Health. 2005;95(4):689–695. [DOI] [PMC free article] [PubMed]
- 14.Wright D, Gfoerer J, Epstein J. The use of external data sources and ratio estimation to improve estimates of hardcore drug use from the NHSDA. NIDA Research Monograph. Vol 167. Rockville, MD: Office of Applied Studies, Substance Abuse and Mental Health Services Administration; 1997. [PubMed]
- 15.Wright D, Gfoerer J. Estimation of hardcore drug users. J Off Statistics. 1997;13:401–416.
- 16.Archibald CP, Jayaraman GC, Major C, Patrick DM, Houston SM, Sutherland D. Estimating the size of hard-to-reach populations: a novel method using HIV testing data compared to other methods. AIDS. 2001;15(Suppl 3):S41–S48. [DOI] [PubMed]
- 17.Korf D, Reijneveld S, Toet J. Estimating the number of heroin users: a review of methods and empirical findings from the Netherlands. Int J Addict. 1994;29:1393–1417. [DOI] [PubMed]
- 18.Substance Abuse and Mental Health Services Administration. Results from the 2005 National survey on drug use and health: national findings. Rockville, MD: SAMHSA; 2006.
- 19.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. National Survey on Drug Use & Health Methodology Reports and Questionnaires; 2007.
- 20.US Department of Justice Office of Justice Programs Bureau of Justice Statistics. Additional Corrections Facts at a Glance: Demographic Trends in Correctionals Populations. http://www.ojp.usdoj.gov/bjs/gcorpop.htm#CorrPopRace. Accessed January 19, 2007.
- 21.US Department of Housing and Urban Development. Homelessness: Programs and the People They Serve. Washington, DC: Department of Housing and Urban Development; 1999.
- 22.Fendrich M, Johnson TP. Race/ethnicity differences in the validity of self-reported drug use: Results from a household survey. J Urban Health. 2005;82(Suppl 3):iii67–iii81. [DOI] [PMC free article] [PubMed]
- 23.Johnson T, Bowman P. Cross-cultural sources of measurement error in substance use surveys. Subst Use Misuse. 2003;38:1447–1490. [DOI] [PubMed]
- 24.Cox S, Shipley M. Counting the uncatchable? An epidemiological method for counting drug misusers. Soc Psychiatry Psychiatr Epidemiol. 1997;32:19–23. [DOI] [PubMed]
- 25.Hickman M, Cox S, Harvey J, et al. Estimating the prevalence of problem drug use in inner London: a discussion of three capture-recapture studies. Addict. 1999;94:1653–1662. [DOI] [PubMed]
- 26.Larson A, Stevens A, Wardlaw G. Indirect estimates of ‘hidden’ populations: capture-recapture methods to estimate the numbers of heroin users in the Australian capital territory. Soc Sci Med. 1994;39(6):823–831. [DOI] [PubMed]
- 27.Substance Abuse and Mental Health Administration. Treatment Episode Data Set (TEDS): SAMHSA; 2007.
- 28.US Department of Health and Human Services. AIDS Public Information Dataset (APID): US DHHS; 2007.
- 29.Centers for Disease Control and Prevention. HIV Counseling and Testing in Publicly Funded Sites: CDC; 2006.
- 30.Cooper HL, Friedman SR, Tempalski B, Friedman R, Keem M. Racial/ethnic disparities in injection drug use in large US metropolitan areas. Ann Epidemiol. 2005;15(5):326–334. [DOI] [PubMed]
- 31.Galea S, Nandi A, Vlahov D. The social epidemiology of substance use. Epidemiol Rev. 2004;26:36–52. [DOI] [PubMed]
- 32.Diez-Roux A. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91:1783–1789. [DOI] [PMC free article] [PubMed]
- 33.Berkman LF, Kawachi I. Social Epidemiology. New York: Oxford University Press; 2000.
- 34.Kawachi I, Berkman LF. Neighborhoods and Health. New York: Oxford University Press; 2003.
- 35.Nurco D, Shaffer J, Cisin I. An ecological analysis of the interrelationships among drug abuse and other indices of social pathology. Int J Addict. 1984;19:441–451. [DOI] [PubMed]
- 36.Hunt L, Chambers C. The heroin epidemics: a study of heroin use in the United States, 1965–1975. New York: Spectrum; 1976.
