Abstract
Disc degeneration is a common disorder. Although the back pain that can develop in association with this is rarely life-threatening, the annual cost in terms of morbidity, lost productivity, medical expenses and workers’ compensation benefits is significant. Surgical intervention as practised currently is directed towards removing the damaged or altered tissue. Development of new treatment modalities is critical as there is a growing consensus that the strategies used currently for symptomatic degenerative disc disease may not be effective. Accordingly, there is a need to develop an entirely new way to treat this disorder; regenerative medicine and tissue engineering approaches appear particularly promising in this regard. This paper reviews some of the challenges that currently are limiting the clinical application of this approach to the treatment of disc degeneration.
Keywords: Tissue engineering, Intervertebral disc, Implantation, Review
Introduction
The human spine consists of 33 vertebral bodies which with the exception of C1 and C2, and the sacrum, are separated by an intervertebral disc (IVD) [17]. This is a specialized structure consisting of three interdependent tissues, the annulus fibrosus (AF) and the nucleus pulposus (NP) which are sandwiched in part between two cartilage endplates (CEP) that are integrated to the adjacent vertebral bodies [43, 104]. The outer annulus fibrosus is responsible for withstanding tensile stresses primarily (circumferential, longitudinal and torsion), the NP compressive stress and the inner annulus a mixture of both [43, 104]. Together these can handle more load than each tissue alone stressing the importance of intact properly integrated structures. The cartilage endplate plays a critical role in maintaining the viability of nucleus pulposus cells [42, 74]. It also prevents protrusion of the nucleus pulposus into the adjacent vertebral body. Thus all three tissues are critical to the proper functioning of the disc.
Disc degeneration is a common disorder. In an autopsy study, 97% of individuals 50 years or older showed disc degeneration, a disease process that involves the annulus fibrosus, nucleus pulposus, and CEP [69]. The aetiology of this disorder is unknown but may be due to the relative avascularity of the tissue, mineralization of or trauma to the cartilage endplate, mechanical factors, vertebral body microfracture and loss of notochordal cells. Whatever the exact pathway, disc degeneration is certainly associated with aging and has a strong genetic component [1, 10, 96, 109, 115, 120]. The back pain that can develop either in association with or as a result of this disease has a lifetime prevalence of 80%. Approximately 1 in 50 Canadians become disabled by back pain and 40% of all workplace absences in Canada are due to back pain [46, 59, 86]. Although back pain is rarely life-threatening, the annual cost in terms of lost productivity, medical expenses and workers’ compensation benefits was estimated at $50 billion in 1998 in the United States alone [27]. Surgical intervention as practised currently is directed towards removing the damaged or altered tissue. Discectomy, which although may relieve pain, does not restore disc height or its original load bearing capacity [83], the loss of which has been implicated in the pathogenesis of this disease. Spinal fusion, a commonly performed surgical procedure (200,000 spinal fusions in 2002 in the US [16]), is not an optimal treatment as it is not always successful [53] and can result in limited flexibility, in addition to possibly inducing degenerative changes in adjacent vertebrae [48, 70, 79]. In an attempt to preserve motion, other approaches have been developed [89] such as partial or total disc replacement [53, 87, 103, 107, 119, 126]. As there are numerous clinical contraindications to artificial disc implant surgery and as the complications can be catastrophic, the confirmation of the utility of this approach awaits long term outcome data.
Tissue engineering and the intervertebral disc
New treatment modalities are critical as there is a growing consensus that the strategies used currently for symptomatic degenerative disc disease are not effective. Accordingly, there is a need to develop an entirely new way to treat this disorder; regenerative medicine and tissue engineering approaches appear particularly promising in this regard [6, 19, 78, 89].
Cell-based therapies avoid the functional impact on the adjacent tissues, in terms of the consequences of metal fatigue and reaction to wear debris that can result after prosthesis implantation, and importantly the regenerated tissue can remodel and respond to load in a way synthetic prostheses can not. Clearly unlike articulating joints, where only cartilage and in some settings bone, need to be repaired, in the disc there are three tissues, the nucleus pulposus, annulus fibrosus, and the CEP, all of which can be involved in the disease process, and may require repair. This adds a level of complexity to biological disc repair. Nevertheless there are now both animal and human studies into cell therapies for the disc, suggesting that this approach may be feasible. It has been shown that reinsertion of autologous nucleus pulposus cells or stem cells delays degeneration in experimental models of disc degeneration [32, 39, 65, 80, 98, 108] and injection of autologous nucleus pulposus cells are being used in humans with herniated discs [65]. However, it is not yet clear what the optimal implant will be. For example it could be that transplantation of tissue rather than cells may be a better approach as a study in rabbits demonstrated that insertion of nucleus pulposus tissue was more effective than nucleus pulposus cells in delaying the development of degenerative disc disease [77]. However, implantation of cells alone, or even nucleus pulposus tissue, will not reverse changes such as CEP calcification, which may be the cause of the disease process nor will it be able to restore structural functionality when all three tissues are degenerated. In these settings the optimal approach would be to replace the entire disc. Furthermore, an IVD replacement will restore load sharing and natural kinematics and so by preserving spinal motion will prevent development of adjacent segment disease. Thus, although repairing the nucleus pulposus may be effective in the short term replacement of the entire spinal unit may be necessary for long-term efficacy in some settings.
