Abstract
Low back pain is an extremely common symptom, affecting nearly three-quarters of the population sometime in their life. Given that disc herniation is thought to be an extension of progressive disc degeneration that attends the normal aging process, seeking an effective therapy that staves off disc degeneration has been considered a logical attempt to reduce back pain. The most apparent cellular and biochemical changes attributable to degeneration include a decrease in cell density in the disc that is accompanied by a reduction in synthesis of cartilage-specific extracellular matrix components. With this in mind, one therapeutic strategy would be to replace, regenerate, or augment the intervertebral disc cell population, with a goal of correcting matrix insufficiencies and restoring normal segment biomechanics. Biological restoration through the use of autologous disc chondrocyte transplantation offers a potential to achieve functional integration of disc metabolism and mechanics. We designed an animal study using the dog as our model to investigate this hypothesis by transplantation of autologous disc-derived chondrocytes into degenerated intervertebral discs. As a result we demonstrated that disc cells remained viable after transplantation; transplanted disc cells produced an extracellular matrix that contained components similar to normal intervertebral disc tissue; a statistically significant correlation between transplanting cells and retention of disc height could displayed. Following these results the Euro Disc Randomized Trial was initiated to embrace a representative patient group with persistent symptoms that had not responded to conservative treatment where an indication for surgical treatment was given. In the interim analyses we evaluated that patients who received autologous disc cell transplantation had greater pain reduction at 2 years compared with patients who did not receive cells following their discectomy surgery and discs in patients that received cells demonstrated a significant difference as a group in the fluid content of their treated disc when compared to control. Autologous disc-derived cell transplantation is technically feasible and biologically relevant to repairing disc damage and retarding disc degeneration. Adipose tissue provides an alternative source of regenerative cells with little donor site morbidity. These regenerative cells are able to differentiate into a nucleus pulposus-like phenotype when exposed to environmental factors similar to disc, and offer the inherent advantage of availability without the need for transporting, culturing, and expanding the cells. In an effort to develop a clinical option for cell placement and assess the response of the cells to the post-surgical milieu, adipose-derived cells were collected, concentrated, and transplanted under fluoroscopic guidance directly into a surgically damaged disc using our dog model. This study provides evidence that cells harvested from adipose tissue might offer a reliable source of regenerative potential capable of bio-restitution.
Keywords: Autologous disc cell transplantation, Adipose-derived stem cells, Degenerative disc disease, Cell transplantation, Biological repair
Introduction
Low back pain is an extremely common symptom, affecting nearly three-quarters of the population sometime in their life. While 90% of the population recovers within 3 months, in some patients chronic back or leg pain leads to long term physical disability and a reduced quality of life. Disc anatomy would be expected to play a pivotal role in the underlying pain, yet abnormal spine and disc morphology including disc herniation has been described as a normal component of an asymptomatic population [6]. Why is it that some patients remain asymptomatic, and is it possible to treat patients with degenerative change that become symptomatic?
Given that disc herniation is thought to be an extension of progressive disc degeneration that attends the normal aging process, seeking an effective therapy that staves disc degeneration has been considered a logical attempt to reduce back pain. Previous studies have validated genetic factors [1, 16, 24, 33], and implicated nutrition [31] as relevant to the degenerative process. However, the high prevalence across diverse populations suggests that a myriad of unidentified factors likely contribute to similar symptoms.
As no effective therapies to retard or reverse disc degeneration have yet been devised, a variety of surgical procedures have been developed to treat disc degeneration and back pain. Unfortunately, the procedures currently available fail to offer an outcome that is prosthetic and at the same time physiologic. Surgery tends to limit motion, and fusion in particular seems to shunt excessive stresses to adjacent spinal segments. Equally concerning in selecting fusion as an option is the fact that non-unions have been reported in 5–35% of patients [5, 29], and that patients undergoing a repeat fusion for failed surgery in the lumbar spine may still have a clinical failure rate as high as 40% [10, 34, 35]. The advent of tissue engineering has broadened the options for considering treatments that tailor repair to distinct anatomy. In particular, the use of cell and gene therapy to endow specific properties or repair specific tissues is widely considered an emerging modality for effecting treatment.
Numerous scientific studies have provided observations concerning the biochemistry and biomechanics of the disc, offering insights and theories into structure–function-failure relationships [8, 13, 16]. The most apparent cellular and biochemical changes attributable to degeneration include a decrease in cell density in the disc that is accompanied by a reduction in synthesis of cartilage-specific extracellular matrix components such as Type II collagen and aggrecan. As the proteoglycan content of the disc decreases, the resulting loss of water-binding capacity by the disc matrix coupled with a subsequent reduced capacity for dissipating spinal forces are thought to lead to disc disease [5, 17, 21].
Collagen plays a key load-bearing role in the disc, and changes in its extracellular matrix content have been attributed to aging as well as to the pathology of degeneration [2]. In normal intervertebral discs, at least seven different types of collagen are present (i.e., Types I, II, III, V, VI, IX, and XI), although Types I and II are the most abundant [3, 4, 9, 27, 28, 36]. The annulus fibrosus contains more Type I collagen than Type II, whereas the nucleus pulposus is composed mainly of Type II collagen.
Calcification of the vertebral endplates is another factor thought to be relevant to disc degeneration. The passage of nutrients and waste products across the endplate depends on fluid flowing into the disc (during the night at bed rest) and flowing out during the day when we walk about [18]. Thus, shortcomings of permeability would be expected to adversely affect chondrocyte metabolism [7, 19, 20].
While cells constitute only 1% of the adult disc tissue by volume, their role in matrix synthesis and metabolic turnover is vital. Most assessments of intervertebral disc failure have focused on degenerative, morphologic changes in disc tissue morphology that affect the biomechanical performance of the motion segment [13, 32]. In this consideration, mechanical failure is little more than a corollary of matrix structure, which in turn depends on balanced cell metabolism for efficient maintenance of the disc matrix. Given the value of cells to the metabolic health of the disc, one therapeutic strategy would be to replace, regenerate, or augment the intervertebral disc cell population, with a goal of correcting matrix insufficiencies and restoring normal segment biomechanics.
Recent work has shown that disc aging and degeneration are accompanied by a decline in the number of cells in the disc, a change attributable to both necrosis and apoptosis [14]. Perhaps a more important outcome of this work and that of others has been to demonstrate that disc cells retain an ability to respond to both genetic endowment and appropriate in vivo stimulation, and that when returned to the disc under controlled conditions integrate with the surrounding tissue [11, 14, 22, 31].
With this in mind, we designed a study (using the dog as our model) to investigate the hypothesis that (1) repair of the damaged disc is technically feasible, (2) autologous cells can be reproducibly cultured under defined and controlled conditions, (3) percutaneous delivery is possible, and that (4) disc cells will integrate with the surrounding tissue, produce the appropriate intervertebral disc extracellular matrix, and potentially provide a functional solution to disc repair.
Canine trial of chondrocyte transplantation
The goal of this study was to test the hypothesis that restoration of intervertebral disc morphology could be achieved by transplantation of cultured autologous chondrocytes into the nucleus pulposus. As a natural model of degeneration has not been described in a large mammal, this study was fashioned after established work demonstrating that degeneration can be stimulated by damaging the outer annulus [23]. Under institutional guidelines of the Institutional Animal Care and Use Committee (IACUC), 18 purpose-bred, 2-year old female dogs, weighing between 20 and 25 Kg, were studied to see whether the introduction of cultured autologous disc-derived cells would repair a damaged disc and inhibit degenerative changes. Prior to surgery, 125 ml of blood was obtained from each of the dogs to serve as a serum supplement for autologous cell culture. As blood loss was insignificant during the surgical procedure, this approximate 6–8% loss of total blood volume was not considered an additional risk to the animals.
The dogs were divided into two basic groups; four animals receiving autologous cells containing bromodeoxyuridine (BrdU) as a nuclear marker, the other 14 receiving autologous cells without a nuclear marker. Animals were radiographed to establish a baseline for pre-existing spine pathology. Under general anesthesia, a minimal invasive approach was made to the posterolateral aspect of the canine lumbar spine. Lumbar intervertebral discs at three levels (L1/L2, L2/L3, and L3/L4) were identified as study levels for the procedure and disc tissue was collected. Approximately 200 mg of tissue was collected from the lateral aspect of the annulus, 100 mg of annulus material, and 100 mg of nucleus pulposus material.
The sampled disc cells were expanded in culture through several passages, with a goal of establishing a population of disc cell capable of producing matrix and sustaining an expanded volume within the damaged disc. The average number of cells expanded and transplanted in each L3–L4 disc was approximately 6 million cells. This procedure was done by the Co.don AG Teltow/Germany.
In this study, the L1–L2 intervertebral disc had tissue removed but did not receive chondrocyte transplantation, the L2–L3 disc was approached but not violated and served as a surgical control, and the L3–L4 level had disc material removed and received chondrocyte transplantation 12 weeks later. The wound sites were closed with resorbable suture and the animals returned to their holding area. None of the animals developed problems related to the surgery and all regained full function.
An important criterion for evaluating the success of cell transplantation in the disc repair procedure was identifying that matrix regeneration was attributable to transplanted in culture expanded disc cells rather than a result of inherent disc capacity for self-repair. BrdU, an analog nucleotide of thymidine, was incorporated into the nucleus during DNA synthesis and could later be identified by immunohistochemical techniques. As such, it was possible to analyze morphology in situ after repair, and delineate cells that were transplanted from those already present in the host tissue. To verify the source of disc repair and matrix regeneration, BrdU was used as a cell marker in four animals.
During the last 4 days in monolayer culture, the cells in passage two were tagged by adding a small concentration of BrdU (1:1,000) to the culture medium. To perform growth curves, monolayer cells in passage 1 were cultivated in 6-well plates and the cell number in each well was determined daily. Viability of the cells was assessed by staining with trypan blue.
Twelve weeks after disc tissue had been harvested; the autologous disc cell cell cultures were transplanted at L3–L4 on each of the dogs. The intervertebral disc between L1 and L2 served as the control for untreated degeneration. Cells were shipped from Teltow, Germany, overnight at 4–8°C for transplantation. Animals were anesthetized, placed in right lateral recumbence, and the L3–L4 level was located by fluoroscopic imaging. As the previous surgeries had been performed from the right lateral side, the cultured cells were introduced through the left side of the annulus.
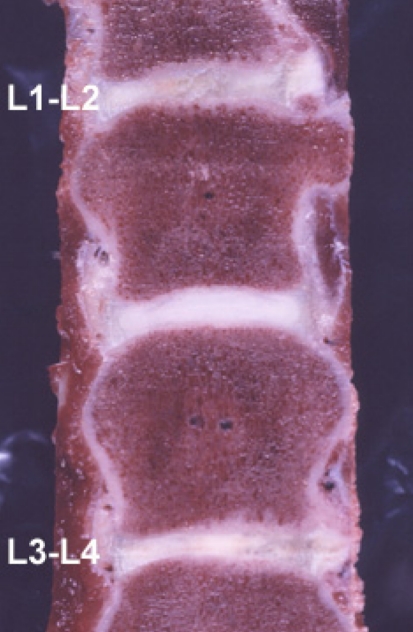
The animals were humanely euthanized 3 months (3 dogs), 6 months (7 dogs), 9 months (4 dogs), and 12 months (4 dogs) following the cell transplantation. Immediately after the dogs were killed, their lumbar spines were removed and the tissue analyzed (Fig. 1), MRI and X-ray analysis and coronal slices of the spinal column were performed to interpreting the disc height.
Fig. 1.
Gross pathology 12 month follow-up after autologous chondrocyte transplantation in the Canine model Level L3–L4 was transplanted, level L1–L2 received no treatment and displayed more scar tissue, L2–L3 was the control level with a normal intervertebral disc
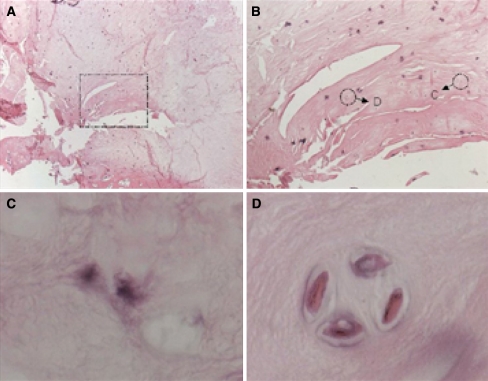
Tissue analyses included light microscopy and immunohistochemistry for assessing BrdU content (Fig. 2) and collagen expression.
Fig. 2.
Staining of paraffin sections of the regenerated intervertebral disc 6 months following cell transplantation. BrdU containing chondrocytes were detected and stained by immunohistochemical procedures using DAB as the chromogen. Sections were counterstained by Eosin. BrdU positive cells are colored black. a Nucleus regenerate overview (25×), b BrdU stained transplanted cells (200×), c, d single BrdU stained transplanted chondrocytes, pericellular de novo synthesis of nucleus matrix (1,000×)
The canine study evaluated whether autologous disc cell transplantation might be an appropriate therapeutic treatment to repair disc damage and inhibit degeneration. In this context, several important observations emerged:
Autologous disc cells were expanded in culture and returned to the disc by a minimally invasive procedure after 12 weeks. Under defined conditions, it was possible to assure phenotype and assess metabolic capacity of the cells prior to transplantation.
Disc cells remained viable after transplantation as shown by BrdU incorporation and maintained a capacity for proliferation after transplantation as depicted by histology.
Transplanted disc cells produced an extracellular matrix that contained components similar to normal intervertebral disc tissue. Positive evidence of proteoglycan content was supported by accepted histochemical staining techniques such as safranin O-Fast Green.
Both Type II and Type I collagens were demonstrated in the regenerated intervertebral disc matrix by immunohistochemistry following chondrocyte transplantation.
There was a statistically significant correlation between transplanting cells and retention of disc height that was demonstrated at longer intervals following transplantation.
Although a morphotypic nucleus pulposus was not generated, cells that could appropriately be considered disc cells were identified in the intervertebral discs that had received disc cell transplantation. The observed matrix to cell ratio suggested strongly that these cells were elaborating a cartilage specific matrix that was appropriate with respect to both collagen and proteoglycan components. No evidence of necrotic change was present, nor were there any active signs of tissue vascularisation. Absence of bone in the intervertebral space, and the productive matrix synthesis suggested that active remodeling and expression were guided by the demands of the anatomy, and that cell response after transplantation was dependent on both phenotypic identity of the cells and the biomechanical cues of the anatomy.
Cell viability and their capacity for matrix synthesis were particularly encouraging outcomes of this study. In the light of a 12-week interval between disc tissue sampling and cell transplantation, cells were placed into an environment that had fundamentally changed in both composition and function. Under the provision of central delivery and pressurized containment, the transplanted cells were primed in the vein of the nucleus pulposus. The high cell to volume ratio of the transplanted cells, the deformable nature of the regional anatomy, and the inherent capacity of the cells to respond to new loading regimens all supported the vitality of the transplant conditions.
Extracellular matrix change, biomechanical variation, altered morphology, and cell viability are acknowledged steps leading to intervertebral disc degeneration. In invigorating the population of vital disc cells and achieving matrix transformation, positive action in addressing the morphology of the disc has been demonstrated. The ability to control cell conditions, potentially to imbue the cells with additional genetic capacity, and the availability of autologous tissue from discectomy procedures make this a technology that is available, effective and attractive.
After these positive and promising results the Euro Disc Randomized Trial was initiated to embrace a representative patient group, examining not only the traumatic, less degenerative disc, but also to include patients with persistent symptoms that had not responded to conservative treatment where an indication for surgical treatment was given.
Euro disc randomized trial
Interventional surgery for disc herniation is one of the most widely used and effective treatments for back pain that emerges within the broad scope of disc degeneration. Successful removal of impinging tissue offers the individual patient substantial relief for associated pain. However, the reduction of tissue involved in the surgical procedure anatomically compromises the function of the affected disc, and affects a load transfer to adjacent discs.
Biological restoration with interventional cell therapy offers a potential for accentuating disc metabolism with an underlying intent to restore spine mechanics.
Included patients having exclusively one level requiring surgical intervention were eligible for participation in the trial; patients requiring treatment at more than one level were excluded from the study. Prior to their participation, all patients were advised of the potential risks and signed a letter of consent. No placebo group was committed to this study; each patient participating in the clinical trial will undergo surgical treatment for their disc prolapse, and the prospective basis of cell transplantation will constitute and separate the active treatment from the control group. Patients were not blinded to their treatment. Randomization was done after the open microdiscectomy. Eligibility was limited to patients between 18 and 60 years of age, with a body mass index (BMI) below 28. Exclusion criteria for participating in the study included sclerotic changes, edema, Modic changes of grade II or III, and spondylolisthesis among other accepted criteria such as pregnancy, etc.
Operative procedure was done as a minimal invasive open sequestrectomy done by an experienced neurosurgeon under general anesthesia. The harvested cells from the sequestered disc material were cultured by the Co.don AG Teltow/Germany under GMP conditions. In the solution for the transplantation are more than 5 million living disc cells included.
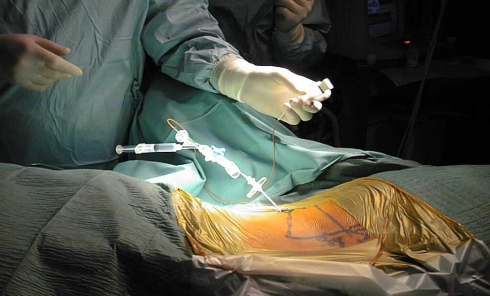
A single puncture with a minimal caliber cannula was used to obtain a precise delivery with minimal trauma to the patient and to the annulus (Fig. 3). The technique was developed with respect to literature that has demonstrated a size-specific correlation of annular injury to disc degeneration. A simple, minimally-invasive technique was necessary to reduce the wound site trauma and effectively support cell injection without further injury to the annulus. Cells are transplanted approximately 12 weeks following sequestrectomy to assure that the annulus has healed and will contain the cells. Using a pressure–volume test prior to the delivery of any chondrocytes, cells could be placed with confidence that they would be retained at the site of delivery.
Fig. 3.
Intraoperative picture of the fluoroscopic guided minimal invasive puncture of the intervertebral disc form the opposite side, pressure–volume-test and transplantation
One hundred and twelve patients have been enrolled in the Euro Disc Study; the primary criteria follow-up was intended to occur at 1 year, an interim analysis scheduled at 2 years, and the final analysis will be completed at 4 years. The primary clinical evaluation criterion is the Oswestry Low Back Pain Disability Questionnaire. Secondary criteria include the SF-36, Prolo Score [26], Quebec Back Pain Disability Scale, MRI, and X-ray evaluation. Use of the Oswestry disability questionnaire in clinical trials is recommended by the German Orthopedic Society (DGOT); demonstrating acceptable test quality and satisfactory test–retest reliability. The Quebec back pain disability scale, another self-rating scale, was professionally developed using factor analysis comprising with high internal consistency, high item discriminability, and high test-retest reliability. Finally, the SF-36, an often used scale to assess patients’ general condition and quality of life, and a VAS will be used to standardize measurable pain.
An interim analysis, made by a cut in January of 2006 to assess whether intervention was correlated with positive clinical outcomes, forms the basis for this report. Within the analysis, successive 3-month, 6-month, 12-month, and 24-month assessments are stratified within the continuum of study. The information within this study allows a broad interpretation of the general progress made over 2 years following a clinical intercession with autologous disc cells. Interim analysis was performed on the first 28 patients who reached 24 months follow-up to the autologous disc cell transplantation (ADCT). These first 28 patients were randomized in three different centers.
For descriptive analysis of efficacy, the total sum score as well as the disability index of the Oswestry Low Back Pain Disability Questionnaire (OPDQ) and the total sum score of the Quebec Back-Pain Disability Scale (QBPD) were taken into account from the initial presurgical presentation through the 2-year follow-up. The outcomes are depicted in (Table 1). Based on the mean total sum score as well as the disability index of the OPDQ, differences in initial presentations between the control group and those receiving autologous cells were not minimal. Surgery as an intervention was a positive experience, and as expected substantially reduced the patient’s disability and pain. The trend in reduction of the total sum score continued to decrease in the patients whose treatment was supplemented by cell transplantation, while the control group did not sustain continual improvement. At V4, 2 years following the therapeutic intervention with cells, both the total sum score as well as the disability index of the OPDQ were plainly lower in the ADCT group compared with the control.
Table 1.
Total sumscore and disability Index of the OPDQ based on patients who had been followed for 2 years after autologous disc chondrocyte transplantation
| N | Mean | SD | Min. | Lower quartile | Median | Upper quartile | Max. | ||
|---|---|---|---|---|---|---|---|---|---|
| Total sumscore | |||||||||
| Visit −1 | |||||||||
| ADCT | 12 | 28.42 | 9.30 | 13.00 | 20.00 | 29.50 | 36.00 | 45.00 | |
| Control | 16 | 26.88 | 9.99 | 14.00 | 18.00 | 25.50 | 34.00 | 46.00 | |
| Visit 0.5 | |||||||||
| ADCT | 12 | 8.00 | 6.89 | 0.00 | 2.50 | 7.50 | 12.50 | 24.00 | |
| Control | 15 | 8.40 | 4.69 | 1.00 | 4.00 | 9.00 | 13.00 | 15.00 | |
| Visit 1 | |||||||||
| ADCT | 11 | 6.73 | 8.56 | 0.00 | 0.00 | 5.00 | 12.00 | 28.00 | |
| Control | 14 | 7.14 | 6.36 | 0.00 | 1.00 | 5.50 | 13.00 | 19.00 | |
| Visit 2 | |||||||||
| ADCT | 10 | 9.10 | 10.72 | 0.00 | 1.00 | 6.50 | 12.00 | 35.00 | |
| Control | 14 | 7.79 | 7.42 | 0.00 | 2.00 | 6.50 | 12.00 | 26.00 | |
| Visit 3 | |||||||||
| ADCT | 11 | 7.82 | 8.46 | 0.00 | 2.00 | 4.00 | 15.00 | 25.00 | |
| Control | 14 | 7.07 | 5.94 | 0.00 | 1.00 | 7.00 | 12.00 | 19.00 | |
| Visit 4 | |||||||||
| ADCT | 12 | 6.00 | 8.89 | 0.00 | 0.00 | 2.00 | 8.50 | 29.00 | |
| Control | 16 | 7.56 | 6.52 | 0.00 | 2.50 | 6.00 | 13.00 | 19.00 | |
| Disability index (%) | |||||||||
| Visit −1 | |||||||||
| ADCT | 12 | 56.83 | 18.60 | 26.00 | 40.00 | 59.00 | 72.00 | 90.00 | |
| Control | 16 | 53.75 | 19.97 | 28.00 | 36.00 | 51.00 | 68.00 | 92.00 | |
| Visit 0.5 | |||||||||
| ADCT | 12 | 16.06 | 13.73 | 0.00 | 5.33 | 15.00 | 25.00 | 48.00 | |
| Control | 15 | 16.80 | 9.37 | 2.00 | 8.00 | 18.00 | 26.00 | 30.00 | |
| Visit 1 | |||||||||
| ADCT | 11 | 13.45 | 17.11 | 0.00 | 0.00 | 10.00 | 24.00 | 56.00 | |
| Control | 14 | 14.29 | 12.72 | 0.00 | 2.00 | 11.00 | 26.00 | 38.00 | |
| Visit 2 | |||||||||
| ADCT | 10 | 18.64 | 21.53 | 0.00 | 2.00 | 13.89 | 26.67 | 70.00 | |
| Control | 14 | 15.62 | 14.80 | 0.00 | 4.44 | 13.00 | 24.00 | 52.00 | |
| Visit 3 | |||||||||
| ADCT | 11 | 15.64 | 16.92 | 0.00 | 4.00 | 8.00 | 30.00 | 50.00 | |
| Control | 14 | 14.14 | 11.88 | 0.00 | 2.00 | 14.00 | 24.00 | 38.00 | |
| Visit 4 | |||||||||
| ADCT | 12 | 12.00 | 17.79 | 0.00 | 0.00 | 4.00 | 17.00 | 58.00 | |
| Control | 16 | 15.19 | 12.99 | 0.00 | 5.50 | 12.00 | 26.00 | 38.00 | |
Visit −1, sequestrectomy; visit 0.5, ADCT/Control; visit 1, 3 months after ADCT/Control visit 0.5; visit 2, 6 months after ADCT/Control visit 0.5; visit 3, 12 months after ADCT/Control visit 0.5; visit 4, 24 months after ADCT/Control visit 0.5
Descriptive analyses of the mean total sum score of the QBPD prior to sequestrectomy, prior to ADCT/control, and 3 months after ADCT/control demonstrated a decrease in mean and median sum scores in both groups. Although the mean and median values for both the ADCT and the control group decreased between 1 (V3) and 2 years (V4), the assessments for the ADCT group were clearly lower (Table 2). Patient global assessment of pain demonstrated some fluctuation although both groups received substantial relief from the surgical intervention. However, as patients were tracked over the course of the V4, or 2-year follow-up, changes emerged that suggest that the ADCT-treated patients have a lower assessment of their pain (Table 3).
Table 2.
Total sumscore of the QBPD based on patients with at least 2 years follow-up after autologous disc chondrocyte transplantation
| N | Mean | SD | Min. | Lower quartile | Median | Upper quartile | Max. | ||
|---|---|---|---|---|---|---|---|---|---|
| Total sumscore | |||||||||
| Visit −1 | |||||||||
| ADCT | 12 | 45.08 | 17.60 | 23.00 | 31.50 | 42.00 | 55.00 | 82.00 | |
| Control | 16 | 46.69 | 18.69 | 21.00 | 34.00 | 45.00 | 65.00 | 81.00 | |
| Visit 0.5 | |||||||||
| ADCT | 12 | 14.75 | 16.07 | 0.00 | 4.50 | 8.50 | 17.50 | 50.00 | |
| Control | 15 | 18.27 | 11.04 | 1.00 | 6.00 | 19.00 | 25.00 | 38.00 | |
| Visit 1 | |||||||||
| ADCT | 11 | 10.64 | 16.05 | 0.00 | 1.00 | 4.00 | 15.00 | 55.00 | |
| Control | 14 | 13.29 | 9.72 | 3.00 | 6.00 | 8.50 | 24.00 | 30.00 | |
| Visit 2 | |||||||||
| ADCT | 10 | 15.00 | 20.77 | 0.00 | 1.00 | 10.00 | 19.00 | 70.00 | |
| Control | 14 | 13.93 | 11.76 | 1.00 | 4.00 | 12.50 | 18.00 | 41.00 | |
| Visit 3 | |||||||||
| ADCT | 11 | 11.09 | 16.71 | 0.00 | 2.00 | 4.00 | 19.00 | 57.00 | |
| Control | 14 | 12.71 | 12.55 | 2.00 | 4.00 | 9.50 | 17.00 | 48.00 | |
| Visit 4 | |||||||||
| ADCT | 12 | 9.33 | 15.33 | 0.00 | 0.50 | 3.50 | 12.50 | 55.00 | |
| Control | 16 | 13.94 | 12.61 | 0.00 | 5.00 | 8.00 | 22.50 | 41.00 | |
Visit −1, sequestrectomy; visit 0.5, ADCT/Control; visit 1, 3 months after ADCT/Control visit 0.5; visit 2, 6 months after ADCT/Control visit 0.5; visit 3, 12 months after ADCT/Control visit 0.5; visit 4, 24 months after ADCT/Control visit 0.5
Table 3.
Global assessment of pain based on patients with at least 2 years follow-up after autologous disc chondrocyte transplantation
| N | Mean | SD | Min. | Lower quartile | Median | Upper quartile | Max. | |
|---|---|---|---|---|---|---|---|---|
| Global assessment of pain (100 mm VAS) | ||||||||
| Visit −1 | ||||||||
| ADCT | 11 | 59.45 | 22.76 | 15.00 | 48.00 | 60.00 | 76.00 | 96.99 |
| Control | 16 | 57.31 | 28.51 | 0.00 | 27.00 | 70.00 | 79.50 | 88.98 |
| Visit 0.5 | ||||||||
| ADCT | 12 | 19.17 | 19.37 | 0.00 | 2.50 | 13.00 | 31.50 | 65.00 |
| Control | 15 | 17.20 | 14.70 | 0.00 | 3.00 | 14.00 | 31.00 | 46.00 |
| Visit 1 | ||||||||
| ADCT | 11 | 12.82 | 19.37 | 0.00 | 0.00 | 3.00 | 24.00 | 61.99 |
| Control | 14 | 14.36 | 10.59 | 1.00 | 4.00 | 15.00 | 22.00 | 33.00 |
| Visit 2 | ||||||||
| ADCT | 10 | 21.00 | 22.85 | 0.00 | 8.00 | 16.50 | 23.00 | 78.99 |
| Control | 14 | 14.00 | 16.51 | 1.00 | 2.00 | 5.50 | 19.00 | 51.00 |
| Visit 3 | ||||||||
| ADCT | 11 | 18.00 | 18.73 | 2.00 | 3.00 | 9.00 | 25.00 | 56.00 |
| Control | 14 | 15.07 | 12.16 | 0.00 | 3.00 | 12.00 | 29.00 | 37.00 |
| Visit 4 | ||||||||
| ADCT | 12 | 11.17 | 13.48 | 0.00 | 1.00 | 5.00 | 17.00 | 39.00 |
| Control | 16 | 15.62 | 15.16 | 1.00 | 3.00 | 12.50 | 26.50 | 53.99 |
Visit −1, sequestrectomy; visit 0.5, ADCT/Control; visit 1, 3 months after ADCT/Control visit 0.5; visit 2, 6 months after ADCT/Control visit 0.5; visit 3, 12 months after ADCT/Control visit 0.5; visit 4, 24 months after ADCT/Control visit 0.5
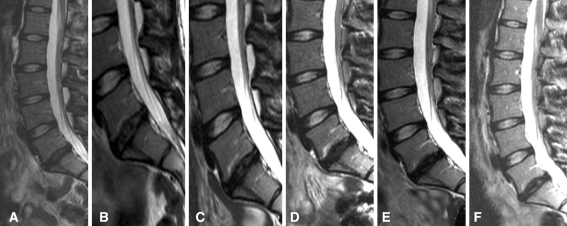
MRI was used to assess the respective disc height along the course of the analyses from the date of the sequestrectomy until the 2-year follow-up (Fig. 4). In addition to the disc height, the content of the liquid component was evaluated as a means of assessing matrix content. Results of the analysis of the inter-vertebral disc height compared affected (treated with surgery, or with surgery and cells) with non-affected adjacent segments in the same patients, and also measured the relative vertebral heights as a means of assessing patient demographics and morphologic variation. Comparison of the mean inter-vertebral disc heights and the vertebral heights revealed no differences between the groups.
Fig. 4.
28 years old woman undergoing discectomy in level L5–S1. The 60-month follow-up in the MRI displays the stable disc high in the transplanted level L5–S1. a Pre transplantation, b 1 day post transplantation, c 3-month post transplantation, d 12-month post transplantation, e 24-month post transplantation, f 60-month post transplantation
An analysis of fluid content of the inter-vertebral disc at each visit demonstrated that more than 80% of the affected segments showed decreased hydration 3 months (V1) following surgery (Table 4). In general, the proportion of affected segments with a decreased content of liquid decreased over the course of the trial. Of particular interest was the outcome at 2 years, where the ADCT treated group showed a substantially higher normalization as a group; 41% normal fluid content compared with only 25% normal content in the control group. Perhaps most interesting of all the data to emerge from this study comes from inspecting discs either one, or two segments from the treated intervertebral disc. Fluid levels at both of these segments showed a substantially higher percentage of normal fluid content despite the fact that they were away from the surgical intervention site.
Table 4.
Analysis of fluid content of the inter-vertebral disc with at least 2 years follow-up after autologous disc chondrocyte transplantation
| N | Affected segment | Non-affected segment | Non-affected segment | ||||
|---|---|---|---|---|---|---|---|
| Normal (%) | Decreased (%) | Normal (%) | Decreased (%) | Normal (%) | Decreased (%) | ||
| Content of liquid | |||||||
| Visit −1 | |||||||
| ADCT | 12 | 16.67 | 83.33 | 83.33 | 16.67 | 83.33 | 16.67 |
| Control | 15 | 13.33 | 86.67 | 86.67 | 13.33 | 46.67 | 53.33 |
| Visit 0.5 | |||||||
| ADCT | 12 | 25.00 | 75.00 | 81.82a | 18.18a | 50.00 | 50.00 |
| Control | 14 | 0.00 | 100.0 | 78.57 | 21.43 | 28.57 | 71.43 |
| Visit 3 | |||||||
| ADCT | 11 | 27.27 | 72.73 | 90.91 | 9.09 | 63.64 | 36.36 |
| Control | 13 | 23.08 | 76.92 | 76.92 | 23.08 | 53.85 | 46.15 |
| Visit 4 | |||||||
| ADCT | 12 | 41.67 | 58.33 | 91.67 | 8.33 | 66.67 | 33.33 |
| Control | 16 | 25.00 | 75.00 | 86.67b | 13.33b | 56.25 | 43.75 |
Visit −1, sequestrectomy; visit 0.5, ADCT/Control; visit 3, 12 months after ADCT/Control visit 0.5; visit 2, 6 months after ADCT/Control visit 0.5; visit 3: 12 months after ADCT/Control visit 0.5; visit 4, 24 months after ADCT/Control visit 0.5
aOnly 11 values available
bOnly 15 values available
This first interims analysis of the Euro Disc study evaluated that:
Disc cells that had been removed as a normal part of sequestrectomy could be expanded in culture under GMP conditions and returned to the patient after the annulus had been allowed to heal for 12 weeks.
Disc cell transplantation could be delivered by the percutaneous technique.
Patients who received autologous disc cell transplantation had greater pain reduction at 2 years compared with patients who did not receive cells following their discectomy surgery.
Discs in patients that received cells demonstrated a significant difference as a group in the fluid content of their treated disc when compared to control.
Adjacent intervertebral discs, both at 1 level or 2 levels from the intervertebral disc that received the cell therapy also demonstrated a difference in fluid content.
The results of this study are encouraging from several perspectives; first to the fact that the morphologic outcomes mirrored that seen in our pre-clinical animal study [12]; and second that the pain relief seen in the pilot study which served as a basis for this clinical trial was sustained for the course of this 2-year interim analysis. This gives cause to the success of the cell-based intervention.
In clinical use chondrocytes are only limited available. To develop prophylactic options for disc regeneration other cell lines are necessary.
Adipose tissue provides an alternative source of regenerative cells with little donor site morbidity. These regenerative cells are able to differentiate into a nucleus pulposus-like phenotype when exposed to environmental factors similar to disc, and offer the inherent advantage of availability without the need for transporting, culturing, and expanding the cells [30, 37].
Canine trial of adipose-derived regenerative cell transplantation
In an effort to develop a clinical option for cell placement and assess the response of the cells to the post-surgical milieu, adipose-derived cells were collected, concentrated, and transplanted under fluoroscopic guidance directly into a surgically damaged disc.
For this study 12 dogs, 2 years of age, were obtained. Adipose cells were harvested from the super-scapular region of the neck (scruff) and adherent cells separated, collected, and labeled with DAPI. Adipose tissue has been known for some time to contain regenerative cells in addition to fat cells [15].Three lumbar intervertebral disc levels in each dog underwent a partial nucleotomy; other levels served as non-operated controls. Levels of intervention as well as the regimen of treatment were dually randomized. Three interventions were used in this study; adipose-derived cells in hyaluronic acid (HA) carrier, HA alone, or no intervention—all deliveries were guided by fluoroscopy. Assessments were made by MRI, radiography, microscopy, RT-PCR, and ELISA.
A total of six dogs were radiographed, received MRI scans (Fig. 5) and then were euthanized by 6 months. The disc tissue was harvested from the lumbar spine in each dog (Fig. 6). Cells were seen to be viable in the tissue (Fig. 7). Matrix composition was assessed; assays were made of aggrecan, Types I and II collagen by both RT-PCR and ELISA to assess and compare matrix regeneration. mRNA and protein from each level were presented with respect to normal values defined as the 100% expression (Table 5).
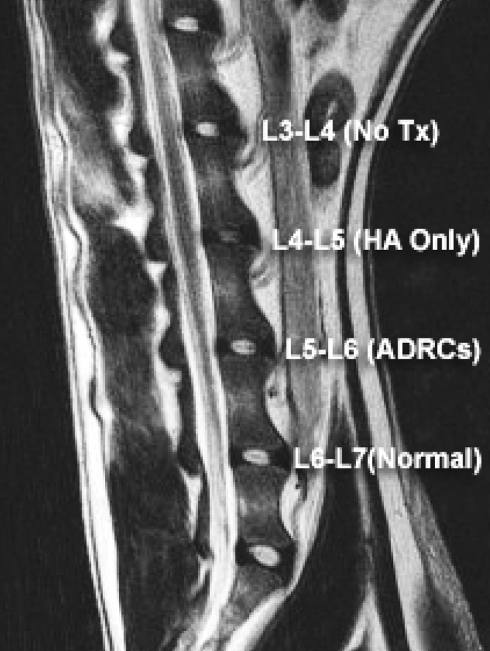
Fig. 5.
6-month follow-up with MRI sagittal section (same dog as in Fig. 2)
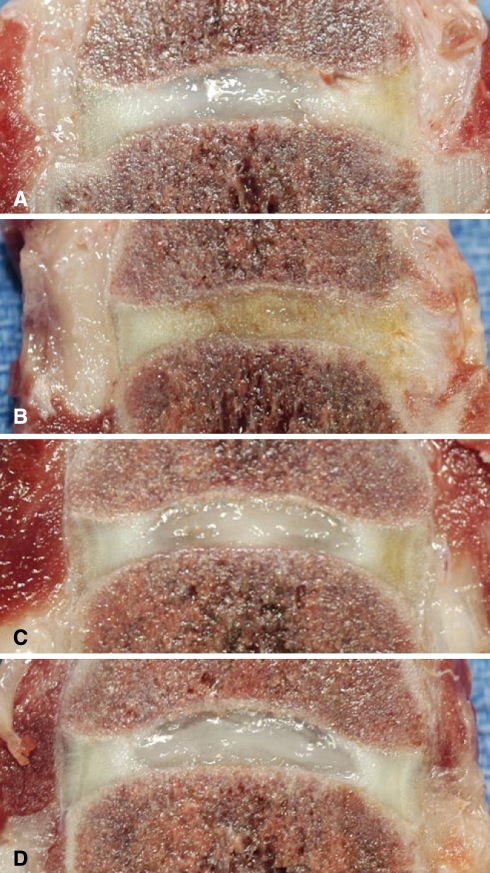
Fig. 6.
6-month follow-up gross section pathology a L3–L4 no treatment, b L4–L5 hyaluronic acid alone, c L5–L6 adipose-derived stem cells in hyaluronic acid, d L6–L7 normal disc
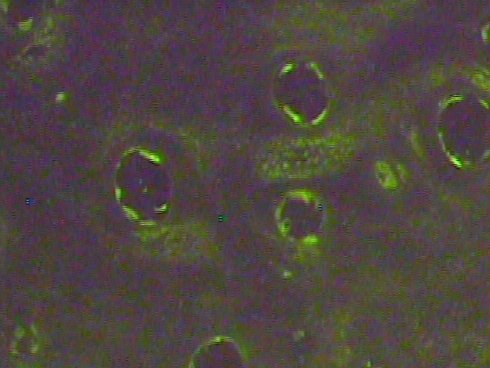
Fig. 7.
Cell viability and cell sustenance were judged positive by DAPI-stained nuclei that were present 6-month following transplantation of the adipose-derived stem cells
Table 5.
Relative mRNA for specific matrix proteins—comparison between treatments
| Control | Hyaluronic acid alone | Adipose-derived stem cells with hyaluronic acid | No intervention | |
|---|---|---|---|---|
| Aggrecan | 100 | 43.6 | 85.6 | 37.9 |
| Type-I Col | 100 | 73.2 | 87.1 | 67.2 |
| Type-II Col | 97.5 | 41.5 | 82.8 | 41.35 |
Table 6 depicts the relative protein levels as measured by ELISA.
Table 6.
Relative protein for specific matrix proteins—comparison between treatments
| Control | Hyaluronic acid alone | Adipose-derived stem cells with hyaluronic acid | No intervention | |
|---|---|---|---|---|
| Aggrecan | 100 | 62.3 | 83.0 | 58.4 |
| Type-I Col | 97.7 | 74.9 | 88.4 | 71.2 |
| Type-II Col | 99 | 55 | 85 | 53 |
The data were calculated with two samples t-test, comparing the control with interventions at P < 0.05 and P < 0.01. Statistical differences were found between the control and each intervention at P < 0.01, whereas the difference between control and HA pluscells was only significant at P < 0.05. No significant difference could be shown between HA alone and No Intervention. These evaluations and other morphometric assessments support:
Cells viability follows implantation.
- Supplementing adipose cells following injury supports regeneration.
- Morphology was maintained.
- Intervertebral disc height was not lost.
- MRI signal remained similar to native control.
Hyaluronic acid was insufficient to prevent disc degeneration or desiccation.
Lack of intervention resulted in progressive degeneration.
A limited nucleotomy procedure, similar to that which would be experienced following clinical micro-discectomy, resulted in prolapsed annulus tissue into the central space of the nucleus pulposus.
No significant regeneration of cells or matrix occurred without treatment.
The study will be finalized with a 12-month follow-up data in another six dogs.
These first results of the study provide evidence that cells harvested from adipose tissue might offer a reliable source of regenerative potential capable of bio-restitution. Key strengths make the case for using adipose-derived cells; first, cells can be transplanted percutaneously; and second cells survive and functionally adapt and produce appropriate matrix. The span of this study was sufficient to show that freshly isolated cells will survive the trauma associated with post-surgical inflammation. The time to treat, the cell carrier, and the ability of the cells to integrate into the disc matrix were all certainly convincing.
Summary
Cell transplantation in degenerative disc disease is possible.
Autologous disc cell transplantation after sequestrectomy is a save and technical feasible procedure. Transplanted chondrocytes are viable in situ and create a functional matrix.
The interims results of the Euro DISC study give strong evidence for the safety and efficiency of the disc-derived cell transplantation applied following sequestrectomy to delay or inhibit ongoing processes of disc degeneration. After transplantation a statistically significant decrease in OPDQ, QBPD and VAS in ADCT treated patients for disability and pain is measured. In the MRI-analysis less decreased liquid content in affected intervertebral discs in the ADCT group could be displayed. A statement for the ideal group of patients who profits most from the autologous disc cell transplantation is at this time not possible.
The technique of autologous disc cell transplantation is only possible for patients who underwent a sequestrectomy. It might be better to transplant disc cells in earlier stage of degeneration without any loose of matrix from the intervertebral disc, but for harvesting cell material to culture disc cells an operation is necessary. But for an operative intervention only to harvest cell material form a beginning degenerative intervertebral disc for culture procedure with the risk of infection of the disc and the bone, the risk of nerve root injury and its following problems for the patient there is no ethical or medical foundation.
For patients with signs of disc degeneration in the MRI concerning to the Pfirrmann classification 1–3 [25] and a root nerve compression by sequestered nucleus pulposus prolapse which need to be treated by minimal invasive operative procedure we discuss the possibility of autologous disc cell transplantation.
The following study provide evidence that adipose-derived stem and regenerative cells can be transplanted at surgery using fluoroscopic guidance, and that these cells can be injected directly into the intervertebral disc with the expectation that they will remain viable and produce appropriate, tissue-specific matrix.
If the future results of the adipose-derived stem and regenerative cell study present the good expectations we have a very save and easy technique producing cells for minimal invasive transplantation into a degenerative intervertebral disc without open operative intervention.
Conflict of interest statement
None of the authors has any potential conflict of interest.
References
- 1.Annunen S, Paassilta P, Lohiniva J, Perala M, Pihlajamaa T, Karppinen J, Tervonen O, Kroger H, Lahde S, Vanharanta H, Ryhanen L, Goring HH, Ott J, Prockop DJ, Ala-Kokko L. An allele of COL9A2 associated with intervertebral disc disease. Science. 1999;285:409–412. doi: 10.1126/science.285.5426.409. [DOI] [PubMed] [Google Scholar]
- 2.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beard HK, Roberts S, O’Brien JP. Immunofluorescent staining for collagen and proteoglycan in normal and scoliotic intervertebral discs. J Bone Joint Surg Br. 1981;63B:529–534. doi: 10.1302/0301-620X.63B4.6170646. [DOI] [PubMed] [Google Scholar]
- 4.Beard HK, Ryvar R, Brown R, Muir H. Immunochemical localization of collagen types and proteoglycan in pig intervertebral discs. Immunology. 1980;41:491–501. [PMC free article] [PubMed] [Google Scholar]
- 5.Bibby SR, Jones DA, Lee RB, Yu J, Urban JPG. The pathophysiology of the intervertebral disc. Joint Bone Spine. 2001;68:537–542. doi: 10.1016/S1297-319X(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 6.Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:1178–1184. [PubMed] [Google Scholar]
- 7.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Doers TM, Kang JD. The biomechanics and biochemistry of disc degeneration. Curr Opin in Orthop. 1999;10:117–121. doi: 10.1097/00001433-199904000-00006. [DOI] [Google Scholar]
- 9.Eyre DR. Collagens of the disc. In: Ghosh P, editor. The Biology of the intervertebral disc. Boca Raton: CRC Press; 1988. pp. 171–188. [Google Scholar]
- 10.Flynn JC, Hoque MA. Anterior fusion of the lumbar spine. End-result study with long-term follow-up. J Bone Joint Surg Am. 1979;61:1143–1150. [PubMed] [Google Scholar]
- 11.Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, Hutton WC. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine. 2003;28:2609–2620. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 12.Ganey TM, Meisel HJ. A potential role for cell-based therapeutics in the treatment of intervertebral disc herniation. Eur Spine J. 2002;11(Suppl 2):S206–S214. doi: 10.1007/s00586-002-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruber HE, Hanley EN., Jr Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine. 1998;23:751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 14.Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G, Banks D, Phieffer L, Coldham G, Hanley EN., Jr Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine. 2002;27:1626–1633. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 15.Katz AJ, Llull R, Hedrick MH, Futrell JW. Emerging approaches to the tissue engineering of fat. Clin Plast Surg. 1999;26:587–603. [PubMed] [Google Scholar]
- 16.Kawaguchi Y, Osada R, Kanamori M, Ishihara H, Ohmori K, Matsui H, Kimura T. Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine. 1999;24:2456–2460. doi: 10.1097/00007632-199912010-00006. [DOI] [PubMed] [Google Scholar]
- 17.Lauerman WC, Bradford DS, Ogilvie JW, Transfeldt EE. Results of lumbar pseudarthrosis repair. J Spinal Disord. 1992;5:149–157. doi: 10.1097/00002517-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Malko JA, Hutton WC, Fajman WA. An in vivo MRI study of the changes in volume (and fluid content) of the lumbar intervertebral disc after overnight bed rest and during an 8-hour walking protocol. J Spinal Disord Tech. 2002;15:157–163. doi: 10.1097/00024720-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–130. [PMC free article] [PubMed] [Google Scholar]
- 20.Nerlich AG, Schleicher ED, Boos N. 1997 Volvo Award winner in basic science studies. Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine. 1997;22:2781–2795. doi: 10.1097/00007632-199712150-00001. [DOI] [PubMed] [Google Scholar]
- 21.Norwig J, Josimovic-Alasevic O, Fritsch KG, Steinof K, Siodla W. Integrated isolator technology-based sterile production of cell-based drugs. Pharm Ind. 2000;63:780–784. [Google Scholar]
- 22.Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res. 2000;18:988–997. doi: 10.1002/jor.1100180620. [DOI] [PubMed] [Google Scholar]
- 23.Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine. 1990;15:762–767. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- 24.Paassilta P, Lohiniva J, Goring HH, Perala M, Raina SS, Karppinen J. Identification of a common risc factor for lumbar disk disease. JAMA. 2000;285:1843–1849. doi: 10.1001/jama.285.14.1843. [DOI] [PubMed] [Google Scholar]
- 25.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 26.Prolo DJ, Oklund SA, Butcher M. Toward uniformity in evaluating results of lumbar spine operations. A paradigm applied to posterior lumbar interbody fusions. Spine. 1986;11:601–606. doi: 10.1097/00007632-198607000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Roberts S, McCall IW, Menage J, Haddaway MJ, Eisenstein SM. Does the thickness of the vertebral subchondral bone reflect the composition of the intervertebral disc? Eur Spine J. 1997;6:385–389. doi: 10.1007/BF01834064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts S, Menage J, Duance V, Wotton SF. Type III collagen in the intervertebral disc. Histochem J. 1991;23:503–508. doi: 10.1007/BF01041176. [DOI] [PubMed] [Google Scholar]
- 29.Steinmann JC, Herkowitz HN (1992) Pseudarthrosis of the spine. Clin Orthop Relat Res 284:80–90 [PubMed]
- 30.Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Hurtig M, Pilliar RM, Grynpas M, Kandel RA. Characterization of nucleus pulposus-like tissue formed in vitro. J Orthop Res. 2001;19:1078–1084. doi: 10.1016/S0736-0266(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 32.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Videman T, Leppavuori J, Kaprio J, Battie MC, Gibbons LE, Peltonen L, Koskenvuo M. Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine. 1998;23:2477–2485. doi: 10.1097/00007632-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Waddell G, Kummel EG, Lotto WN, Graham JD, Hall H, McCulloch JA. Failed lumbar disc surgery and repeat surgery following industrial injuries. J Bone Joint Surg Am. 1979;61:201–207. [PubMed] [Google Scholar]
- 35.West JL, 3rd, Bradford DS, Ogilvie JW. Results of spinal arthrodesis with pedicle screw-plate fixation. J Bone Joint Surg Am. 1991;73:1179–1184. [PubMed] [Google Scholar]
- 36.Wu JJ, Eyre DR, Slayter HS. Type VI collagen of the intervertebral disc. Biochemical and electron-microscopic characterization of the native protein. Biochem J. 1987;248:373–381. doi: 10.1042/bj2480373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuk PA, Zhu M, Ashjian P, Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]