- 37.Boardman JD, Finch B, Ellison C, Williams DR, Jackson JS. Neighborhood disadvantage, stress, and drug use among adults. J Health Soc Behav. 2001;42:151–165. [PubMed]
- 38.Friedman SR, Tempalski B, Cooper HL, Keem M, Friedman R, Flom PL. Structural factors to guide structural intervention: predictors of IDU population size, HIV Prevalence Among IDUs, and Prevention Program Coverage for IDUs. XV International AIDS Conference. Bangkok Thailand; 2004.
- 39.Deborah W, Wallace R. A plague on your houses. New York: Verso; 1998.
- 40.Lillie-Blanton M, Anthony J, Schuster C. Probing the meaning of racial/ethnic group comparison in crack cocaine smoking. JAMA. 1993;269(8):993–997. [PubMed]
- 41.Cooper HL, Friedman SR, Tempalski B, Friedman SR, Keem M. Residential segregation and the prevalence of injection drug use among Black adult residents of US metropolitan areas. Am J Public Health. 2007;97(2):344–352. [DOI] [PMC free article] [PubMed]
- 42.Office of Management and Budget. Standards for defining metropolitan and micropolitan statistical areas. Federal Register. 2000;65:8228–82238.
- 43.U.S. Bureau of the Census. State and metropolitan area data book, 1997–1998. Washington, DC: US Bureau of the Census; 1998.
- 44.Pierce T. Gen-X Junkie: Ethnographic research with young white heroin users in Washington, DC. Subst Use Misuse. 1999;34:2095–2114. [DOI] [PubMed]
- 45.Wallace R, Wallace D. Socioeconomic determinants of health: community marginalisation and the diffusion of disease and disorder in the United States. BMJ. 1997;314:1341–1345. [DOI] [PMC free article] [PubMed]
- 46.Census USBot. Historical metropolitan area definitions. Washington, DC: US Bureau of the Census; 2005.
- 47.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Treatment Episode Data Set (TEDS). Highlights—2005. National admissions to substance abuse treatment services. Rockville, MD: SAMHSA; 2006.
- 48.Interuniversity Consortium for Political and Social Research (ICPSR). Treatment Episode Data Set (TEDS) Series: Summary. http://webapp.icpsr.umich.edu/cocoon/SAMHDA-SERIES/00056.xml. Accessed June 4, 2007.
- 49.Centers for Disease Control & Prevention, National Center for HIV S, and TB Prevention, Divisions of HIV/AIDS Prevention, Statistics & Data Management Branch. AIDS Public Information Data Set—Manual, Data Through December 2002. Rockville, MD: Centers for Disease Control & Prevention; 2005:51.
- 50.Klevens RM, Fleming PL, Li J, et al. The completeness, validity, and timeliness of AIDS surveillance data. Ann Epidemiol. 2001;11(7):443–449. [DOI] [PubMed]
- 51.Centers for Disease Control and Prevention. CTS User's Guide, CTS Version 4.0. Rockville, MD: Centers for Disease Control and Prevention; 1999 March 1999.
- 52.US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Treatment Episode Data Set, 1992–2005 (Concatenated): Data Collection Description; 2007.
- 53.Cleveland W. Visualizing Data. Summitt, NJ: Hobart Press; 1993.
- 54.Wells K, Klap R, Koike A, Sherbourne C. Ethnic disparities in unmet need for alcoholism, drug abuse, and mental health care. Am J of Psychiatry. 2001;158:2027–2032. [DOI] [PubMed]
- 55.Johnson DF, Sorvillo FJ, Wohl AR, et al. Frequent failed early HIV detection in a high prevalence area: implications for prevention. AIDS Patient Care. 2003;17(6):277–282. [DOI] [PubMed]
- 56.Metzger D, Navaline H. Human immunodeficiency virus prevention and the potential of drug abuse treatment. Clin Infect Dis. 2003;37:S451–S456. [DOI] [PubMed]
- 57.Hoffman J, Klein H, Clark D, Boyd F. The effect of entering drug treatment on involvement in HIV-related risk behaviors. Am J Drug Alcohol Abuse. 1998;24:259–284. [DOI] [PubMed]
- 58.Hartel D, Schoenbaum E. Methadone treatment protects against HIV infection: two decades of experience in the Bronx, New York City. Public Health Rep. 1998;113:105–115. [PMC free article] [PubMed]
- 59.Janssen R, Holtgrave D, Valdisseri R, Shepherd M, Gayle H, De Cok K. The serostatus approach to fighting the HIV epidemic: preventive strategies for infected individuals. Am J Public Health. 2001;91:1019–1024. [DOI] [PMC free article] [PubMed]
- 60.Centers for Disease Control and Prevention. Cases of HIV infection and AIDS in the United States, by Race/Ethnicity, 1998–2002. Atlanta, Georgia: Centers for disease control and prevention; 2004.
