Abstract
Degenerative disc disease has been implicated as a major component of spine pathology. The current major clinical procedures for treating disc degeneration have been disappointing, because of altered spinal mechanics leading to subsequent degeneration at adjacent disc levels. Disc pathology treatment is shifting toward prevention and treatment of underlying etiologic processes at the level of the disc matrix composition and integrity and the biomechanics of the disc. The ability to perform such treatment relies on one’s ability to accurately and objectively assess the state of the matrix and the effectiveness of treatment by a non-invasive technique. In this review, we will summarize our advances in efforts to develop an objective, accurate, non-invasive diagnostic tool (quantitative MRI) in the detection and quantification of matrix composition and integrity and of biomechanical changes in early intervertebral disc degeneration.
Keywords: Magnetic resonance imaging, Intervertebral disc, Disc degeneration, Diagnostic tool
Introduction
Despite a relentless search for adequate and effective treatment, low back pain is one of the most prevalent [9] and costly illness in today’s society [29, 55]. It has long been recognized that the integrity of the intervertebral disc (IVD), part of a three-joint complex that determines the motion segment of the spine, is an essential component of normal spinal function. Alterations in the disc’s architecture and biochemistry may cause back pain and referred pain, with or without neurological impairment. In addition, this can significantly compromise the biomechanical integrity of the motion segment [15, 17, 37].
Degenerative disc disease has been implicated as a major component of spine pathology. Currently, the two major clinical procedures for treating disc degeneration are disc excision and spinal fusion. These treatment modalities have been disappointing because of altered spinal mechanics leading to subsequent degeneration at adjacent disc levels. Disc pathology treatment is shifting toward prevention and treatment of underlying etiologic processes at the level of the disc matrix composition and integrity. The ability to perform such treatment relies on one’s ability to accurately and objectively assess the state of the matrix and the effectiveness of treatment by a non-invasive technique. Quantitative MRI has the potential to become a very important diagnostic and treatment assessment tool in determining the IVD matrix functional state. MRI analysis can reflect macromolecular structure concentrations in the disc and can be exploited to reflect the structural integrity of the matrix. MRI can make non-invasive and repeatable measurements and can be an important tool for clinical diagnosis and basic research.
In this review, we will summarize our advances in efforts to develop an objective, accurate, non-invasive diagnostic tool (quantitative MRI) in the detection and quantification of matrix composition and integrity and of biomechanical changes in early IVD degeneration. Our group identified that quantitative MR parameters (relaxation times, magnetization transfer, apparent diffusion coefficient) are influenced by both IVD matrix composition (water, proteoglycan, and collagen) and matrix integrity (collagen denaturation). Targeted enzyme matrix denaturation allowed us to determine the effect of altered matrix integrity on quantitative MRI, while maintaining the matrix content constant. Our work has also led us to demonstrate that quantitative MRI is highly influenced by the application of a mechanical load upon IVDs. We have recently shown that quantitative MRI can be used to describe alterations in material properties, specifically the hydraulic permeability and compressive modulus, of the discs. Our recent work suggests that T1rho (time constant of transverse magnetization decay) is a useful parameter in quantitative MRI analysis that correlates predominantly with disc water content and its compressive properties.
Basic properties of the disc relevant to MRI studies
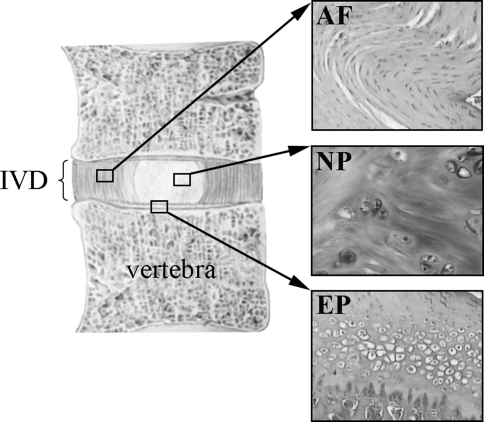
Healthy IVDs are composed of two distinct structural regions, the peripheral annulus fibrosus (AF) consisting of 15–25 concentric lamellae, which are abundant collagen fibers arranged parallel to one another [39], and the central nucleus pulposus (NP) rich in proteoglycan [42]. The IVD lies between thin layers of normally avascular cartilaginous endplates which separate the NP and the AF from the adjacent vertebral bone. Figure 1 shows the general structure of the IVD with histologic views of structured AF, less organized NP, and the cartilaginous endplate which in humans acts as a growth plate for the vertebral bodies.
Fig. 1.
The intervertebral disc. The intervertebral disc (IVD) is composed of three components: The annulus fibrosus (AF) is a fibrocartilage organized with the fibroblast-like cells in dense concentric lamellae. The nucleus pulposus (NP) is a less structured gelatinous substance with chondrocyte-like cells. The IVD lies between cartilaginous endplates (EP)
The composition and organization of the extracellular matrix of the IVD governs the disc’s mechanical function and varies with age and location within the disc [4, 54]. The mechanical role and the water content of the disc is dependent on both the proteoglycans and collagens. Water content is related to the osmotic pressure generated by the fixed negative charges on the proteoglycans and the unique fibrillar structure of collagens provides the resistance to swelling. The proteoglycan and water content of the disc increases on progressing from the outer AF to the NP, while the collagen content decreases from the outer AF to the NP.