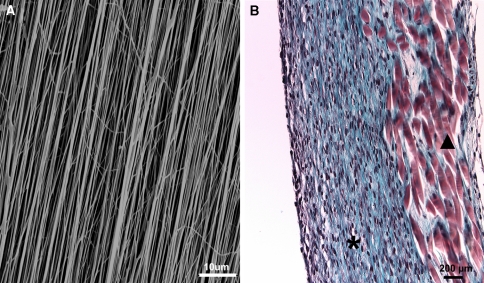
IVD and vertebral body graft transplants have been used successfully in animal models [26, 61, 81] and more recently in humans [95] and serve as proof-of-concept for biological disc replacement. As autografts are not an option and use of allografts is limited by availability and their ability to transmit disease, research groups have been developing methods to generate a functional spinal unit using tissue-engineering principles. Mizuno et al. have generated a model of an intervertebral disc using nude mice as the bioreactor. However, similar to many animal studies, it has not been possible to translate this knowledge into clinical practice [71, 72]. Others have taken a different approach and are working towards generating an IVD in vitro. For example, Kandel and co-workers have been able to show that it is possible to form the inner portion of this disc consisting of nucleus pulposus tissue adherent to a subjacent layer of cartilaginous tissue (representing the CEP), which is integrated with a biodegradable porous bone substitute material [36, 101]. The bone substitute will allow for fixation of the construct into the vertebral body by bone ingrowth into the pores, not filled by cartilage, following implantation. However, forming the multilamellar annulus fibrosus has been more challenging because of the complex organization of this tissue [82] but ongoing studies (Fig. 1) suggest that this will be possible via synthesis of aligned and oriented scaffolds. For example, weaving or electrospinning can produce aligned fibres, such as those found in natural annulus fibrosus (Fig. 1). These techniques show promise for providing scaffolds mimicking the highly organised natural annulus fibrosus and in some settings can have mechanical properties approximating that of a single annulus lamella (oriented silk fibres or polyurethane naofibrous scaffolds; unpublished data) [76].
Fig. 1.
Annulus fibrosus tissue engineering: a Scanning electron micrograph showing aligned electrospun polyurethane nanofibrous scaffold. b Annulus fibrosus tissue formation (asterisk) in vitro on aligned silk fibrous scaffolds (filled triangle). (Photographs courtesy of G. Chang, R. Kandel et al.)
At present there is no consensus as to which approach, whether implantation of cells, tissues or an entire disc, will be optimal. It is likely that the treatment offered will be personalized to the individual and reflect the extent of disease. For example when early symptomatic degeneration is present and changes occur only in the nucleus pulposus then it may be appropriate to use cells alone. However, when the disease affects both the nucleus pulposus and annulus fibrosus, the use of cells or nucleus pulposus alone is unlikely to be sufficient and in that setting disc replacement may be more suitable. Clearly we are only in the infancy of biological disc repair and more studies are required before we can delineate the appropriate treatment paradigm. Concomitant with these studies it will also be important to understand the mechanism(s) leading to back pain to ensure that biological treatment will be effective in ameliorating this symptom.
Source of cells
One of the major issues limiting the clinical application of biological disc repair is the identification of a suitable source of cells. There are clearly many aspects to consider. Should the cells be autologous, allogeneic or even xenogeneic? Should they be cultured extensively in vitro to provide a large number of cells, or should they be primed in any way or ‘conditioned’ by growing in an environment similar to that into which they will be implanted? Should the implanted cells be sourced from the same location that we are trying to repair? Should stem cells be used and if so, from what source: embryonic, or adult and if it is the latter from which site should they be obtained bone marrow, adipose tissue, or from some other location? As yet there is no consensus of the optimal cell type or source, as each potential option has some apparent limitations as discussed below.
Disc cells
The intervertebral disc as a source of autologous cells poses some difficulties, partly due to the low cell number of the tissue (especially the nucleus pulposus), the variety of cell phenotypes within the disc and also the difficulty of accessing the cells. A biopsy of healthy disc from which to extract the cells, as happens in autologous chondrocyte implantation (ACI) for cartilage repair [20], would inevitably mean puncturing the annulus fibrosus and nucleus pulposus thereby damaging structures and thus mechanical function. In addition, removal of disc tissue might be likely to have detrimental effects and lead to further nuclear degeneration, if the situation in the human resembles several animal species where this is used as a means to induce disc degeneration [5]. There are believed to be at least two discrete populations of cells within the adult human disc, the more fibroblastic cells of the annulus fibrosus and the more rounded cells of the nucleus pulposus [93]. These two cell types differ considerably, not only in their morphology, but also in their metabolism [41]. In addition the cartilage endplate is likely to be populated by another distinct cell type. Hence the phenotype sought for cell therapy would ultimately depend on what approach was being taken to tissue engineering and what region(s) of the disc was to be repaired or regenerated.
The viability and metabolic activity which could be anticipated from adult human disc cells is another cause for concern if autologous disc cells are to be used. A large proportion of disc cells are reported to be dead or dying [18], either via necrosis [114] or apoptosis [30]. Cell senescence has been demonstrated as a frequent occurrence in adult human disc cells [30, 31, 56, 92], a phenomenon which is known to alter cell metabolism, often unfavourably. Interestingly, a recent study demonstrated that matrix retention by nucleus pulposus cells obtained from cows (adolescent) is impaired compared to those obtained from younger animals (calves). Furthermore disc cell matrix gene expression is decreased. If these changes occur in human cells as well, the use of disc cells could be limited [52].