- 61.Durant T, McDavid K, Hu X, Sullivan P, Janssen R, Fenton K. Racial/ethnic disparities in diagnoses of HIV/AIDS—33 States, 2001–2005. MMWR. 2007;56(9):189–193. 17347642
- 62.Cohen M, Cook J, Young M, et al. Medically eligible women who do not use HAART: the importance of abuse, drug use, and race. Am J Public Health. 2004;94(7):1147–1151. [DOI] [PMC free article] [PubMed]
- 63.Fleishman JA, Hellinger FH. Recent trends in HIV-related inpatient admissions 1996–2000: a 7-state study. JAIDS. 2003;34(1):102–110. [DOI] [PubMed]
- 64.Anderson RN, Bozzette S, Shapiro M, et al. Access of vulnerable groups to antiretroviral therapy among persons in care for HIV disease in the United States. HSR: Health Serv Res. 2000;35(2):389–416. [PMC free article] [PubMed]
- 65.Ghani A, Donnelly C, Anderson R. Patterns of antiretroviral use in the United States of America: analysis of three observational databases. HIV Med. 2003;4:24–32. [DOI] [PubMed]
- 66.Frischer M, Hickman M, Kraus L, Nariani F, Wiessing L. A comparison of different methods for estimating prevalence of problematic drug misuse in Great Britain. Addict. 2001;96:1465–1476. [DOI] [PubMed]
- 67.Hickman M, Hope V, Platt L. Estimating the prevalence of injecting drug use: a comparison of multiplier and capture-recapture methods in cities in England and Russia. Drug Alcohol Rev. 2006;25:131–140. [DOI] [PubMed]
- 68.Brady JE, Friedman SR, Cooper HLF, Flom PL, Tempalski B, Gostnell K. Estimating the prevalence of injection drug users in the U.S. and in large U.S. Metropolitan areas from 1992 to 2002. J Urban Health. 2008;8(3):323–351. [DOI] [PMC free article] [PubMed]
- 69.Friedman SR, Tempalski B, Cooper HL, et al. Estimating numbers of injecting drug users in metropolitan areas for structural analyses of community vulnerability and for assessing relative degrees of service provision for injecting drug users. J Urban Health. 2004;81(3):377–400. [DOI] [PMC free article] [PubMed]
- 70.Holmberg S. The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. Am J Public Health. 1996;86(5):642–654. [DOI] [PMC free article] [PubMed]
- 71.Census USBot. Population Estimates Program Dataset: U.S. Bureau of the Census; 2007.
- 72.U.S. Bureau of the Census, Population Division. Estimates and projections area methodology county population estimates by age, sex, race, and hispanic origin for July 1, 2005. Washington, DC: US Bureau of the Census; 2006.
- 73.US Bureau of the Census. Estimates And Projections Area Methodology County Population Estimates By Age, Sex, Race, And Hispanic Origin For July 1, 2005. http://www.census.gov/popest/topics/methodology/2005_co_char_meth.html. Accessed May 31, 2007.
- 74.National Institutes of Health, Hepatitis C Virus Concensus Development Panel. Management of Hepatitis C: 2002. Washington, DC: National Institute of Health; 2002.
- 75.U.S. Dept. of Health and Human Services, National Center for Health Statistics. Multiple Cause of Death File, 1992–2002 [Computer file]: U.S. Dept. of Health and Human Services, National Center for Health Statistics; 2004.