The ability of disc tissue to retain water, restrict water flux, and resist compressive forces is largely due to their high aggrecan content, although other proteoglycans, such as decorin, versican, fibromodulin, perlecan, lumican, and biglycan are present in the disc. Figure 2 shows a schematic representation of the proteoglycan aggrecan and its interaction with collagen fibers and hyaluronic acid in the AF (Fig. 2a) and NP (Fig. 2b) tissues. In human adults, most of the proteoglycans are non-aggregated and undergo extensive proteolytic processing. However, the cleaved fragments are not lost, but are entrapped into the matrix and can still temporarily contribute to resisting compressive loads. As disc degeneration advances, the mechanical strength of the tissue ultimately weakens.
Fig. 2.
Schematic representation of the extracellular matrix of the annulus fibrosus and nucleus pulposus. The annulus fibrosus is composed predominantly of type I collagen organized in dense concentric lamellae forming a fibrous collagen network that maintain the disc shape. The nucleus pulposus is rich in proteoglycans and in a type II collagen. In both structures, proteoglycans interact with hyaluronic acid to form proteoglycan aggregates that are designed to resist compressive loads
The disc contains many different collagen types whose abundance changes with age [54]. The AF is composed primarily of type I collagen but, to a lesser extent, it also contains types II, III, V, VI, IX, and XI collagens. The NP is rich in type II collagen, but also contains collagen types I, VI, and IX [20, 21, 23, 25, 44]. Types I and II collagens constitute about 80% of the collagens in the IVD [20]. They are particularly important to the integrity of the disc, because they form the fibrous framework of the tissue.
IVD mechanical behavior is highly non-linear [47, 49] and is dependent on loading history and hydration [27, 52]. IVD may be considered as a biphasic tissue with poroelastic properties: a porous and permeable, fiber-reinforced solid phase and a fluid phase of water mixed with ions. As the composition and structure varies with region, so do the mechanical properties. In general, proteoglycans of the solid phase provide much of the compressive stiffness through electrostatic repulsion and osmotic pressurization of the abundant glycosaminoglycan (GAG) negative charges, whereas collagen helps to immobilize proteoglycans within the tissue and provides tensile and shear properties. Complex molecular structures and interactions, however, suggest interrelationships between the mechanical roles of these major structural proteins. Fluid flow through the solid matrix is governed by the tissue permeability.
The most dramatic changes in IVD with ageing and degeneration are the loss of fluid pressurization and hydration, and the altered biochemical composition and matrix structure. These dramatic alterations are accompanied by specific changes in the disc material properties, including alteration of the compressive modulus, of the hydraulic permeability, and of the tensile properties of the AF, and alteration of the elastic modulus, of the shear modulus, and of the swelling pressure of the NP [43, 48].
Matrix degrading proteinases of the IVD
Extensive studies have confirmed earlier indications that IVD disc degradation results primarily from the extracellular activity of tissue proteinases [24, 53]. Disc cells can synthesize and secrete a large number of these proteinases which, together and not singly degrade the disc. Their activities on matrix proteins are summarized below.
Cleavage of collagen
Matrix metalloproteinases (MMPs) are a family of enzymes that are capable of degrading the major structural components of the IVD, and are thus involved in both the normal turnover and the degradation of the extracellular matrix [30]. MMP-1, -8, and -13 (collagenases) can cleave the triple helical part of the collagens types I, II, and III. MMP-13 preferentially cleaves type II collagen. MMP-2 is one of the two gelatinases that can further degrade the cleaved collagens. However, because of the extensive covalent cross-linking within the collagen fibrils, the extracellular matrix retains collagen fragments (reviewed in [54]). The damaged collagen molecules are then entrapped in the fibril structure and accumulate due in part to the slow collagen turnover. This eventually leads to a weakening of the biomechanics of the disc with age and degeneration. Similarly, the mature human disc contains a high proportion of non-aggregating proteoglycans, derived by proteolytic degradation and retained in the disc.
Cleavage of aggrecan
Along with type II collagen, aggrecan forms a major structural component of the disc (Fig. 2). Recent studies have examined the cleavage of the core protein of aggrecan and link protein. These molecules form macromolecular aggregates with hyaluronic acid. The binding occurs through the G1 globular domain of aggrecan. Proteases capable of degrading aggrecans are called aggrecanases and they are members of the ADAM (A Disintegrin And Metalloprotease) family of proteins [19]. MMP-1 and ADAMTS-4 are expressed in few cells of normal discs, while MMP-3 and -13 are absent [36, 53, 58]. MMP-2 is present in early phases of disc degradation and MMP-1, -3, -9, and -13, as well as ADAMTS-4, have been observed in degenerated IVDs, specifically in the NP and inner AF cells [36, 53, 58]. MMP-1, -3, and -13 and ADAMTS-4 expression appear to increase with the severity of disc degeneration [36, 53, 58]. The release of aggrecan from articular cartilage can also be arrested by a hydroxamate inhibitor of MMPs suggesting that they are also directly implicated in aggrecan cleavage [40].
Determining the matrix composition of the disc using MRI
Water is the principal source of MR-sensitive protons in the disc. Studies have demonstrated that the water content fluctuates in the disc [12, 13]. Thus, to understand disc degeneration, we need to correlate quantitative MR data to matrix composition and integrity.
The time constant, which describes how the longitudinal magnetization returns to its equilibrium value, is called the spin–lattice relaxation time (T1). T2 is the spin–spin relaxation time and is always less than or equal to T1. T1 has been predominantly correlated to water content, while T2 is influenced by tissue anisotropy (orientation of collagen fibers), collagen concentration, and water content in tissues [60]. The transverse relaxation time has also been correlated with proteoglycan content in tissues, but reports vary between studies [56, 57]. T1 and T2 are higher in the NP than in the AF of the disc, and can be used to visualize structural changes of the disc [3]. T1 has also been shown to decrease in the NP with disc degeneration [3].
T1rho is the recent alternate contrast MR mechanism which represents the time constant of transverse magnetization decay, which occurs in the presence of a spin-lock radio-frequency field. The T1rho time constant is directly related to interactions between macromolecules and bulk water, as well as changes in macromolecular content, without problems of spin dephasing due to inhomogeneous magnetic fields inherent of T2 contrast. With the increased dynamic range obtained with the T1rho technique, T1rho has been shown to be related to proteoglycan content in cartilage and in the disc [6, 10, 28, 45, 59]. These recent studies have also shown that T1rho correlates with water content and inversely with age and degeneration in the disc tissue [6, 28, 45] and that T1rho has a strong potential as a quantitative biomarker of the mechanical function of the nucleus pulposus [45].
Magnetization transfer (MT) technique improves soft-tissue contrast and characterization. It measures the cross-relaxation and chemical exchange processes between free and macromolecule-bound water protons in tissues [46]. More recent studies have demonstrated that the rate of MT between free-water protons and the macromolecule-bound protons was elevated in degenerated discs [46]. A positive correlation was found between disc collagen and total protein concentration and the rate of transfer, while the contrast of normal and degenerated discs on MT images was not altered significantly. One of the main applications of MT is the computation of the MT ratio (MTR), which consists of the collection of two images, one with MT saturation and one without. MTR at 3T in most tissues varies only slightly when compared to that of 1.5T [33]. MTR is predominantly dependent upon collagen concentration in cartilage, but the structural integrity of the matrix also plays a role [34]. MT has been shown to increase with degeneration in the disc [5].
MRI can measure molecular diffusion resulting from random thermal, or so-called Brownian, translational motion of molecules [5, 7, 12]. Since freedom of motion is restricted by interactions with other molecules, diffusion imaging can reflect molecular mobility and hence, microscopic changes in tissue organization [1, 30, 32]. Diffusion, measured as the apparent diffusion coefficient (ADC), is independent of the magnetic field strength, and MRI is the only in vivo technique presently capable of measuring diffusion directly from molecular displacements. It has been shown that the ADC decreases in degenerated discs and that it correlates in a direction-dependent manner with disc water, proteoglycan content, and disc matrix integrity [1, 30, 31].
Physiological loading and MRI imaging
Load-induced changes in IVD geometry can be detected and measured using MRI. The mean relative volume change between MRI scans taken after 6-h bed rest and after a full day activity was 16.2% [13], whereas the average volume increase 3 h after a highly compressive load is removed was 5.4% [38]. Between evening and morning MRI scans, T1 was found to decrease, whereas T2 was found to increase [11]. An expansion of the disc area associated with a modest increase in the transverse relaxation time during overnight or longer bed rest [35], and a greater creep in the lumbar spine while in an extended posture than while in a flexed posture were also reported [26]. Higher T1 and ADC were found in loaded discs than in the unloaded discs [18]. After compression of the IVD, the water diffusion either increases in creep (load steps) experiments [18], or decreases in stress–relaxation (displacement steps) experiments [16]. Significant correlations were found between nucleus zone migration, as measured by MRI, and flexion–extension movements of the spine [8, 14, 22]. Correlations were also found between nucleus zone migration and IVD wedging in scoliosis [51]. These experiences prove that loading alone significantly influences quantitative MRI, and that the intricate relation between quantitative MRI to matrix composition and integrity undergoing physiologic loading simulation must be understood and characterized.
Studies on human discs
Growth, maturation, ageing, and degeneration in the human lumbar IVD
Careful regulation of matrix synthesis and degradation (together these processes represent turnover) is an integral requirement for the maintenance of a healthy, fully functional disc. Very little was known about the turnover of extracellular matrix in the human IVD. We measured concentrations of specific molecules reflecting matrix synthesis and degradation in predetermined regions of human lumbar IVDs and correlated them with ageing and Thompson grade of degeneration [4]. We identified three matrix turnover phases (Fig. 3). Phase I (growth) is characterized by active synthesis of matrix molecules such as type II collagen and active denaturation of type II collagen up to 15 years old. Phase II (maturation and ageing; 15–40 years old) is distinguished by a progressive drop in synthetic activity and a progressive reduction in denaturation of type II collagen. Phase III (degeneration and fibrotic, over 40 years old) is illustrated by evidence for a lack of increased synthesis of type II procollagen, but also by an increase in collagen type II denaturation. The pattern of type I collagen and aggrecan synthesis was similar to what was observed for type II collagen. Recently, we showed that the GAG to hydroxyproline ratio within the NP of young adults is approximately 27:1, whereas the ratio within the hyaline cartilage endplate of the same aged individuals is about 2:1 [42]. The production of an extracellular matrix with a high proteoglycan to collagen ratio can thus be used in vivo to distinguish NP cells from chondrocytes.
Fig. 3.
Matrix turnover phases in IVD degeneration. Three matrix turnover phases related to age were identified in human discs as illustrated by collagen II synthesis (pro type II collagen) and matrix degradation (denaturated type II collagen)
Quantitative MRI in the assessment of human degenerative disc disease
In this study, we analyzed T1 and T2 signal patterns in the NP of human discs with increasing Thompson morphological grade and revealed a significant reduction related to grade (Fig. 4). This significant decrease in T1 and T2 in the NP with grade 4 degeneration and the corresponding low correlation coefficients with respect to the content of individual matrix molecules suggested that matrix integrity plays an important and distinct role in determining T1 and T2 signal. Contrary to what was observed for T1 and T2, magnetization transfer (MTR) increased in Thompson grade 4 degenerated discs. No significant changes were observed in the AF of human discs. This study presented the first clear evidence that quantitative magnetic resonance analysis reflects not only the disc matrix composition, but also the structural integrity of the matrix of the disc in humans [5].
Fig. 4.
Relaxation times in the assessment of degenerative disc disease. Relaxation times T1 and T2 were measured in human NPs of IVDs with different Thompson grades of degeneration. *P < 0.05 versus grade 2
Quality of the nutritional supply to the IVD at various ages and levels of degeneration
The lack of a vascular system in the disc results in diffusion of solutes from the peripheral parts of the AF and the center of the end plates as being the sole means of nutritional supply for both the AF and the NP. Disturbances in the osmotic pressure and in the hydrostatic pressure of the disc affect the nutritional pathway and eventually lead to alterations within the IVD. Quantitative MR was used to determine if the quality of the nutritional supply to the IVD at various ages and levels of degeneration could be assessed through measurement of the apparent diffusion coefficients (ADCs) [2]. Modifications of the NP matrix content, specifically of water and glycosaminoglycan (GAG) contents, with age and disc degeneration, were reflected in correlating changes in the ADCs. We found that decreases in GAG or water contents in the NPs resulted in direct decreases in the ADCs (Fig. 5). Changes in matrix integrity, as evidenced by the percentage of denatured collagen, were also detected in the NP with a low positive correlation to the ADC along the height of the disc and an inverse statistically significant regression to the ADC along the anterior to posterior axis of the disc.
Fig. 5.
Apparent diffusion coefficient in the assessment of degenerative disc disease. Apparent diffusion coefficient (ADC) was measured in NPs of human IVDs with different Thompson grades of degeneration and compared to water (H2O) and proteoglycans (GAG) contents
Studies related to the mechanism of disc degeneration using bovine discs
Static, unloaded and closed system
We recently examined the targeted structural degradation of collagens and proteoglycans of the NP using hyaluronidase, collagenase, and trypsin, while keeping the total content of matrix macromolecules constant using quantitative MRI. The approach allowed us to differentiate the contribution of the matrix integrity to the MR signal from that of the total matrix content [3]. The bovine coccygeal NPs were injected with either enzyme or buffer, incubated at 37°C as static, unloaded and closed 3-disc segments, and analyzed by a 1.5-T MR scanner to measure MR parameters. Immediately after the injection protocol, all discs were embedded in paraffin blocks for the purpose of maintaining constant within the discs segment the total content of matrix macromolecules (Fig. 6). We believe that the absence of loading would minimize transport of matrix constituents. Results showed that the relaxation times T1 and T2 significantly decreased in NPs only after collagenase treatment, while hyaluronidase and trypsin had no effect (Fig. 7). Similarly, the enzymes had no effect on MTR either. The collagenase-induced degradation products of the NP were prevented from diffusing out of the disc which led to a decrease in T2.
Fig. 6.
MRI setup for paraffin-embedded disc segments for the study of enzyme-induced disc degeneration. Disc segments were embedded in paraffin and placed in a specially designed Plexiglass container that fits in the MRI head coil array. Note that the vials of standards are situated below one of the disc segments
Fig. 7.
Effect of enzyme treatment on relaxation times. Bovine NPs were injected with collagenase (Coll.), hyaluronidase (HA), and trypsin, and disc segments were analyzed by MRI as illustrated in Fig. 6. *P < 0.05 versus control (Ctl) untreated discs
Overall, this study allowed us to identify and characterize the effect that altered IVD matrix integrity has on quantitative MR measurements. Given that matrix content remained constant, it has been shown that quantitative MRI is not just a reflection of disc matrix content, but is influenced by both disc matrix content and structural integrity. If we assume that in early disc degeneration matrix integrity is lost first (changes in structural integrity) and only later, molecules and protein fragments will be free to diffuse out and be lost (changes in matrix content), this study suggests that it might be potentially possible to detect such early degenerative changes by MRI, namely, when proteins are being cleaved.
Dynamic, axial cyclic compression and open system
Since the human spine is subjected to mechanical loading, we chose to determine the effects of simulated physiologic loading on the correlation between quantitative MR and disc matrix composition and integrity. We thus tested the hypotheses that (1) mechanical loading and enzyme treatment will induce changes in quantitative MR parameters, mechanical properties and biochemical contents of the IVD and (2) the mechanical properties of the IVD are quantitatively related to MR parameters. Bovine caudal discs were subjected to trypsin injection, wrapped in saline, mounted in a specially designed plastic tube with low friction spacers around the circumference of the vertebral bodies to prevent buckling under compression, and loaded with cyclic compression (50–300–50 N at 1 Hz) as illustrated on Fig. 8. MRI parameters, mechanical properties, and biochemical contents of disc tissue were analyzed on the NP. Results after 2 h of cyclic compression loading showed that trypsin treatment decreased the compressive modulus (HA) and increased the hydraulic permeability (k), while loading decreased T1 and T2, and increased MTR [50]. Biochemically, trypsin treatment affected water, GAG, and total collagen contents, while loading decreased water and GAG contents. The significant effect of trypsin treatment on the mechanical properties but not on the MR parameters suggests that the mechanical properties are more sensitive to the structural changes with trypsin treatment than are MR parameters. It is also possible that if the AF remained intact after trypsin injection, the consistency of the NP remained unchanged; therefore, T1 and T2 would remain more or less the same. However, the smaller GAG fragments may affect the mechanical properties.
Fig. 8.
Disc segment setup for the analysis of cyclic compression loading effect on IVD properties. Both ends of the segment are potted in PMMA and mounted on a MTS-858 Mini Bionix system (MTS Systems Corporation, Eden Prairie, MN, USA). The three-ball bearings are equally positioned around the disc segments to prevent buckling while minimizing axial friction
In view of the previous results, the influence of a day of 16 h of compression loading was evaluated on the disc structure and composition, and the MR parameters [41]. Our results confirmed that MR parameters (T1, T2, MTR, ADC) (Fig. 9) and the biochemical properties (water, collagen, aggrecan) of the NP tissues are sensitive to loading. On the other hand, trypsin affects more strongly the swelling pressure (Psw), compressive modulus, and hydraulic permeability of the NP tissue (Fig. 10). In regards to AF tissues, we demonstrated that loading decreased T1 but had no effect on T2 and MTR. NP treatment with trypsin neither significantly affected MR parameters nor the biochemical content of the AF tissues [41]. These results strongly suggested that loading history is an important parameter to consider in efforts to develop quantitative MRI as a diagnostic tool of IVD matrix composition and integrity and of biomechanical changes in early IVD degeneration.
Fig. 9.
Effect of cyclic compression loading on the MRI properties of IVDs. Bovine IVDs were subjected to 16-h compression loading as illustrated in Fig. 8 and the MRI properties of the NPs were evaluated as illustrated in Fig. 6. *P < 0.05 versus unloaded segments
Fig. 10.
Effect of trypsin on the compressive properties of IVDs. Bovine NPs were injected with trypsin and their compressive properties were analyzed in a nonpermeable confined compression chamber. Psw = swelling pressure; HA = compressive modulus; k = hydraulic permeability. *P < 0.05 versus control (Ctl) untreated discs
Conclusion
This work may lead to new and exciting advances in the field of spine research. The inception of such a diagnostic tool will be a foundation for future work in diagnosing and treating spine pathology. In future, we plan to use this technology in a clinical setting using normal volunteers and comparing the results to patients with disc pathology. We also foresee its use in the assessment of new and established therapeutic modalities in the treatment of disc pathology.
Acknowledgments
The authors thank Caroline C. Demers, Tapas Goswami, and Arthur J. Michalek for their precious technical help, Laura Epure for the preparation of the figures, and Dr. Alain Petit for going through the manuscript and providing helpful discussion. We also thank Arijitt Borthakur from the Department of Radiology at the University of Pennsylvania for the T1rho protocol. Funding for this work was provided by the Canadian Institutes of Health Research, the McGill William Dawson Scholar Award, the Whitaker Foundation, the North American Spine Society and the AO Foundation (Switzerland, Funding #F-06-31A).
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.An HS, Anderson PA, Haughton VM, Iatridis JC, Kang JD, Lotz JC, Natarajan RN, Oegema TR, Jr, Roughley P, Setton LA, Urban JP, Videman T, Andersson GB, Weinstein JN. Introduction: disc degeneration: summary. Spine. 2004;29:2677–2678. doi: 10.1097/01.brs.0000147573.88916.c6. [DOI] [PubMed] [Google Scholar]
- 2.Antoniou J, Demers CN, Beaudoin G, Goswami T, Mwale F, Aebi M, Alini M. Apparent diffusion coefficient of intervertebral discs related to matrix composition and integrity. Magn Reson Imaging. 2004;22:963–972. doi: 10.1016/j.mri.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Antoniou J, Mwale F, Demers CN, Beaudoin G, Goswami T, Aebi M, Alini M. Quantitative magnetic resonance imaging of enzymatically induced degradation of the nucleus pulposus of intervertebral discs. Spine. 2006;31:1547–1554. doi: 10.1097/01.brs.0000221995.77177.9d. [DOI] [PubMed] [Google Scholar]
- 4.Antoniou J, Nelson F, Steffen T, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoniou J, Pike GB, Steffen T, Baramki H, Poole AR, Aebi M, Alini M. Quantitative magnetic resonance imaging in the assessment of degenerative disc disease. Magn Reson Med. 1998;40:900–907. doi: 10.1002/mrm.1910400616. [DOI] [PubMed] [Google Scholar]
- 6.Auerbach JD, Johannessen W, Borthakur A, Wheaton AJ, Dolinskas CA, Balderston RA, Reddy R, Elliot DM. In vivo quantification of human lumbar disc degeneration using T1ρ-weighted magnetic resonance imaging. Eur Spine J. 2006;15(Suppl 3):S338–S344. doi: 10.1007/s00586-006-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battie MC, Haynor DR, Fisher LD, Gill K, Gibbons LE, Videman T. Similarities in degenerative findings on magnetic resonance images of the lumbar spines of identical twins. J Bone Joint Surg [Am] 1995;77A:1662–1670. doi: 10.2106/00004623-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Beattie PF, Brooks WM, Rothstein JM, Sibbitt WL, Jr, Robergs RA, MacLean T, Hart BL. Effect of lordosis on the position of the nucleus pulposus in supine subjects. A study using magnetic resonance imaging. Spine. 1994;19:2096–2102. doi: 10.1097/00007632-199409150-00017. [DOI] [PubMed] [Google Scholar]
- 9.Biering-Sorensen F. Low back trouble in a general population of 30-, 40-, 50-, and 60-year- old men and women. Study design, representativeness and basic results. Dan Med Bull. 1982;29:289–299. [PubMed] [Google Scholar]
- 10.Blumenkrant G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 11.Boos N, Wallin A, Aebi M, Boesch C. A new magnetic resonance imaging analysis method for the measurement of disc height variations. Spine. 1996;21:563–570. doi: 10.1097/00007632-199603010-00006. [DOI] [PubMed] [Google Scholar]
- 12.Boos N, Wallin A, Gbedegbegnon T, Aebi M, Boesch C. Quantitative MR imaging of lumbar intervertebral disks and vertebral bodies: influence of diurnal water content variations. Radiology. 1993;188:351–354. doi: 10.1148/radiology.188.2.8327677. [DOI] [PubMed] [Google Scholar]
- 13.Botsford DJ, Esses SI, Ogilvie-Harris DJ. In vivo diurnal variation in intervertebral disc volume and morphology. Spine. 1994;19:935–940. doi: 10.1097/00007632-199404150-00012. [DOI] [PubMed] [Google Scholar]
- 14.Brault JS, Driscoll DM, Laakso LL, Kappler RE, Allin EF, Glonek T. Quantification of lumbar intradiscal deformation during flexion and extension, by mathematical analysis of magnetic resonance imaging pixel intensity profiles. Spine. 1997;22:2066–2072. doi: 10.1097/00007632-199709150-00002. [DOI] [PubMed] [Google Scholar]
- 15.Brisby H. Pathology and possible mechanisms of nervous system response to disc degeneration. J Bone Joint Surg [Am] 2006;88A(Suppl 2):68–71. doi: 10.2106/JBJS.E.01282. [DOI] [PubMed] [Google Scholar]
- 16.Burstein D, Gray ML, Hartman AL, Gipe R, Foy BD. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J Orthop Res. 1993;11:465–478. doi: 10.1002/jor.1100110402. [DOI] [PubMed] [Google Scholar]
- 17.Butler D, Trafimow JH, Andersson GB, McNeill TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15:111–113. doi: 10.1097/00007632-199002000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Chiu EJ, Newitt DC, Segal MR, Hu SS, Lotz JC, Majumdar S. Magnetic resonance imaging measurement of relaxation and water diffusion in the human lumbar intervertebral disc under compression in vitro. Spine. 2001;26:E437–E444. doi: 10.1097/00007632-200110010-00017. [DOI] [PubMed] [Google Scholar]
- 19.East CJ, Stanton H, Golub SB, Rogerson FM, Fosang AJ. ADAMTS-5 deficiency does not block aggrecanolysis at preferred cleavage sites in the chondroitin sulfate-rich region of aggrecan. J Biol Chem. 2007;282:8632–8640. doi: 10.1074/jbc.M605750200. [DOI] [PubMed] [Google Scholar]
- 20.Eyre DR. Biochemistry of the intervertebral disc. Int Rev Connect Tissue Res. 1979;8:227–291. doi: 10.1016/b978-0-12-363708-6.50012-6. [DOI] [PubMed] [Google Scholar]
- 21.Eyre DR, Muir H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem J. 1976;157:267–270. doi: 10.1042/bj1570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fennell AJ, Jones AP, Hukins DW. Migration of the nucleus pulposus within the intervertebral disc during flexion and extension of the spine. Spine. 1996;21:2753–2757. doi: 10.1097/00007632-199612010-00009. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh P, Taylor TK, Braund KG, Larsen LH. The collagenous and non-collagenous protein of the canine intervertebral disc and their variation with age, spinal level and breed. Gerontology. 1976;22:124–134. doi: 10.1159/000212129. [DOI] [PubMed] [Google Scholar]
- 24.Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine. 1998;23:1612–1626. doi: 10.1097/00007632-199807150-00021. [DOI] [PubMed] [Google Scholar]
- 25.Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20:107–121. doi: 10.1016/S0945-053X(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 26.Hedman TP, Fernie GR. In vivo measurement of lumbar spinal creep in two seated postures using magnetic resonance imaging. Spine. 1995;20:178–183. doi: 10.1097/00007632-199501150-00008. [DOI] [PubMed] [Google Scholar]
- 27.Janevic J, Ashton-Miller JA, Schultz AB. Large compressive preloads decrease lumbar motion segment flexibility. J Orthop Res. 1990;9:228–236. doi: 10.1002/jor.1100090211. [DOI] [PubMed] [Google Scholar]
- 28.Johannessen W, Auerbach JD, Wheaton AJ, Kurji A, Borthakur A, Reddy R, Elliott DM. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine. 2006;31:1253–1257. doi: 10.1097/01.brs.0000217708.54880.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg [Am] 2006;88A(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 30.Kerttula LI, Jauhiainen JP, Tervonen O, Suramo IJ, Koivula A, Oikarinen JT. Apparent diffusion coefficient in thoracolumbar intervertebral discs of healthy young volunteers. J Magn Reson Imaging. 2000;12:255–560. doi: 10.1002/1522-2586(200008)12:2<255::AID-JMRI7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 31.Kerttula L, Kurunlahti M, Jauhiainen J, Koivula A, Oikarinen J, Tervonen O. Apparent diffusion coefficients and T2 relaxation time measurements to evaluate disc degeneration. A quantitative MR study of young patients with previous vertebral fracture. Acta Radiol. 2001;42:585–591. doi: 10.1080/028418501127347241. [DOI] [PubMed] [Google Scholar]
- 32.Kimura JH, Hardingham TE, Hascall VC, Solursh M. Biosynthesis of proteoglycans and their assembly into aggregates in cultures of chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1979;254:2600–2609. [PubMed] [Google Scholar]
- 33.Kuo R, Panchal M, Tanenbaum L, Crues JV., 3rd 3.0 Tesla imaging of the musculoskeletal system. J Magn Reson Imaging. 2007;25:245–261. doi: 10.1002/jmri.20815. [DOI] [PubMed] [Google Scholar]
- 34.Laurent D, Wasvary J, Yin J, Rudin M, Pellas TC, O’Byrne E. Quantitative and qualitative assessment of articular cartilage in the goat knee with magnetization transfer imaging. Magn Reson Imaging. 2001;19:1279–1286. doi: 10.1016/S0730-725X(01)00433-7. [DOI] [PubMed] [Google Scholar]
- 35.LeBlanc AD, Evans HJ, Schneider VS, Wendt RE, 3rd, Hedrick TD. Changes in intervertebral disc cross-sectional area with bed rest and space flight. Spine. 1994;19:812–817. doi: 10.1097/00007632-199404000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 37.Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J Bone Joint Surg [Am] 2006;88A(Suppl 2):76–82. doi: 10.2106/JBJS.E.01448. [DOI] [PubMed] [Google Scholar]
- 38.Malko JA, Hutton WC, Fajman WA. An in vivo magnetic resonance imaging study of changes in the volume (and fluid content) of the lumbar intervertebral discs during a simulated diurnal load cycle. Spine. 1999;24:1015–1022. doi: 10.1097/00007632-199905150-00016. [DOI] [PubMed] [Google Scholar]
- 39.Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine. 1990;15:402–410. doi: 10.1097/00007632-199005000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Mort JS, Dodge GR, Roughley PJ, Liu J, Finch SJ, DiPasquale G, Poole AR. Direct evidence for active metalloproteinases mediating matrix degradation in interleukin 1-stimulated human articular cartilage. Matrix. 1993;13:95–102. doi: 10.1016/s0934-8832(11)80068-5. [DOI] [PubMed] [Google Scholar]
- 41.Mwale F, Demers CN, Michalek AJ, Beaudoin G, Goswami T, Beckman L, Iatridis JC, Antoniou J. Evaluation of quantitative magnetic resonance imaging, biochemical and mechanical properties of trypsin-treated intervertebral discs. J Magn Res Imaging. 2008;27:563–573. doi: 10.1002/jmri.21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–64. doi: 10.22203/ecm.v008a06. [DOI] [PubMed] [Google Scholar]
- 43.Nachemson AL. Disc pressure measurements. Spine. 1981;6:93–97. doi: 10.1097/00007632-198101000-00020. [DOI] [PubMed] [Google Scholar]
- 44.Newall JF, Ayad S. Collagen IX isoforms in the intervertebral disc. Biochem Soc Trans. 1995;23:517S. doi: 10.1042/bst023517s. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen AN, Johannessen W, Yoder JH, Wheaton AJ, Vresilovic EJ, Borthakur A, Elliott DM. Noninvasive quantification of human nucleus pulposus pressure with use of T1ρ-weighted magnetic resonance imaging. J Bone Joint Surg [Am] 2008;90-A:796–802. doi: 10.2106/JBJS.G.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paajanen H, Komu M, Lehto I, Laato M, Haapasalo H. Magnetization transfer imaging of lumbar disc degeneration. Correlation of relaxation parameters with biochemistry. Spine. 1994;19:2833–2837. doi: 10.1097/00007632-199412150-00018. [DOI] [PubMed] [Google Scholar]
- 47.Panjabi MM, Brand RAJ, White AA. Mechanical properties of the human thoracic spine as shown by three-dimensional load-displacement curves. J Bone Joint Surg [Am] 1976;58A:642–652. [PubMed] [Google Scholar]
- 48.Panjabi M, Brown M, Lindahl S, Irstam L, Hermens M. Intrinsic disc pressure as a measure of integrity of the lumbar spine. Spine. 1988;13:913–917. doi: 10.1097/00007632-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Panjabi MM, Oxland TR, Yamamoto I, Crisco JJ. Mechanical behavior of the human lumbar and lumbosacral spine as shown by three-dimensional load-displacement curves. J Bone Joint Surg [Am] 1994;76A:413–424. doi: 10.2106/00004623-199403000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Perie D, Iatridis JC, Demers CN, Goswami T, Beaudoin G, Mwale F, Antoniou J. Assessment of compressive modulus, hydraulic permeability and matrix content of trypsin-treated nucleus pulposus using quantitative MRI. J Biomech. 2006;39:1392–1400. doi: 10.1016/j.jbiomech.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 51.Perie D, Sales de Gauzy J, Curnier D, Hobatho MC. Intervertebral disc modeling using a MRI method: migration of the nucleus zone within scoliotic intervertebral discs. Magn Reson Imaging. 2001;19:1245–1248. doi: 10.1016/S0730-725X(01)00452-0. [DOI] [PubMed] [Google Scholar]
- 52.Race A, Broom ND, Robertson P. Effect of loading rate and hydration on the mechanical properties of the disc. Spine. 2000;25:662–669. doi: 10.1097/00007632-200003150-00003. [DOI] [PubMed] [Google Scholar]
- 53.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 54.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 55.Spengler DM, Bigos SJ, Martin NA, Zeh J, Fisher L, Nachemson A. Back injuries in industry: a retrospective study. I. Overview and cost analysis. Spine. 1986;11:241–245. doi: 10.1097/00007632-198604000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Tertti M, Paajanen H, Laato M, Aho H, Komu M, Korman M. Disc degeneration in magnetic resonance imaging. A comparative biochemical, histologic, and radiologic study in cadaver spines. Spine. 1991;16:629–634. doi: 10.1097/00007632-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Weidenbaum M, Foster RJ, Best BA, Saed-Nejad F, Nickoloff E, Newhouse J, Ratcliffe A, Mow VC. Correlating magnetic resonance imaging with the biochemical content of the normal human intervertebral disc. J Orthop Res. 1992;10:552–561. doi: 10.1002/jor.1100100410. [DOI] [PubMed] [Google Scholar]
- 58.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. Expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wheaton AJ, Dodge GR, Borthakur A, Kneeland JB, Schumacher HR, Reddy R. Detection of changes in articular proteoglycan by T(1rho) magnetic resonance imaging. J Orthop Res. 2005;23:102–108. doi: 10.1016/j.orthres.2004.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol. 2000;35:602–621. doi: 10.1097/00004424-200010000-00007. [DOI] [PubMed] [Google Scholar]