Stem cells
Autologous stem cells could be obtained from bone marrow (marrow stromal cells, MSCs) or adipose tissue (adipose-derived stem cells; ADSC) or possibly other tissues [4, 15, 127]. MSCs are known to have the capability to differentiate into several different cell types including hyaline cartilage, bone, adipose tissue or muscle. There is little known about the characteristic molecular profile of the AF and nucleus pulposus cells so it is not known whether MSCs can differentiate into the disc cell phenotypes. Steck et al. has suggested that MSCs can adopt a gene expression profile resembling native disc cells but the molecules they examined for are also found in cartilage tissue [111]. Co-culturing MSC with disc cells appears to speed up the conversion to a chondrogenic/discogenic phenotype [57, 90, 118], possibly via paracrine effects or cell–cell interactions. The effect of implantation of MSCs or adipose derived stem cells have been studied in vivo in animal models and appear promising but have not as yet been used to repair human discs [97, 108, 125]. Haematopoetic precursor stem cells, however, have been used clinically. Cells isolated from bone marrow (but with no characterisation of the cells) were injected into human discs of individuals with discogenic pain [38]. This treatment was followed by 2 weeks of hyperbaric oxygen therapy, nominally to improve the oxygen supply to the implanted cells. Twelve months following treatment there was no relief in back pain in the treated patients and 75% progressed to a spinal fusion indicating that these are not likely to be an appropriate source of cells. Embryonic stem cells (hESC) have the capacity to produce intervertebral disc tissue, though very little work has been carried out in this area. It is obviously a source of cells totally unacceptable to a large proportion of the world’s population, due to religious and ethical reasons [4]. Stem cells from umbilical cord blood may be more acceptable, and increasingly available as cord blood banks are established, but again little is known about their capacity to undertake a disc cell phenotype [15]. Similarly inducible progenitor cells hold much promise but their role in disc tissue engineering has not yet been investigated [75, 124].
Notochordal cells
Another group of cells which have been investigated in terms of tissue engineering, is notochordal cells. These cells are present in the embryonic human nucleus pulposus [44]. Soon after birth, however, the number diminishes very rapidly in humans and by early adulthood they have virtually disappeared. In contrast, in some animals, such as rabbits and rodents, they are present throughout life [5]. Some breeds of dogs which do not present commonly with disc prolapse and degenerative disc disease, non-chondrodystrophoid dogs, also retain notochordal cells all through adulthood. This, together with the fact that they have been shown to stimulate the production of proteoglycans when co-cultured with mature nucleus pulposus cells [3], has given rise to a belief that they may be a useful cell type to consider in disc tissue engineering. In reality, however, there is no human source for these cells readily available [44].
Identification of disc cells
Research into directing stem cells or notochordal cells towards a discogenic phenotype is hindered by lack of specific markers which can distinguish nucleus pulposus (or even annulus fibrosus or CEP) cells from articular chondrocytes or nucleus cells from annulus cells [23, 58]. Markers commonly used for showing conversion of stem cells to a disc cell phenotype are expression of aggrecan and type II collagen and of SOX-9 [91]; these are no different, however, from those used to identify articular chondrocytes. Other markers such as HIF-1α and GLUT-1 expression have been suggested but these are constitutively expressed by most cell types and their use as markers for disc cells has not been validated, nor have other markers which have been suggested such as CD-44 and cytokeratin [58, 85, 91]. It is important that cells used for repairing the disc differentiate to the correct phenotype as even though the major macromolecular components of the nucleus pulposus, cartilage endplate and articular cartilage are very similar, the organisation of the extracellular matrix and their biomechanical properties are very different in the three tissues. Several groups are currently searching for specific markers to identify disc cell phenotypes but whether these will be the same across species remains to be seen. This is particularly important as factors such as co-culture, growth factors and/or mechanical stresses could be used to influence stem cell differentiation to disc cells, as has been done for chondrocytes. However, as we have not yet delineated characteristic biomarkers of either the annulus fibrosus or nucleus pulposus phenotypes we do not yet know which factors will be effective in influencing differentiation into disc cells.
Expansion of cell number and in vitro culture
In cell therapies for other musculoskeletal tissues the number of cells introduced per defect is in the range of 5–10 million cells [7]. If similar numbers of cells are required for cell therapy of one disc there would be a requirement to expand cells in culture, both MSC or differentiated cells, typically by growing them in monolayer to encourage cell proliferation. In ACI the number of passages is restricted to 2, or at maximum to 3 (representing approximately 3–6 population doublings), prior to cell implantation. This could be difficult to accomplish for autologous disc cell therapy, due to the low cell density in the tissue. If nucleus pulposus cells are being cultured in monolayer then there is likely to be divergence from the nucleus pulposus phenotype, with increasing culture time in monolayer which might alter the way the cells behaved when re-implanted into the disc. Simply changing the morphology of the cells in vitro (from rounded in vivo to flattened cells in monolayer) leads to dedifferentiation and alters matrix production [41]. Mignotte et al. [68] has also shown that cells can dedifferentiate in regard to energy metabolism when cultured in monolayer; however, what happens when these cells are then placed in a 3D culture is not known.
There are additional concerns with extensive culture and population doublings in that cells can become senescent or the cell karyotype can even become altered. For example, a subpopulation of cells in human MSC culture was noted to appear morphologically distinct from typical MSCs [122]. These cells showed a high level of telomerase activity compared with typical MSCs and formed tumours with aggressive growth when transplanted into NOD/SCID mice.
Other potential problems with growing the cells in vitro prior to implantation could arise due to their contact with animal cells or products. The most commonly used animal product in cell culture is foetal calf serum; this could pose a risk of cross-contamination, induce an immune response, or be an infection risk [37]. There is great interest in developing alternatives to calf serum to avoid these problems such as culturing the cells in the patient’s own serum [37, 55, 102] or using human platelet lysate as an alternative [100]. hESC are commonly cultured on a layer of murine feeder cells which at present together with issues of histocompatability restricts their clinical use currently.
Any way of minimising the time that cells, to be implanted into a patient, spend in culture, and the degree of manipulation (especially if under different conditions from that which the cells experience in vivo), may be advantageous in terms of patient safety. The exact risks remain poorly characterised at this time and are likely to be better identified as experience with cell therapies increases.
What is the right scaffold?
Various approaches have been developed to form the different tissues of the disc and can be divided broadly into two groups, scaffold-free or scaffold-containing. The scaffold can have a variety of roles such as to help retain the cells in the desired location, to provide mechanical properties (sufficient for weight bearing as the disc is always loaded) and/or biochemical cues to the tissue as it is developing or to facilitate and guide tissue ingrowth [45].
The choice of scaffold is critical as it can affect the tissue that forms. Scaffold fibre diameter and stiffness can influence cell function and proliferation. It can also influence cell orientation and the molecules by which the cell attaches, all of which can have profound effects on the cell [9, 24, 60]. In addition the presence of the scaffold can influence the recipient tissue reaction after implantation. For example the polylactic/glycolic acid systems used currently for tissue engineering purposes release acid as they degrade and the lowered pH can affect cell synthesis [88].
It is clear that different scaffolds will be required for tissue engineering repair of nucleus, annulus and endplate but no rational choice is yet possible as at present information on optimal cell seeding numbers, how cells react to different scaffolds, and how the scaffolds affect matrix accumulation is still very limited. Moreover, most studies on scaffolds for repair of the nucleus, for example, have not considered the biomechanical requirements of this tissue. To date, a variety of scaffolds have been utilized to support formation of the different components of the disc such as chitosan, silk, collagen, alginate, and fibrin just to list a few [21, 33, 35, 78, 94]. The suitability of these scaffolds for the most part have been determined in vitro by evaluating parameters such as collagen and aggrecan gene expression, proteoglycan and collagen content, features that provide limited assessment of the cells/tissue and how they will function after implantation. There have been several studies in which cell seeded scaffolds were placed in vivo and then evaluated [71, 99]. In one study an IVD was developed; a polylactic acid scaffold was used for the annulus fibrosus and was seeded with annulus fibrosus cells whereas the nucleus was generated using agarose seeded with nucleus pulposus cells. After one day of culture the construct was implanted subcutaneously in athymic mice [71, 72]. After 12 weeks the cells were viable, formed tissue but the collagen content was low, and the mechanical properties were about 50% of the in vivo disc. There is clearly a long way to go before we identify the optimal scaffold.
It may be that under certain conditions it is not even necessary to use a scaffold. Scaffold-free tissue engineering is particularly suitable for proteoglycan-rich tissues, such as the nucleus pulposus and CEP, where the thickness of the tissue to be regenerated is limited. It has been possible to generate scaffold-free nucleus pulposus tissue in vitro [101, 112]. Seguin et al. [101], using a calcium polyphosphate substrate on which the cells grow, showed that the scaffold-free tissue that formed had proteoglycan content and compressive mechanical properties similar to the tissue from which the cells were obtained. This approach has also been utilized to generate cartilaginous tissue in vitro as well [121]. In vivo sheep studies have demonstrated that following implantation into focal defects in the joint, cartilage tissue thickness increases and its mechanical properties improve, confirming the utility of a scaffold-free tissue engineering approach [51]. Interestingly this approach can be used to form composite tissues, such as nucleus pulposus/cartilage (CEP) which is necessary for disc tissue engineering [36]. In vivo testing in the spine is required to confirm the success of this approach.
Methods of insertion
The method of insertion of a tissue engineered product to the site of delivery will depend largely on whether it is a complete disc or only part of it. For example, if a complete intervertebral disc has been engineered, with a composite nucleus pulposus and annulus fibrosus structure, it will require more invasive surgery to implant than, for example, cell therapy to replace the nucleus alone. The latter could simply be delivered via a needle, into the central nucleus pulposus, if the carrier was adequately fluid. Such a method of disc cell therapy is currently being utilized [65].
Implantation of a tissue engineered annulus fibrosus alone to the in vivo tissue could prove more challenging, and the requirements to accomplish this would depend to some extent on the clinical situation. If the product was being applied to ‘seal’ the AF after disc herniation, it may require some form of ‘patch’ which could be inserted and applied via ‘keyhole’ type of instrumentation. Tissue engineered annulus fibrosus may be designed to retain a nuclear implant aimed at restoring disc height. Any such ‘patch’, however, would require good adherence to the native annulus fibrosus in order to retain the inserted implant and would also have to be strong enough to survive the mechanical forces generated in the disc during loading and movement.
The technique required to insert a complete tissue engineered intervertebral disc would also depend on its exact structure. If it contained something resembling a bony ‘endplate’ [36] then its insertion would require surgical interventions similar to that used for prosthetic disc implants, usually via anterior exposure and surgery. It would be anticipated that the endplate would interface and ultimately integrate with the vertebral bone by bone ingrowth and ensure the correct location and attachment, at least superiorly and inferiorly. Composite structures of annulus fibrosus and nucleus pulposus alone may be inserted into a disc space, relying on being ‘contained’ between the two vertebral bodies. The insertion and application of tissue engineered products in vivo is an important area which is perhaps not always given adequate consideration in the overall design of tissue engineered constructs. It will certainly have a great influence on whether the tissue engineered product succeeds in the clinic.
Some in vivo challenges
Cell therapies for intervertebral disc repair will only be successful if transplanted cells or tissue engineered disc are introduced into an environment conducive to maintaining and encouraging cell activity. Unlike other organs, ingrowth of vessels into the implanted tissue is not a requirement as the adult healthy disc is largely avascular. However, the local environment is important. Two environmental factors which need to be considered are nutrient supply and mechanical load; these are generally not troublesome in cell or tissue culture in vitro but could be of major concern in vivo as discussed below.
Disc nutrition
Disc cells require nutrients to function and maintain viability. Mature disc nucleus cells appear to generate ATP virtually only by glycolysis. They thus use glucose and produce lactic acid at a high rate [13, 110]. Although they do not require oxygen to remain alive, cellular activity and matrix synthesis is reduced significantly at low oxygen concentrations [42, 47]. Cells from the annulus also appear to require little oxygen and like nucleus cells produce their energy primarily by glycolysis [2, 40, 47]. In vitro studies have shown that because of the high demand for glucose, the viability of disc cells in 3D culture is compromised if glucose levels fall below 0.2 mM glucose and some cell death is seen even below 0.5 mM glucose [14]. Low pH arising from accumulation of lactic acid (pH < 6.7) is also detrimental to disc cell survival [14]. Maintenance of adequate levels of glucose and pH in cell culture in the laboratory can be readily achieved; however, this is not necessarily the case in the disc in situ. Indeed loss of nutrient supply leading to fall in glucose levels and accumulation of lactic acid is thought to contribute to the development of disc degeneration [74].
As the adult disc is avascular, the cells rely on blood vessels outside the disc to supply them with the glucose and oxygen (and other nutrients) they require and to remove metabolic wastes [34]. Most of the disc, apart from the outer annulus, is supplied with nutrients by capillaries arising from blood vessels of the vertebral body; these capillaries penetrate the subchondral plate and terminate at the cartilaginous endplate. Nutrients then diffuse through this thin layer of cartilage and through the dense disc matrix to the cells which may lie as much as 8 mm from the nearest blood vessels. The nutritional situation of these cells is thus precarious and loss of nutrient supply has long been thought to be a major cause of cell death and disc degeneration [116]. This hypothesis has been confirmed by a recent in vivo MRI study on 150 human discs which showed that nutrient supply is diminished significantly even in moderately degenerated discs although it can increase markedly in end stage disease as the result of vascular invasion [84]. This loss of nutrient supply appears to arise mainly because of calcification of the cartilaginous endplate or because of endplate sclerosis which increases with age and degree of degeneration [11].
Calcification of the endplate thus poses a major problem for the success of cell-based therapies for regenerating or repairing the disc, even if moderately degenerated. If degeneration occurred or progressed because the calcified endplates blocked nutrient supply to the cells which then became inactive or died, implanted cells will suffer the same fate. In line with this, injection of factors to stimulate endogenous cell biosynthesis will also not be effective as the cells still have inadequate nutrition. Thus unless it can be assured that a degenerate disc has a nutrient supply which can support the implanted cells, treatment using a cell therapy approach is both pointless and unethical. The way forward in the short-term is to identify patients who have an adequate nutrient supply and hence may be able to benefit from these treatments. In the long term treatment may be possible for others through replacement or repair of the calcified endplate and nutrient supply sources.
Mechanical loading
In vivo, the disc is always under load from body weight and muscle activity. Even when lying down the pressure in the lumbar discs is ~0.2 MPa [73, 123]; it increases 3–5 fold when rising and changes with posture and movement. The disc thus has to withstand these loads and allow the spine to bend, extend and rotate; maintenance of normal biomechanical responses to loading is one of the main rationales behind tissue engineering of the disc. However, disc biomechanical behaviour ultimately depends on the organisation and composition of the macromolecules which make up the tissue; full physiological responses to external loads may not thus be achieved in a tissue-engineered disc until concentrations and network architecture of the various macromolecules reach those found in vivo. Matrix production and turnover is slow in normal and degenerate human discs with aggrecan half-lives being ~12 and ~8 years, respectively and that of collagen >90 years [105, 106]. In animals, repair of the outer layers of the annulus is similarly slow [66]. Thus it may take the implanted cells months or perhaps years to regenerate the nucleus pulposus so that it has a similar amount of matrix molecules to that present in healthy adult animal discs [28], although the level of regeneration required to achieve a clinically successful repair is as yet unknown.
The large loads the disc experiences in vivo also provide a challenge for success of cell therapies. If biomechanically immature tissues or cells alone are implanted, the disc may not be able to withstand the loads routinely experienced and may collapse if not protected; the consequent abnormal forces on the disc cells may lead them to produce an abnormal matrix. In addition, other spinal structures such as the facet joints would also be exposed to inappropriate loads during the extended period of time before disc height and properties were restored, possibly leading to their degeneration also [50]. Growing the disc in vitro until sufficiently mature to carry load is possible. Further development is required before such an approach can be used clinically. Another approach would be to create a scaffold, biodegradable or otherwise, capable of contributing to biomechanical function until the tissue has regrown sufficiently to support disc loading.
How do we assess success?
At present, there has been little discussion on how the performance of disc cell therapies can be monitored in humans in vivo. Apart from assessment of the effect of these therapies on the clinical symptoms of the patients, it would be useful if changes in disc composition, disc cell number and viability, and disc biomechanical behaviour could be monitored in relation to the original condition of the disc and time after treatment. This information would give an indication of whether the cells were alive and functioning and whether they were producing appropriate matrix macromolecules to restore disc height and biomechanical behaviour. Cartilage repair has been assessed through biopsies but since even needle puncture, depending on its size, can lead to disc degeneration [25, 54] biopsies are unlikely to be used to assess disc repair clinically.
Changes in disc height are one method of assessing success and can be carried out by radiological imaging. This method has been used for monitoring changes in animal models of disc regeneration [63]. However, repeated X-rays for monitoring changes in disc height are not possible for assessing repair rates in humans and the sensitivity may be inadequate for the slow rate of repair in humans if only one or two time points can be imaged.
MRI seems to be an ideal method for assessing changes in disc hydration and composition because of its non-invasive nature and routine clinical use [64]. However, it has proved difficult to correlate proton T1 and T2 images directly with aggrecan, water or collagen contents because of the complex relationships between these parameters and matrix biochemistry and organization [29]. T1rho-weighted MRI images are claimed to be able to assess aggrecan content of tissues; they do appear sensitive to degenerative changes in human discs and indeed detect changes before morphological signs are visible [49] but whether variations in the signal can be accounted for by variations in aggrecan alone is questionable [67]. Thus MRI imaging in its present state, though able to assess some qualities of the newly produced matrix, does not appear able to determine whether implanted cells or tissues are producing a matrix of the desired composition. MRI moreover, is usually measured when lying prone when the discs are relatively unloaded. Thus standard MRIs give little information on the biomechanical behaviour of discs treated with cell therapy and also on whether they are able to maintain disc height under load. Standing or upright MRIs, available in only a few centres at present, would give more useful information in this regard and a trial should be considered once cell therapies are used clinically.
Even though future developments in MRI might give more accurate information about the composition of the disc, because turnover of matrix is very slow [105, 106], MRIs cannot give information on the current state of the cells. Indeed, even if most of the disc cells were dead, it might be several years before the composition of the tissue changed enough for it to be detected biochemically by MRI. For instance the GAG/collagen ratio measured in scoliotic and non-scoliotic discs was similar even though scoliotic discs contained very few viable cells [117]. For monitoring success, measures of viability are thus essential but are difficult partly because of the disc’s low cell density. One possible method of measuring cell viability is through the determination of nutrient gradients. These are steep in normal healthy discs but are flat in discs with no functioning cells. Nutrient gradients have been measured in humans in vivo and in discs in vitro using needle microelectrodes [8, 12]. The technique is however, invasive (though no more than discography); whether it would be ethically acceptable for assessing the health of tissue engineered discs in vivo if these treatments come into routine clinical use is questionable. Clinically useful means of assessing cellular activity in vivo thus still requires development.
Recently methods of tracking cells, using MRI or optical imaging methods, have been shown to be possible in settings such as heart attacks and stroke [22, 62, 113]. This holds much promise for evaluating where injected cells end up residing which will be particularly important for assessing the validity of cell-based treatments.
It should be noted however, that whether the disc is repaired or not, success of these biological techniques must also be evaluated by determining the benefit to the patient and whether there has been relief of symptomatology. Thus some effort should be directed toward determining which patients will actually benefit from these treatments.
Summary
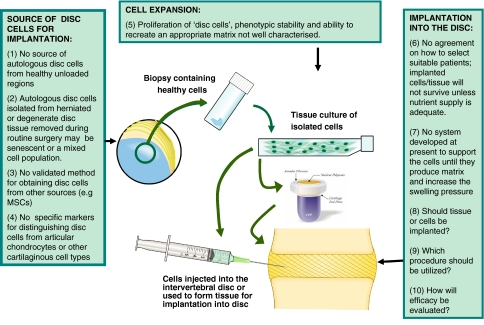
Clearly many challenges will have to be overcome before biological repair of the intervertebral disc is successful and can be used clinically for routine treatment of back pain and other spinal disorders (Fig. 2). Issues as discussed in this review, such as cell source, cell expansion, the necessity for and the type of scaffold, nutrient supply, and clinical assessment of effectiveness both structurally and in terms of patient morbidity are just some of them. Importantly all work done in disc tissue engineering to date has assumed that repairing the disc will automatically relieve symptoms including pain. As we are just beginning to understand the pathogenesis of back pain, it is essential that an approach able to identify those patients who will respond should be developed. Otherwise treatments may be offered to patients who will not benefit from it, placing a potentially useful procedure into disrepute.
Fig. 2.
Challenges for disc tissue engineering. Schematic showing some areas requiring development before this technique can be applied to the disc as a routine clinical treatment. CPP indicates substrate used as a bone substitute (e.g. calcium polyphosphate)
Acknowledgments
This work was supported by EU “MyJoint” (FP6: 28861(NEST)) (SR and JU) and CIHR and NIHR21 (RK). We thank Godfrey Chang for his help in preparing Fig. 1 and Andy Biggs for Fig. 2.
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31(18):2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, Risbud MV. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293(2):C621–C631. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- 3.Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246(1):129–137. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed N, Stanford WL, Kandel RA. Mesenchymal stem and progenitor cells for cartilage repair. Skeletal Radiol. 2007;36(10):909–912. doi: 10.1007/s00256-007-0333-3. [DOI] [PubMed] [Google Scholar]
- 5.Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson DG, Risbud MV, Shapiro IM, Vaccaro AR, Albert TJ. Cell-based therapy for disc repair. Spine J. 2005;5(6 Suppl):297S–303S. doi: 10.1016/j.spinee.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Bajada S, Harrison PE, Ashton BA, Cassar-Pullicino VN, Ashammakhi N, Richardson JB. Successful treatment of refractory tibial nonunion using calcium sulphate and bone marrow stromal cell implantation. J Bone Joint Surg Br. 2007;89(10):1382–1386. doi: 10.1302/0301-620X.89B10.19103. [DOI] [PubMed] [Google Scholar]
- 8.Bartels EM, Fairbank JC, Winlove CP, Urban JP. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine. 1998;23(1):1–7. doi: 10.1097/00007632-199801010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Bashur CA, Dahlgren LA, Goldstein AS. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(d, l-lactic-co-glycolic acid) meshes. Biomaterials. 2006;27(33):5681–5688. doi: 10.1016/j.biomaterials.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Battie MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(Suppl 2):3–9. doi: 10.2106/JBJS.E.01313. [DOI] [PubMed] [Google Scholar]
- 11.Benneker LM, Heini PF, Alini M, Anderson SE, Ito K. 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine. 2005;30(2):167–173. doi: 10.1097/01.brs.0000150833.93248.09. [DOI] [PubMed] [Google Scholar]
- 12.Bibby SR, Fairbank JC, Urban MR, Urban JP. Cell viability in scoliotic discs in relation to disc deformity and nutrient levels. Spine. 2002;27(20):2220–2228. doi: 10.1097/00007632-200210150-00007. [DOI] [PubMed] [Google Scholar]
- 13.Bibby SR, Jones DA, Ripley RM, Urban JP. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine. 2005;30(5):487–496. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 14.Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13(8):695–701. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bieback K, Kern S, Kocaomer A, Ferlik K, Bugert P. Comparing mesenchymal stromal cells from different human tissues: bone marrow, adipose tissue and umbilical cord blood. Biomed Mater Eng. 2008;18(1 Suppl):S71–S76. [PubMed] [Google Scholar]
- 16.Boden SD. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine. 2002;27(16 Suppl 1):S26–S31. doi: 10.1097/00007632-200208151-00007. [DOI] [PubMed] [Google Scholar]
- 17.Bogduk N. The inter-body joints and the intervertebral discs. In: Bogduk N, editor. Clinical anatomy of the lumbar spine and sarcum. New York: Churchill Livingstone; 1997. pp. 13–31. [Google Scholar]
- 18.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27(23):2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Brisby H, Tao H, Ma DD, Diwan AD. Cell therapy for disc degeneration—potentials and pitfalls. Orthop Clin North Am. 2004;35(1):85–93. doi: 10.1016/S0030-5898(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 20.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 21.Chang G, Kim HJ, Kaplan D, Vunjak-Novakovic G, Kandel RA. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur Spine J. 2007;16(11):1848–1857. doi: 10.1007/s00586-007-0364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang NK, Jeong YY, Park JS, Jeong HS, Jang S, Jang MJ, et al. Tracking of neural stem cells in rats with intracerebral hemorrhage by the use of 3T MRI. Korean J Radiol. 2008;9(3):196–204. doi: 10.3348/kjr.2008.9.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006;15(Suppl 3):S303–S311. doi: 10.1007/s00586-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Patra PK, Warner SB, Bhowmick S. Role of fiber diameter in adhesion and proliferation of NIH 3T3 fibroblast on electrospun polycaprolactone scaffolds. Tissue Eng. 2007;13(3):579–587. doi: 10.1089/ten.2006.0205. [DOI] [PubMed] [Google Scholar]
- 25.Elliott DM, Yerramalli CS, Beckstein JC, Boxberger JI, Johannessen W, Vresilovic EJ. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine. 2008;33(6):588–596. doi: 10.1097/BRS.0b013e318166e0a2. [DOI] [PubMed] [Google Scholar]
- 26.Frick SL, Hanley EN, Jr, Meyer RA, Jr, Ramp WK, Chapman TM. Lumbar intervertebral disc transfer. A canine study. Spine. 1994;19(16):1826–1834. doi: 10.1097/00007632-199408150-00006. [DOI] [PubMed] [Google Scholar]
- 27.Frymoyer JW, Cats-Baril WL. An overview of the incidences and costs of low back pain. Orthop Clin North Am. 1991;22(2):263–271. [PubMed] [Google Scholar]
- 28.Garvin PJ, Jennings RB, Smith L, Gesler RM. Chymopapain: a pharmacologic and toxicologic evaluation in experimental animals. Clin Orthop Relat Res. 1965;41:204–223. doi: 10.1097/00003086-196500410-00023. [DOI] [PubMed] [Google Scholar]
- 29.Gold GE, Beaulieu CF. Future of MR imaging of articular cartilage. Semin Musculoskelet Radiol. 2001;5(4):313–327. doi: 10.1055/s-2001-19042. [DOI] [PubMed] [Google Scholar]
- 30.Gruber HE, Hanley EN., Jr Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine. 1998;23(7):751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 31.Gruber HE, Ingram JA, Davis DE, Hanley EN Jr (2008) Increased cell senescence is associated with decreased cell proliferation in vivo in the degenerating human annulus. Spine J (in press) [DOI] [PubMed]
- 32.Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G, et al. Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine. 2002;27(15):1626–1633. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 33.Gruber HE, Leslie K, Ingram J, Norton HJ, Hanley EN. Cell-based tissue engineering for the intervertebral disc: in vitro studies of human disc cell gene expression and matrix production within selected cell carriers. Spine J. 2004;4(1):44–55. doi: 10.1016/S1529-9430(03)00425-X. [DOI] [PubMed] [Google Scholar]
- 34.Grunhagen T, Wilde G, Soukane DM, Shirazi-Adl SA, Urban JP. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88(Suppl 2):30–35. doi: 10.2106/JBJS.E.01290. [DOI] [PubMed] [Google Scholar]
- 35.Halloran DO, Grad S, Stoddart M, Dockery P, Alini M, Pandit AS. An injectable cross-linked scaffold for nucleus pulposus regeneration. Biomaterials. 2008;29(4):438–447. doi: 10.1016/j.biomaterials.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton DJ, Seguin CA, Wang J, Pilliar RM, Kandel RA. Formation of a nucleus pulposus-cartilage endplate construct in vitro. Biomaterials. 2006;27(3):397–405. doi: 10.1016/j.biomaterials.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Harrison PE, Ashton IK, Johnson WE, Turner SL, Richardson JB, Ashton BA. The in vitro growth of human chondrocytes. Cell Tissue Bank. 2000;1(4):255–260. doi: 10.1023/A:1010131729208. [DOI] [PubMed] [Google Scholar]
- 38.Haufe SM, Mork AR. Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006;15(1):136–137. doi: 10.1089/scd.2006.15.136. [DOI] [PubMed] [Google Scholar]
- 39.Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, et al. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26(5):589–600. doi: 10.1002/jor.20584. [DOI] [PubMed] [Google Scholar]
- 40.Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8(2):101–119. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 41.Horner HA, Roberts S, Bielby RC, Menage J, Evans H, Urban JP. Cells from different regions of the intervertebral disc: effect of culture system on matrix expression and cell phenotype. Spine. 2002;27(10):1018–1028. doi: 10.1097/00007632-200205150-00004. [DOI] [PubMed] [Google Scholar]
- 42.Horner HA, Urban JP. 2001 Volvo Award Winner in Basic Science Studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26(23):2543–2549. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 43.Hukins DW (1988) Disc structure and function. In: Ghosh P (ed) Biology of intervertebral disc. CRC Press, Boca Raton, pp 2–37
- 44.Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9(4):667–677. doi: 10.1089/107632703768247368. [DOI] [PubMed] [Google Scholar]
- 45.Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3(10):589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iron KS, Manuel DG, Williams J. Using a linked data set to determine the factors associated with utilization and costs of family physician services in Ontario: effects of self-reported chronic conditions. Chronic Dis Can. 2004;24(4):124–132. [PubMed] [Google Scholar]
- 47.Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17(6):829–835. doi: 10.1002/jor.1100170607. [DOI] [PubMed] [Google Scholar]
- 48.Javedan SP, Dickman CA. Cause of adjacent-segment disease after spinal fusion. Lancet. 1999;354(9178):530–531. doi: 10.1016/S0140-6736(99)00201-9. [DOI] [PubMed] [Google Scholar]
- 49.Johannessen W, Auerbach JD, Wheaton AJ, Kurji A, Borthakur A, Reddy R, et al. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine. 2006;31(11):1253–1257. doi: 10.1097/01.brs.0000217708.54880.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalichman L, Hunter DJ. Lumbar facet joint osteoarthritis: a review. Semin Arthritis Rheum. 2007;37(2):69–80. doi: 10.1016/j.semarthrit.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Kandel RA, Grynpas M, Pilliar R, Lee J, Wang J, Waldman S, et al. Repair of osteochondral defects with biphasic cartilage-calcium polyphosphate constructs in a sheep model. Biomaterials. 2006;27(22):4120–4131. doi: 10.1016/j.biomaterials.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Kandel RA, Hamilton D, Seguin C, Li SQ, Arana C, Pilliar R. An in vitro tissue model to study the effect of age on nucleus pulposus cells. Eur Spine J. 2007;16(12):2166–2173. doi: 10.1007/s00586-007-0467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim PK, Branch CL., Jr The lumbar degenerative disc: confusion, mechanics, management. Clin Neurosurg. 2006;53:18–25. [PubMed] [Google Scholar]
- 54.Korecki CL, Costi JJ, Iatridis JC. Needle puncture injury affects intervertebral disc mechanics and biology in an organ culture model. Spine. 2008;33(3):235–241. doi: 10.1097/BRS.0b013e318175cae7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 2007;213(1):18–26. doi: 10.1002/jcp.21081. [DOI] [PubMed] [Google Scholar]
- 56.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9(4):R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le VC, Kim SW, Tateno K, Sieber AN, Kostuik JP, Leong KW. Interaction of human mesenchymal stem cells with disc cells: changes in extracellular matrix biosynthesis. Spine. 2006;31(18):2036–2042. doi: 10.1097/01.brs.0000231442.05245.87. [DOI] [PubMed] [Google Scholar]
- 58.Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, et al. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16(12):2174–2185. doi: 10.1007/s00586-007-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee P. The economic impact of musculoskeletal disorders. Qual Life Res. 1994;3(Suppl 1):S85–S91. doi: 10.1007/BF00433381. [DOI] [PubMed] [Google Scholar]
- 60.Li WJ, Jiang YJ, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12(7):1775–1785. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 61.Luk KD, Ruan DK, Chow DH, Leong JC. Intervertebral disc autografting in a bipedal animal model. Clin Orthop Relat Res. 1997;337:13–26. doi: 10.1097/00003086-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Mani V, Adler E, Briley-Saebo KC, Bystrup A, Fuster V, Keller G, et al. Serial in vivo positive contrast MRI of iron oxide-labeled embryonic stem cell-derived cardiac precursor cells in a mouse model of myocardial infarction. Magn Reson Med. 2008;60(1):73–81. doi: 10.1002/mrm.21642. [DOI] [PubMed] [Google Scholar]
- 63.Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30(1):5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 64.Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine. 2006;31(7):742–754. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 65.Meisel HJ, Siodla V, Ganey TM, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation. A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24(1):5–21. doi: 10.1016/j.bioeng.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Melrose J, Roberts S, Smith S, Menage J, Ghosh P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine. 2002;27(12):1278–1285. doi: 10.1097/00007632-200206150-00007. [DOI] [PubMed] [Google Scholar]
- 67.Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51(3):503–509. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 68.Mignotte F, Champagne AM, Froger-Gaillard B, Benel L, Gueride M, Adolphe M, et al. Mitochondrial biogenesis in rabbit articular chondrocytes transferred to culture. Biol Cell. 1991;71(1–2):67–72. doi: 10.1016/0248-4900(91)90052-O. [DOI] [PubMed] [Google Scholar]
- 69.Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13(2):173–178. doi: 10.1097/00007632-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Mirza SK, Deyo RA. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine. 2007;32(7):816–823. doi: 10.1097/01.brs.0000259225.37454.38. [DOI] [PubMed] [Google Scholar]
- 71.Mizuno H, Roy AK, Vacanti CA, Kojima K, Ueda M, Bonassar LJ. Tissue-engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine. 2004;29(12):1290–1297. doi: 10.1097/01.BRS.0000128264.46510.27. [DOI] [PubMed] [Google Scholar]
- 72.Mizuno H, Roy AK, Zaporojan V, Vacanti CA, Ueda M, Bonassar LJ. Biomechanical and biochemical characterization of composite tissue-engineered intervertebral discs. Biomaterials. 2006;27(3):362–370. doi: 10.1016/j.biomaterials.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 73.Nachemson A. Lumbar intradiscal pressure. