Abstract
Disc degeneration is deeply associated with many spinal disorders and thus has a significant clinical impact on society. The currently available surgical treatment often necessitates removing a pathological disc and spinal fusion. However, it is also well known that these surgical treatments have many potential problems including invasion and cost. Therefore, biological approaches for regenerating these pathological discs have received much attention. Gene therapy is one of these biological approaches. Gene therapy involves the transfer of genes to cells so the recipient cells express these genes and thereby synthesize the RNA and protein they encode in a continuous fashion. One of the significant advantages of gene therapy is that we can expect a lasting duration of biological effect which is potentially beneficial for most disc degeneration associated disorders, as they are, by nature, chronic conditions. Originally, gene therapy was mediated by viral vectors, but recent technological progress has enabled us to opt for non-virus-mediated gene therapy for the disc. Furthermore, the development of the RNA interference technique has enabled us to down-regulate a specific gene expression in the disc opening the door for a new generation of intradiscal gene therapy.
Keywords: Disc degeneration, Gene therapy, Non-viral vectors, RNAi
Introduction
As society continues to age, intervertebral disc degeneration and associated spinal disorders including so-called discogenic low back pain present a growing problem. Once the degeneration process starts, it is difficult to stop or reverse it using currently available techniques due to the limited ability of disc tissue to regenerate [4, 14]. Accordingly, current methods for treating degenerative disc diseases often necessitate removing the pathological disc and spinal fusion with or without instrumentation. Although the use of instrumentation for spinal fusion decreases the possibility of pseudo-union, a number of problems including breakage or loosening of the instruments have been reported [3, 44]. Furthermore, recent reports have shown an acceleration of disc degeneration adjacent to fused segments, which is considered another formidable problem [1, 8, 13, 22]. Semi-rigid fixation of functional spinal units [2, 10] or insertion of artificial intervertebral discs [18, 24] are recently becoming more readily available techniques, which may provide better results in minimizing the problems of adjacent disc diseases. However, these new methods still incur large costs and necessitate relatively major surgery using expensive artificial implants. Therefore, biological approaches for regenerating the degenerated disc are currently receiving much attention.
Basically, these biological approaches for disc regeneration can be divided into three major groups: 1) to inject growth factors with or without using a carrier, 2) to use cells (including so-called stem cells) with or without a scaffold, and 3) to genetically modify intradiscal gene expression via gene therapy. This review paper focuses on the potential applications of gene therapy in the treatment of spinal disorders, particularly those disorders associated with disc degeneration.
What is gene therapy and why is it needed?
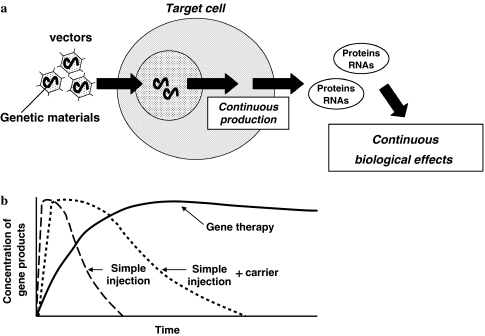
Gene therapy is usually defined as the use of nucleic acid transfer, either RNA or DNA, to treat or prevent a disease. Diseases targeted for treatment by gene therapy have traditionally been inheritable, classic genetic disorders many of which are fatal. However, recent technical advances have suggested the potential use of gene therapy for other type of diseases including acquired chronic disorders. Since gene therapy proposes transfer of “the gene of interest (Table 1)” into the target cells using a so-called vector, once the transferred gene is successfully operating in the target cells, these genetically modified cells can produce the desired gene products (RNAs or proteins) in a continuous fashion (Fig. 1a). Thus, one of the significant advantages of the gene therapy approach is that we can expect a longer-lasting effect compared with a single injection of RNAs or proteins to the target organ (Fig. 1b). The disc degeneration process and its associated disorders are obviously chronic conditions and accordingly are good candidates for gene therapy. However, when we take the complex technical requirements of the gene therapy approach into consideration, a simple injection of proteins with or without their carriers might be more beneficial for acute or sub-acute diseases including injury associated disorders.
Table 1.
Reported genetic materials
| Growth (anabolic) factors |
| TGF-β1 (transforming growth factor-β1) [28, 33] |
| GDF-5 (growth and differentiation factor-5) [6] |
| BMP-2 (bone morphogenetic proteins-2) [28] |
| Transcriptional factors |
| Sox-9 (Sry-type high-mobility group box transcription factor-9 [36]) |
| Inhibitors |
| IL-1Ra (interleukin-1 receptor anatagonist) [23] |
| TIMPs (tissue inhibitors of metalloproteinases) [41] |
| Others |
| hTERT (human telomerase reverse transcriptase) [5] |
Fig. 1.
Schematic representation of gene therapy. a Gene therapy proposes a transfer of “the gene of interest” into the target cells using a so-called vector. Once the transferred gene works successfully in the target cells, these genetically modified cells can produce the desired gene products (RNAs or proteins) in a continuous fashion. b One of the significant advantages of the gene therapy approach is that we can expect a longer-lasting effect compared with single injection of RNAs or proteins to the target organ. This characteristic of gene therapy would make it suitable for treating chronic diseases such as disc degenerated diseases
For successful gene therapy, there are some technical issues that need consideration. Usually genetic materials are not well received by cells; therefore, vehicles known as vectors are required to deliver the gene of interest into the target cells. There are two different types of vectors, viral vectors and non-viral vectors. Viruses are very efficient vectors because their entry into cells and the subsequent expression of their genes is part of the natural viral life cycle; thus much gene therapy research has used viral vectors; intradiscal gene therapy field, retrovirus [43], adenovirus [32], adeno-associated virus [19], and baculovirus [25] have been reported. Although these viral vectors have been shown to be basically safe, safety concerns remain. For this reason, non-viral vectors, which have a much better safety profile, have been receiving much attention [12]. However, the biggest disadvantage of non-virus-mediated gene transfer is that the transfection efficiency of non-viral vectors is usually significantly lower compared with viral vectors. Recently, the ultrasound transfection method with micro-bubbles significantly enhanced transfection efficiency of plasmid DNA into the nucleus pulposus cells in vivo. [35].
Other factors which should be considered are how to deliver these genetic materials to the target organ. One method is to deliver the gene of interest directly into the cells of the target organ in vivo. This method is called in vivo or direct gene transfer. Another method is to harvest and culture cells from the host, deliver the gene of interest into these cultured cells in vitro, and finally reimplant these genetically modified cells into the target organ. This method is called in vitro or indirect gene transfer. Additionally, delivering genes into cells of specific organ is called local gene therapy and delivering genes to a broad area of the body or the whole body is known as systemic gene therapy. The choice of method depends on the character of the target disease, and the anatomical or physiological properties of the target organ. For example, the disc is a good candidate for local, direct gene therapy, since the disc is well encapsulated, and as avascular tissue and degeneration usually occur in a specific disc. Furthermore, avascular tissue inside the disc leads to a biologically harsh environment, which makes it difficult for implanted cells to survive meaning, in vitro, indirect gene transfer to the disc would be less likely to succeed.
Virus-mediated gene therapy
This gene transfer approach to the intervertebral disc was first developed using viral vectors. The University of Pittsburgh Group is a pioneer in this field and has published many articles about virus-mediated intradiscal gene therapy.
Retrovirus
Retrovirus-mediated gene transfer to the cartilaginous endplate in vitro
Wehling and colleagues [43] reported the retrovirus-mediated transfer of two different exogenous genes to cultured chondrocytic cells from bovine intervertebral endplates in vitro. Vertebral end plate tissue was obtained from bovine os coccygis. Chondrocytic cells were isolated and cultured in vitro. The bacterial [beta]-galactosidase (LacZ) gene and, alternatively, the cDNA of the human interleukin-1 receptor antagonist were introduced into the isolated cells by retrovirus-mediated gene transfer. [beta]-Galactosidase activity was determined by staining with 5-bromo-4-chloro-3-indolyl-[beta]-galactosidase (X-Gal), and interleukin-1 receptor antagonist protein was quantitated by enzyme-linked immunosorbent assay (ELISA). Isolation and cultivation of chondrocytic end plate cells is possible. Native cells continue to grow in culture for more than 2 months. Transfer of the [beta]-galactosidase gene to cultured cells resulted in ~1% [beta]-galactosidase positive cells. Transfer of the interleukin-1 receptor antagonist cDNA resulted in the production of 24 ng/ml 10−6 cells interleukin-1 receptor antagonist protein in 48 h. This was the first report of gene therapy targeting the disc in vitro, and the authors indicated the potential use of indirect, ex vivo gene therapy for the degenerated disc by re-injecting genetically modified chondrocytic end plate cells into the disc or surrounding tissue. However, one of the notable disadvantages of a retroviral vector may be the possibility of the insertional mutagenesis and the requirement of cell mitosis to deliver exogenous genes into the cells.
Adenovirus
Adenovirus-mediated transfer of a reporter gene to the intervertebral disc
Nishida and Kang et al. [32] reported the adenovirus-mediated transfer of the lacZ reporter gene to rabbit intervertebral disc cells both in vitro and in vivo. For the in vitro study, cell cultures were established from the nucleus pulposus tissue of New Zealand white rabbits and were transfected with an adenovirus construct encoding the lacZ gene (Ad-lacZ). For the in vivo study, the anterior aspects of lumbar intervertebral discs were surgically exposed and Ad-lacZ in saline solution was injected directly into the nucleus pulposus (direct gene transfer). An equal volume of saline-only solution was injected into control discs. Expression of the transferred gene was detected using X-Gal staining.
The results of these in vitro experiments demonstrated that nucleus pulposus cells were efficiently transduced by an adenoviral vector carrying the lacZ gene. In vivo injection of Ad-lacZ into the nucleus pulposus similarly resulted in the transduction of considerable numbers of cells. Reporter gene expression persisted in vivo at an apparently undiminished level for at least 12 weeks. No X-Gal staining was noted in control discs. Later, a follow-up report using two different reporter genes (X-Gal and Firefly Luciferase) showed the longevity of the transgene expression extended over 1 year [34].
This demonstration of successful transfer of an exogenous reporter gene to the disc alongside sustained, long-term expression in an adult animal model in vivo suggested that the adenoviral vector might be suitable for delivery of therapeutic genes to the disc, using an in vivo, direct gene therapy approach for the treatment of spinal disorders. Additionally, adenoviral vectors used in these studies (Type 5 adenoviral vector) are usually able to facilitate only short-term in vivo transgene expression in most tissues/organs because they elicit immune responses by the host. Therefore, remarkably long-term transgene expression mediated by Type 5 adenoviral vector within the disc without showing obvious cellular immune responses by the host around transfected disc cells has been considered significant evidence of the immune-privilege environment of the disc [34].
Adenovirus-mediated transfer of a therapeutic gene to the intervertebral disc
In their next study, they reported an in vivo study using the rabbit model to determine the feasibility of adenovirus-mediated transfer of a therapeutic gene to the intervertebral disc [33]. These researchers used an adenovirus construct (Ad/CMV-TGFβ1) containing a human TGF-β1 encoding gene. TGF-β1 was selected because it had been previously found by Thompson et al. [39] to increase proteoglycan synthesis in cultured canine disc tissues in vitro. In their study, the anterior aspects of lumbar intervertebral discs of 21 rabbits were surgically exposed, and 15 μl of saline with adenovirus containing the human TGF-β1 cDNA was directly injected into the nucleus pulposus. The supra-adjacent disc served as an intact control for each rabbit. The rabbits were sacrificed 1 week later. Expression of the transferred gene was determined using ELISA, and proteoglycan synthesis was assessed by measurement of sulfate incorporation.
In vivo injection of Ad/CMV-TGFβ1 into the nucleus pulposus was found to result in an approximately sixfold increase in total (i.e., active + latent) TGF-β1 production over that of the intact control discs (P < 0.05). The discs of the therapeutic gene group exhibited a statistically significant two-fold increase in proteoglycan synthesis compared to the intact control discs (P < 0.05).
This study demonstrated the efficacy of adenovirus-mediated transfer of a therapeutic gene to the intervertebral disc in vivo. The observation of a significant increase in proteoglycan synthesis secondary to gene transfer suggests that gene therapy may have potential applications in altering the time-course of degenerative disc disease.
Adeno-associated virus (AAV)
Lattermann et al. [19] reported adeno-associated virus (AAV) vector-mediated gene transfer to intervertebral disc in vivo. AAV vectors are known to be less immunogenic than adenoviral vectors and up to now have not been linked with any disease in humans or mammals. The size of the vector can accommodate the genetic information coding for growth factors such as TGF-β and BMP-2. In their study, Human nucleus pulposus cells were transduced in vitro. Twenty-four rabbits were injected with AAV vectors carrying different marker genes. Transgene expression and the humoral/cellular immune response to the vector was evaluated. The AAV vector efficiently transduced intervertebral disc cells and provided a strong transgene expression even in light of a low-level humoral immune response. The overall transgene expression was approximately half of that seen with the adenovirus, and the in vivo gene expression was associated with a 4–6 week latency period. Whether these quantitative observations are of therapeutic importance is not yet known, because the overall amount of transgene expression needed for a therapeutic effect within the disc is still not known. Nevertheless, in light of growing safety concerns over the use of adenoviral vectors for human gene therapy, the AAV vector might offer a valuable alternative to the adenovirus as a delivery vehicle for therapeutic gene transfer into the intervertebral disc.
Baculovirus
Baculovirus is an insect virus able to deliver exogenous genes to mammalian cells including nondividing cells with no cell toxicity in vitro or in vivo. Liu et al. [25] reported the usefulness of baculovirus for the disc. Intervertebral disc cells cultured in monolayer were treated with 6 different doses of baculovirus carrying the green fluorescence protein gene (Ac-CMV-GFP). Fluorescence microscopy and flow cytometry were used to analyze transgene expression. The Autographa californica nucleopolyhedrovirus/GFP virus was then injected directly into the intervertebral discs of 8 rabbits at 7, 13, 20, and 28 days after injection. The nucleus pulposus tissues of injected discs were evaluated immediately by fluorescence microscopy for GFP expression. Results showed that a dose of Ac-CMV-GFP at a multiplicity of infection of 200 achieved the highest transduction ratio (approximately, 87% of nucleus pulposus cells) and long-term expression without any toxicity to the cells. In vivo assay showed that Ac-CMV-GFP could also mediate GFP expression in rabbit intervertebral disc cells without inducing any symptoms. The GFP expression level at 7 days after transduction was significantly higher than at 21 and 28 days after treatment. They conclude that baculovirus can transfer exogenous genes into rabbit nucleus pulposus cells safely and with high efficiency both in vitro and in vivo. Their results suggest that baculoviruses might be useful as a gene therapy vector for intervertebral disc diseases.
Non-virus-mediated G.T.
From a safety viewpoint, the problem with the retroviral vector is the possibility of insertional mutagenesis, and the problem with the adenoviral vector is the immunogenesity of transduced cells [40, 42]. Furthermore, manufacturing viral vectors requires special facilities and techniques and specialized patient care would be necessary after performing virus-mediated gene therapy. These factors suggest a potentially much higher cost for viral vector-mediated gene therapy and highlight the potential advantages of a non-virus-mediated gene transfer technique due to its safety and overall simplicity [12]. Although various types of non-virus-mediated gene transfer techniques have been developed, a major limitation has been the lower transfection efficiency compared with viral vector-mediated methods.
Microbubble-enhanced ultrasound gene therapy
Recent evidence suggested that an appropriate intensity of ultrasound exposure can make a small transient hole on the cell surface in a phenomenon called sonoporation without causing cell toxicity, which may affect an increase in the permeability of the cell membrane to large molecules such as plasmid DNA [20, 31]. Furthermore, recent reports have also revealed that a type of ultrasonography contrast agent called micro-bubble makes a cavitation with the exposure of ultrasound and this results in a bursting of the micro-bubbles leading to a distribution of material over a specific area of interest. Both of these phenomenon, sonoporation and cavitation, are thought to have synergistic effects for increased transfection efficiency [21].
We demonstrated this micro-bubble enhanced ultrasound gene therapy in the intervertebral disc in vivo [35]. In our study, two different reporter plasmid DNA encoding green fluorescent protein (GFP) and Firefly Luciferase were used. Plasmid DNA was mixed with ultrasonography contrast agent (microbubbles) and injected into coccygeal intervertebral discs of Sprague–Dawley (SD) rats. Therapeutic ultrasound was applied on the surface of injected discs. Rats were sacrificed 1, 3, 6, 12, and 24 weeks after gene transduction. Harvested nucleus pulposus tissues were utilized for evaluation of transgene expression. The intact discs were used as a control. Seven days after gene transfection, considerable numbers of GFP positive cells were observed in the nucleus pulposus from the GFP transfected group. Luciferase assay revealed that the ultrasound group demonstrated approximately an 11-fold increase in luciferase activity over the plasmid DNA-only group. Furthermore, transgene expression mediated by this method was observed, at least up to 24 weeks. The results of the current study demonstrate that ultrasound-mediated destruction of microbubbles loaded with plasmid-DNA is a feasible and efficient technique for local gene delivery within the intervertebral disc. To the best of our knowledge, this was the first report of direct, in vivo transfection to intervertebral disc cells using a non-virus-mediated method.
New strategy for intradiscal gene therapy
Considerations of specific biological property of the disc
The disc is known to be the largest avascular structure in the body with a nucleus pulposus, well encapsulated by cartilagenous end-plates and, a dense fibrous structure, the annulus fibrosus. The main path of nutrition or oxygen supply is passive diffusion via the end-plates, which results in poor nutrition and low oxygen tension, particularly near the center of the disc [11, 15]. As a result of metabolism in a relatively anaerobic environment, lactates are produced, leading to low pH in the nucleus pulposus [30]. In addition, low nutrition and oxygen tension and low pH make the interior of the intervertebral disc a harsh biological environment. Accordingly, nucleus pulposus cells must be highly differentiated to survive in this special environment, resulting in relative stability in terms of cell proliferation and significantly low metabolism with a small cell number compared with the rich extra-cellular matrix.
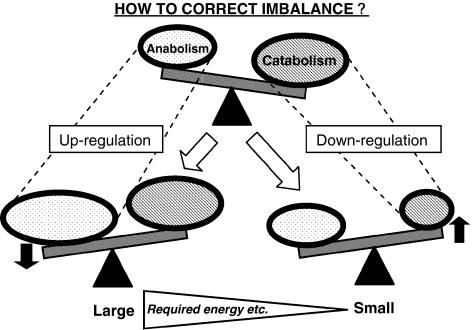
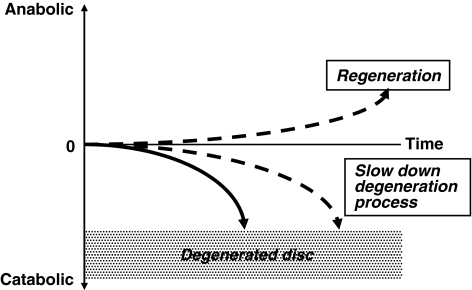
So far, the main focus of intradiscal gene therapy as well as growth factor injection or cell therapy approaches has been to stimulate matrix synthesis. However, when taking the disc environment (little nutrition and oxygen, low pH, overall low cell metabolism) into consideration, methods that require more resources or energy to up-regulate or stimulate matrix synthesis are less likely to result in successful disc regeneration. Therefore, a different approach which requires less energy or fewer resources may have a better chance of promoting regeneration of the disc. One of these approaches is the down-regulation of gene expressions that are potentially harmful for the physiological condition of the disc, and thus may induce degenerative change in the disc (Fig. 2). This type of approach would focus more on prophylactic treatment of disc degeneration. However, theoretically, it could become a regenerative treatment over an extended period (Fig. 3).
Fig. 2.
Two different approaches for disc regeneration. Theoretically, there are two approaches for correcting the imbalance between matrix anabolism and catabolism for disc regeneration. Previous approaches focused on stimulating matrix synthesis using growth (anabolic) factors. Another approach could be to down regulate the matrix catabolism. It is notable that stimulating matrix synthesis demands a lot of energy and resources, thus taking into account what we know of the disc environment, such as limited nutrition and cell activity, down-regulating the matrix catabolism might be more advantageous for the disc
Fig. 3.
Expected effects of genetic modification of the disc cells. If we can correct the imbalance between matrix anabolism and catabolism in disc degeneration either by stimulating matrix synthesis or down-regulating catabolism, we can potentially change the direction of the disc degeneration leading to a delay in the process (focusing on more prophylactic treatment) or even regeneration of the degenerated disc
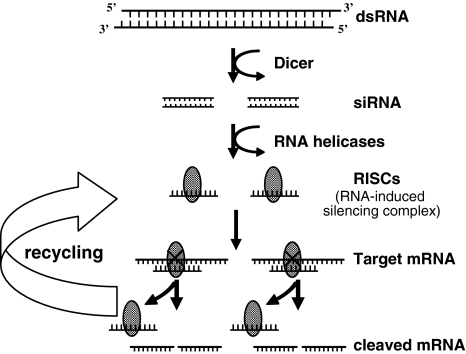
Among advancements in gene silencing technologies, RNA interference (RNAi) has recently emerged as an important biological strategy for specific gene silencing. RNAi was first reported as the phenomenon observed in Caenorhabditis elegans as a biological response to exogenous double-stranded RNA (dsRNA) and a process of sequence-specific, post-transcriptional gene silencing [9]. Later, Elbashir et al. reported that gene-specific suppression in mammalian cells could be achieved by small interfering RNAs (siRNA) of 21 nt in length [7] without stimulating a host interferon response. One of the notable advantages of siRNA-mediated RNAi is its effectiveness. It is reported that even a relatively small amount of siRNA can effectively inhibit specific gene expression because siRNA is incorporated into the RNA-induced silencing complex (RISC) that is stable in the cell [29, 37] and cleaves repeatedly to the target mRNA (Fig. 4) [16].
Fig. 4.
The schema of RNAi pathways. Small Interfering RNAs (siRNAs) are short, double-stranded RNA molecules that can target mRNAs with complimentary sequence. These siRNAs are incorporated into a multicomponent nuclease complex, known as RNA-induced silencing complex (RISC), which cleave target messenger RNAs for degradation [17]
RNA interference
We showed for the first time that the small interfering RNA (siRNA) targeting exogenous reporter gene is effective in silencing this transgene expression in nucleus pulposus cells in humans and rats in vitro [17]. In our study, two reporter luciferase plasmids (Firefly and Renilla) were used. These plasmids were co-transfected with siRNA targeting Firefly luciferase to the nucleus pulposus cells extracted from SD rats and scoliosis patients. The inhibitory effects were evaluated by dual luciferase assay for 3 weeks. The results showed the expression of Firefly Luciferase was drastically inhibited both in rats (94.7%) and in humans (93.7%). The inhibitory effects were maintained for 2 weeks and had disappeared completely by 3 weeks. We showed siRNA-mediated gene silencing in rat and human disc cells for a relatively sustained period, probably due to the stability of the nucleus pulposus cells in terms of cell proliferation.
In our follow-up study, we reported that the effectiveness of the DNA vector-based RNAi technique in vitro in terms of prolonged RNAi effect. Furthermore, we also demonstrated that simple unmodified siRNA-mediated RNAi effect in intervertebral discs in vivo resulted in a long lasting period of up to 24 weeks (168 days) [38].
The demonstration that siRNA transfected by a non-virus-mediated method which can effectively inhibit specific gene expression in nucleus pulposus cells may open the door for exploring the use of siRNA for scientific research purposes and investigating the role of certain gene expressions. Furthermore, this method has potential use as a novel strategy of gene therapy for treatment of disc degenerative diseases (Fig. 3).
Problems and future directions
The disc is a unique organ in our body and this uniqueness poses difficulties for disc regeneration. As described, one of the problems for disc regeneration is very limited nutrition or oxygen especially in the central part of the disc and this means that, theoretically, the disc cannot support many cells especially energy consuming, active type cells. Therefore, a different regeneration approach from ordinary organ/tissue types is needed. Most of the current approaches for regenerating discs do not seem to take enough account of the disc physiology, and this reduces the likelihood of favorable results.
Another potential problem is the animal model being used for research of disc degeneration. So far, there are few disc degeneration models which simulate clinical situations well. Recently, it has been reported that there are physiological difference between the notochordal and non-notochordal disc in terms of cell activity. These researchers showed notochordal disc such as rodent disc have better regeneration abilities than non-notochordal disc like those found in human beings [26]. Therefore, despite the many limitations that exist, a disc degeneration model which simulates the clinical situation, using a bigger animal with non-notochordal disc is required to obtain clinically applicable results.
Additionally, the relationship between clinical symptom such as low back pain and disc degeneration is still obscure. In other words, there is no clear understanding of which kind of disc condition causes low back pain. Therefore, continued efforts are needed to clarify the mechanism of clinical symptoms associated with disc degeneration as well as of disc degeneration processes.
There are many obstacles to be overcome in disc regeneration research before findings can be clinically applied. However, the amount of this research is increasing and broadening so it is not unrealistic to expect a break through from these studies over the next few years.
Acknowledgments
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.Aota Y, Kumano K, Hirabayashi S. Postfusion instability at the adjacent segments after rigid pedicle screw fixation for degenerative lumbar spinal disorders. J Spinal Disord. 1995;8:464–473. doi: 10.1097/00002517-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Asazuma T, Yamugishi M, Sato M, Ichimura S, Fujikawa K, Crock HV. Posterior spinal fusion for lumbar degenerative diseases using the Crock–Yamagishi (C–Y) spinal fixation system. J Spinal Disord Tech. 2004;17:174–177. doi: 10.1097/00024720-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Booth KC, Bridwell KH, Eisenberg BA, Baldus CR, Lenke LG. Minimum 5-year results of degenerative spondylolisthesis treated with decompression and instrumented posterior fusion. Spine. 1999;24:1721–1727. doi: 10.1097/00007632-199908150-00014. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Chung SA, Wei AQ, Connor DE, Webb GC, Molloy T, Pajic M, Diwan AD. Nucleus pulposus cellular longevity by telomerase gene therapy. Spine. 2007;32:1188–1196. doi: 10.1097/BRS.0b013e31805471a3. [DOI] [PubMed] [Google Scholar]
- 6.Cui M, Wan Y, Anderson DG, Shen FH, Leo BM, Laurencin CT, Balian G, Li X. Mouse growth and differentiation factor-5 protein and DNA therapy potentiates intervertebral disc cell aggregation and chondrogenic gene expression. Spine J. 2008;8:287–295. doi: 10.1016/j.spinee.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 8.Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg Spine. 1999;90:163–169. doi: 10.3171/spi.1999.90.2.0163. [DOI] [PubMed] [Google Scholar]
- 9.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 10.Gertzbein SD, Betz R, Clements D, Errico T, Hammerberg K, Robbins S, Shepherd E, Weber A, Kerina M, Albin J, Wolk D, Ensor K. Semirigid instrumentation in the management of lumbar spinal conditions combined with circumferential fusion. A multicenter study. Spine. 1996;21:1918–1925. doi: 10.1097/00007632-199608150-00018. [DOI] [PubMed] [Google Scholar]
- 11.Grunhagen T, Wilde G, Soukane DM, Shirazi-Adl SA, Urban JP. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg. 2006;88-A:30–35. doi: 10.2106/JBJS.E.01290. [DOI] [PubMed] [Google Scholar]
- 12.Herweijer H, Wolff JA. Progress and prospects: naked DNA gene transfer and therapy. Gene Ther. 2003;10:453–458. doi: 10.1038/sj.gt.3301983. [DOI] [PubMed] [Google Scholar]
- 13.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg. 1999;81-A:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch C, Schajowicz F. Studies on structural changes in the lumbar annulus fibrosus. Acta Orthop Scand. 1953;22:184–231. doi: 10.3109/17453675208989006. [DOI] [PubMed] [Google Scholar]
- 15.Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–119. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 16.Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- 17.Kakutani K, Nishida K, Uno K, Takada T, Shimomura T, Maeno K, Kurosaka M, Doita M. Prolonged down regulation of specific gene expression in nucleus pulposus cell mediated by RNA interference in vitro. J Orthop Res. 2006;24:1271–1278. doi: 10.1002/jor.20171. [DOI] [PubMed] [Google Scholar]
- 18.Kostuik JP. Intervertebral disc replacement. Experimental study. Clin Orthop. 1997;337:27–41. doi: 10.1097/00003086-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Lattermann C, Oxner WM, Xiao X, Li J, Gilbertson LG, Robbins PD, Kang JD. The adeno associated viral vector as a strategy for intradiscal gene transfer in immune competent and pre-exposed rabbits. Spine. 2005;30:497–504. doi: 10.1097/01.brs.0000154764.62072.44. [DOI] [PubMed] [Google Scholar]
- 20.Lawrie A, Brisken AF, Francis SE, Tayler DI, Chamberlain J, Crossman DC, Cumberland DC, Newman CM. Ultrasound enhances reporter gene expression after transfection of vascular cells in vitro. Circulation. 1999;99:2617–2620. doi: 10.1161/01.cir.99.20.2617. [DOI] [PubMed] [Google Scholar]
- 21.Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther. 2000;7:2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- 22.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine. 1988;13:375–377. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Le Maitre CL, Hoyland JA, Freemont AJ (2007) Interleukin–1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study. Arthritis Res Ther 9:R83. DOI 10.1186/ar2282 [DOI] [PMC free article] [PubMed]
- 24.Lemaire JP, Skalli W, Lavaste F, Templier A, Mendes F, Diop A, Sauty V, Laloux E. Intervertebral disc prosthesis. Results and prospects for the year 2000. Clin Orthop. 1997;337:64–76. doi: 10.1097/00003086-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Li K, Song J, Liang C, Wang X, Chen X. Efficient and stable gene expression in rabbit intervertebral disc cells transduced with a recombinant baculovirus vector. Spine. 2006;31:732–735. doi: 10.1097/01.brs.0000206977.61305.43. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki T, Kobayashi S, Takeno K, Uchida K, Yayama T, Baba H (2007) Proteoglycan production of notochordal disc and non-notochordal disc. International Society for the Study of the Lumbar Spine, Hong Kong
- 27.Moon SH, Gilbertson LG, Nishida K, Knaub M, Muzzonigro T, Robbins PD, Evans CH, Kang JD. Human intervertebral disc cells are genetically modifiable by adenovirus-mediated gene transfer: implications for the clinical management of intervertebral disc disorders. Spine. 2000;25:2573–2579. doi: 10.1097/00007632-200010150-00006. [DOI] [PubMed] [Google Scholar]
- 28.Moon SH, Nishida K, Gilbertson L, Hall A, Robbins P, Kang JD (2001) Biologic response of human intervertebral disc cell to gene therapy cocktail. International Society for the Study of the Lumbar Spine, San Francisco [DOI] [PMC free article] [PubMed]
- 29.Martinez J, Patkaniowska A, Urlaub H, Lührmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563–574. doi: 10.1016/S0092-8674(02)00908-X. [DOI] [PubMed] [Google Scholar]
- 30.Nachemson A. Intradiscal measurements of pH in patients with lumbar rhizopathies. Acta Orthop Scand. 1969;40:23–42. doi: 10.3109/17453676908989482. [DOI] [PubMed] [Google Scholar]
- 31.Newman CM, Lawrie A, Brisken AF, Cumberland DC. Ultrasound gene therapy: on the road from concept to reality. Echocardiography. 2001;18:339–347. doi: 10.1046/j.1540-8175.2001.00339.x. [DOI] [PubMed] [Google Scholar]
- 32.Nishida K, Kang JD, Suh JK, Robbins PD, Evans CH, Gilbertson LG. Adenovirus-mediated gene transfer to nucleus pulposus cells. Implications for the treatment of intervertebral disc degeneration. Spine. 1998;23:2437–2442. doi: 10.1097/00007632-199811150-00016. [DOI] [PubMed] [Google Scholar]
- 33.Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, Robbins PD, Evans CH. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999;24:2419–2425. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Nishida K, Gilbertson LG, Moon SH, Robbins PD, Evans CH, Kang JD (2000) Immune-privilege of the intervertebral disc: long-term transgene expression following direct adenovirus-mediated gene transfer. International Society for the Study of the Lumbar Spine, Adelaide
- 35.Nishida K, Doita M, Takada T, Kakutani K, Miyamoto H, Shimomura T, Maeno K, Kurosaka M. Sustained transgene expression in intervertebral disc cells in vivo mediated by microbubble-enhanced ultrasound gene therapy. Spine. 2006;31:1415–1419. doi: 10.1097/01.brs.0000219945.70675.dd. [DOI] [PubMed] [Google Scholar]
- 36.Paul R, Haydon RC, Cheng H, Ishikawa A, Nenadovich N, Jiang W, Zhou L, Breyer B, Feng T, Gupta P, He TC, Phillips FM. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine. 2003;28:755–763. doi: 10.1097/00007632-200304150-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz DS, Hutvagner G, Haley B, Zamore PD. Evidence that siRNAs function as guides, not primers, in the Drosophila and human RNAi pathways. Mol Cell. 2002;10:537–548. doi: 10.1016/S1097-2765(02)00651-2. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki T, Nishida K, Kakutani K, Doita M, Kaneyama S, Takada T, Kurosaka M. Long term RNA interference in nucleus pulposus in vivo mediated by native unmodified short interference RNA. San Francisco: Orthopaedic Research Society; 2008. [Google Scholar]
- 39.Thompson JP, Oegema TR, Jr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Tripathy SK, Black HB, Goldwasser E, Leiden JM. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 41.Wallach CJ, Sobajima S, Watanabe Y, Kim JS, Georgescu HI, Robbins P, Gilbertson LG, Kang JD. Gene transfer of the catabolic inhibitor TIMP–1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine. 2003;28:2331–2337. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- 42.Wallach CJ, Kim JS, Sobajima S, Lattermann C, Oxner WM, McFadden K, Robbins PD, Gilbertson LG, Kang JD. Safety assessment of intradiscal gene transfer: a pilot study. Spine J. 2006;6:107–112. doi: 10.1016/j.spinee.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Wehling P, Schulitz KP, Robbins PD, Evans CH, Reinecke JA. Transfer of genes to chondrocytic cells of the lumbar spine. Proposal for a treatment strategy of spinal disorders by local gene therapy. Spine. 1997;22:1092–1097. doi: 10.1097/00007632-199705150-00008. [DOI] [PubMed] [Google Scholar]
- 44.West JL, III, Ogilvie JW, Bradford DS. Complications of the variable screw plate pedicle screw fixation. Spine. 1991;16:576–579. doi: 10.1097/00007632-199105000-00016. [DOI] [PubMed] [Google Scholar]