- 76.European Monitoring Centre for Drugs and Drug Addiction. EMCDDA Statistical Bulletin 2005. http://stats05.emcdda.europa.eu/en/home-en.html. Accessed October 18, 2006.
- 77.Chen CM, Yoon Y-H, Yi H-Y, Lucas DL. Alcohol and hepatitis C mortality among males and females in the United States: a life table analysis. Alcoholism: Clin Exp Res. 2007;31(2):285–292. [DOI] [PubMed]
- 78.Gasiorowwicz M, Hurie M, Russell A, Hoxie N, Vergeront J. Epidemiologic trends in infection, mortality, and transplants related to hepatitis C in Wisconsin. Wisconsin Med J. 2006;105(1):34–39. [PubMed]
- 79.Alter MJ, Kuhnert WL, Finelli L. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. MMWR. 2003;52(RR03):1–16. [PubMed]
- 80.Centers for Disease Control and Prevention. Viral Hepatitis E: Fact Sheet. Accessed May 4 2007.
- 81.National Center for HIV/AIDS VH, STD, and TB Prevention at the Centers for Disease Control and Prevention. Slide set: Hepatitis D. Slide Set: Viral Hepatitis; 2006.
- 82.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd edn. Thousand Oaks, CA: Sage Publications; 2002.
- 83.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. New York: Oxford University Press; 2003.
- 84.SAS/STAT® 9.1. Computer program. Version. Cary, NC: SAS Institute; 2004.
- 85.Friedman SR, Lieb S, Tempalski B, et al. HIV among injection drug users in large US metropolitan areas, 1998. J Urban Health. 2005;82(3):434–445. [DOI] [PMC free article] [PubMed]
- 86.Holmberg S. The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. Am J Public Health. 1996;86:642–654. [DOI] [PMC free article] [PubMed]
- 87.Massey D, Denton N. American apartheid: segregation and the making of the underclass. Boston, MA: Harvard University Press; 1998.
- 88.Smedley B, Smith A, Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2003. [PubMed]
- 89.Easterbrook P, Keruly J, Creagh-Kirk T, Richman D. Racial and ethnic differences in outcome in zidovudine-treated patients with advanced HIV disease. JAMA. 1991;266(19):2713–2718. [PubMed]
- 90.Moore R, Hidalgo J, Sugland B, Chaisson R. Zidovudine and the natural history of the Acquired Immunodeficiency Syndrome. N Engl J Med. 1991;324(20):1412–1416. [DOI] [PubMed]
- 91.Moore R, Stanton D, Gopalan R, Chaisson R. Racial differences in the use of drug therapy for HIV disease in an urban community. N Engl J Med. 1994;330(11):763–768. [DOI] [PubMed]
- 92.Rosenberg P, Gail M, Schrager L, et al. National AIDS incidence trends and the extent of Zidovudine therapy in selected demographic and transmission groups. JAIDS. 1991;4(4):392–401. [PubMed]
- 93.Stein M, Piette J, Mor V, et al. Difference in access to Zidovudine (AZT) among symptomatic HIV-infected persons. J Gen Intern Med. 1991;6:35–40. (January/February) [DOI] [PubMed]
- 94.Woods W, Guydish J, Sorenson J, Coutts A, Bostrom A, Acampora A. Changes in HIV-related risk behaviors following drug abuse treatment. AIDS. 1999;13:2151–2155. [DOI] [PubMed]
- 95.Thiede H, Harris N, McGough J, Roberts B, Khabbaz R, Kaplan J. Prevalence of HTLV Types I and II Among Drug Users in King County, Washington. West J Med. 1994;160:540–544. [PMC free article] [PubMed]
- 96.Kwiatkowski C, Booth R. Methadone maintenance as HIV risk reduction with street-recruited injecting drug users. JAIDS. 2001;26:483–489. [DOI] [PubMed]
- 97.Wells K, Klap R, Koike A, Sherbourne C. Ethnic disparities in unmet need for alcoholism, drug abuse, and mental health care. Am J Psychiatry. 2001;158:2027–2032. [DOI] [PubMed]
- 98.Bernstein KT, Bucciarelli A, Piper TM, Gross C, Tardiff K, Galea S. Cocaine- and opiate-related fatal overdose in New York City, 1990–2000. BMC Public Health. 2007;7:31. [DOI] [PMC free article] [PubMed]
- 99.Kallan JE. Drug abuse-related mortality in the United States: patterns and correlates. Am J Drug Alcohol Abuse. 1998;24(1):103–117. [DOI] [PubMed]
- 100.Office of Applied Studies, Substance Abuse and Mental Health Services Administration. Treatment admissions in urban and rural areas involving abuse of narcotic painkillers:2002 update. Washington, DC: SAMHSA; 2004.
- 101.U.S. Dept. of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Treatment Episode Data Set (TEDS), 1992–2005, Concatenated data [Computer file]: Ann Arbor, MI: Inter-university Consortium for Political and Social Research [producer and distributor]; 2007.
- 102.Hennekens CH, Buring JF. Epidemiology in Medicine. Boston, MA: Little, Brown and Company; 1987.
- 103.US Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for HIV SaTPN. AIDS Public Information Data Set (APIDS) US Surveillance Data for 1981–2002, CDC WONDER On-line Database: CDC; December 2005.
- 104.Louie JK, Hsu LC, Osmond DH, Katz MH, Schwarcz SK. Trends in causes of death among persons with acquired immunodeficiency syndrome in the era of highly active antiretroviral therapy, San Francisco, 1994–1998. J Infect Dis. 2002;186(7):1023–1027. [DOI] [PubMed]
- 105.Harawa NT, Greenland S, Bingham TA, et al. Associations of Race/Ethnicity With HIV Prevalence and HIV-related behaviors among young men who have sex with men in 7 urban centers in the United States. JAIDS. 2004;35(5):526–536. [DOI] [PubMed]
- 106.Kottiri BJ, Friedman SR, Neaigus A, Curtis R, Des Jarlais DC. Risk networks and racial/ethnic differences in the prevalence of HIV infection among injection drug users. JAIDS. 2002;30(1):95–104. [DOI] [PubMed]
- 107.Des Jarlais DC, McKnight C, Milliken J. Public funding of US syringe exchange programs. J Urban Health. 2004;81(1):118–121. [DOI] [PMC free article] [PubMed]
- 108.Lurie P, Reingold A. The public-health impact of needle-exchange programs in the United States and abroad: summary, conclusions, and recommendations. San Francisco: University of California, San Francisco, Institute for Health Policy Studies; 1993.
- 109.Normand J, Vlahov D, Moses L. Preventing HIV transmission: the role of sterile needles and bleach. Washington, DC: National Academy Press/National Research Council/Institute of Medicine; 1995. [PubMed]
- 110.Tempalski B, Flom P, Friedman S, et al. Social and political factors predicting the presence of syringe exchange programs in 96 US metropolitan areas. Am J Public Health. 2007;97(3):437–447. [DOI] [PMC free article] [PubMed]
- 111.Burris S, Welsh J, Ng M, Li M, Ditzler A. State syringe and drug possession laws potentially influencing safe syringe disposal by injection drug users. J Am Pharm Assoc. 2002;42(6 Suppl 2):S94–S98. [DOI] [PubMed]
- 112.Cunningham W, Markson LE, Anderson RM, et al. Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the United States. JAIDS. 2000;25:115–123. [DOI] [PubMed]
- 113.Human Rights Watch. Targeting blacks: drug law enforcement and race in the United States. New York: Human Rights Watch; 2008.
- 114.Blankenship KM, Smoyer AB, Bray SJ, Mattocks K. Black-white disparities in HIV/AIDS: the role of drug policy and the corrections system. J Health Care Poor Underserved. 2005;16(4 Suppl B):140–145. [DOI] [PMC free article] [PubMed]
- 115.Martin YI, James B, Rajeev NR, Terry F. How criminal system racial disparities may translate into health disparities. J Health Care Poor Underserved. 2005;16(4 Supplement B):48–56. [DOI] [PubMed]
- 116.U.S. Department of Justice, Office of Justice Programs, Bureau of Justice Statistics. Incarcerated parents and their children. Washington, DC: US Department of Justice; 2000.
- 117.Kang S-Y, Deren S, Andia J, Colon HM, Robles R, Oliver-Velez D. HIV transmission behaviors in jail/prison among Puerto Rican drug injectors in New York and Puerto Rico. AIDS Behav. 2005;9(93):377–386. [DOI] [PubMed]
- 118.Hammett TM. HIV/AIDS and other infectious diseases among correctional inmates: transmission, burden, and an appropriate response. Am J Public Health. 2006;96(6):974–978. [DOI] [PMC free article] [PubMed]
- 119.Binswanger IA, Stern MF, Deyo RA, et al. Release from prison–a high risk of death for former inmates. N Engl J Med. 2007;356(2):157–165. [DOI] [PMC free article] [PubMed]
- 120.Bird SM, Hutchinson SJ. Male drugs-related deaths in the fortnight after release from prison: Scotland, 1996–99. Addict. 2003;98(2):185–190. [DOI] [PubMed]
- 121.Kerr T, Fairbairn N, Tyndall M, et al. Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend. 2007;87(1):39–45. [DOI] [PubMed]
- 122.Thomas JC, Torrone E. Incarceration as forced migration: effects on selected community health outcomes. Am J Public Health. 2006;96(10):1762–1765. [DOI] [PMC free article] [PubMed]
- 123.Neaigus A, Gyarmathy VA, Miller M, Frajzyngier VM, Friedman SR, Des Jarlais DC. Transitions to injecting drug use among noninjecting heroin users: social network influence and individual susceptibility. JAIDS. 2006;41(4):493–503. [DOI] [PubMed]
- 124.Fuller CM, Vlahov D, Ompad D, Shah N, Aria A, Strathdee SA. High-risk behaviors associated with transition from illicit non-injection to injection drug use among adolescent and young adult drug users: a case-control study. Drug Alcohol Depend. 2002;66(2):189–198. [DOI] [PubMed]
- 125.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the Era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145(6):397–406. [DOI] [PubMed]
- 126.Fordyce EJ, Singh TP, Nash D, Gallagher B, Forlenza S. Survival Rates in NYC in the Era of Combination ART. JAIDS. 2002;30(1):111–118. [DOI] [PubMed]
- 127.Hellinger FJ, Fleishman JA. Location, race, and hospital care for AIDS patients: an analysis of 10 states. Inquiry. 2001;38(3):319–330. [DOI] [PubMed]
- 128.Anderson KH, Mitchell JM. Differential access in the receipt of antiretroviral drugs for the treatment of AIDS and its implications for survival. Arch Internal Medicine. 2000;160(20):3114–3120. [DOI] [PubMed]
- 129.Johnston L, O'Malley P, Bachman J. Demographic subgroup trends for various licit and illicit drugs, 1975–2002. Ann Arbor, MI: Institute for Social Research; 2003.
- 130.US Department of Health and Human Services, National Institutes of Health, National Institute on Drug Abuse, Division of Epidemiology S, and Prevention Research. Drug use among racial/ethnic minorities, revised. Bethesda, MD: NIH; 2003.
- 131.Hamid A, Curtis R, McCoy K, al E. The heroin epidemic in New York City: current status and prognosis. J Psychoactive Drugs. 1999;41:375–391. [DOI] [PubMed]
- 132.Broz D, Ouellet LJ. Racial and ethnic changes in heroin injection in the United States: Implications for the HIV/AIDS epidemic. Drug and Alcohol Dependence. In press. [DOI] [PMC free article] [PubMed]
- 133.Taylor JJB. Factors affecting alcohol consumption in black women, Part II. Int J Addict. 1990;25:1415–1427. [DOI] [PubMed]
- 134.Yen I, Ragland D, Greiner B, Fisher J. Racial discrimination and alcohol-related behavior in urban transit operators: findings from the San Francisco Muni Health and Safety Study. Public Health Rep. 1999;114:448–458. [DOI] [PMC free article] [PubMed]
- 135.Martin J, Tuch S, Roman PJ, Tuch SA, Roman PM. Problem drinking patterns among African-Americans: the Impacts of reports of discrimination, perceptions of prejudice, and “Risky” coping strategies. J Health Soc Behav. 2003;44:408–425. [PubMed]
- 136.Bennet G, Yaus Wolin K, Robinson E, Fowler S, Edwards C. Perceived Racial/Ethnic Harassment and tobacco use among African-American young adults. Am J Public Health. 2005;95:238–240. [DOI] [PMC free article] [PubMed]
- 137.Landrine H, Klonoff E. Racial discrimination and cigarette smoking among blacks: findings from two studies. Ethnicity and Disease. 2000;10:195–202. [PubMed]
- 138.Guthrie B, Young A, Williams D, Boyd C, Kinter E. African American girls’ smoking habits and day-to-day experiences with racial discrimination. Nursing Research. 2002;51:183–190. [DOI] [PubMed]
- 139.Gibbons F, Gerrard M, Cleveland M, Wills T, Brody G. Perceived discrimination and substance use in African American parents and their children: a panel study. Journal of Personality and Social Psychology. 2004;86:517–529. [DOI] [PubMed]
- 140.Iceland J, Weinberg DH, Steinmetz E. Racial and ethnic residential segregation in the United States: 1980–2000. Washington, DC: U.S. Census Bureau; 2002.
- 141.Davidson PJ, McLean RL, Kral AH, Gleghorn AA, Edlin BR, Moss AR. Fatal heroin-related overdose in San Francisco, 1997–2000: a case for targeted intervention. J Urban Health. 2003;80(2):261–273. [DOI] [PMC free article] [PubMed]
- 142.Oxman G, Kowalski S, Drapela L, et al. Heroin overdose deaths—Multnomah county, Oregon, 1993–1999. MMWR. 2000;49(28):633–636. [PubMed]
- 143.Solet D, Hagan H, Nakagawara J, Plough A, Ball J. Unintentional Opiate overdose deaths—King County, Washington, 1990–1999. MMWR. 2000;49(28):636–640. [PubMed]
- 144.Darke S, Roos J, Zador D, Sunjic S. Heroin-related deaths in New South Wales, Australia, 1992–1996. Drug Alcohol Depend. 2000;60:141–150. [DOI] [PubMed]
- 145.Darke S, Hetherington K, Ross J, Lynskey M, Teesson M. Non-injecting routes of administration among entrants to three treatment modalities for heroin dependence. Drug Alcohol Rev. 2004;23:1. [DOI] [PubMed]
- 146.Carpentrer CAC, Stitzer M. Heroin snorters versus injectors: comparison on drug use and treatment outcome in age-matched samples. Drug Alcohol Depend. 1998;53:11–15. [DOI] [PubMed]
- 147.Swift W, Maher L, Sunjic S. Transitions between routes of heroin administration: a study of Caucasian and Indochinese heroin users in southwestern Sydney. Addict. 1999;94:71–82. [DOI] [PubMed]
- 148.Mocroft A, Vella S, Benfield T, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. Lancet. 1998;352:1725–1730. [DOI] [PubMed]
- 149.Hogg R, Heath K, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–454. [DOI] [PubMed]
- 150.Palella FJ, Delaney K, Moorman A, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. [DOI] [PubMed]
- 151.US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for HIV S, and TB Prevention. AIDS Public Information Data Set (APIDS) US Surveillance Data for 1981–2002, CDC Wonder On-line Database: CDC; 2005.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Median Percent of Injectors who are Black in 95 Large US Metropolitan Statistical Areas Over Time (1992-2002) as Estimated Using Each of Four Methods (The "Index" is the mean of the CTS-, TEDS-, & APIDS-based estimates). (PDF 560 KB)
Median Percent of Injectors Who Are White in 95 Large US Metropolitan Statistical Areas Over Time (1992-2002) As Estimated Using Four Methods (The "Index" is the average of the CTS-, TEDS-, & APIDS-based Estimates). (PDF 88 KB)
Median Number of Injection Drug Users Living in 95 Large US Metropolitan Statistical Areas Over Time (1992-2002), as published in Brady et al, 2008 (see article bibliography for full citation). (PDF 72 KB)
Estimated Prevalence of Injection Drug Use per 10,000 Black Adult Residents of 95 Large US Metropolitan Statistical Areas (1992-2002) as Estimated Using the Index. (PDF 560 KB)
Estimated Prevalence of Injection Drug Use per 10,000 White Adult Residents of 95 Large US Metropolitan Statistical Areas (1992-2002) as Estimated Using the Index. (PDF 120 KB)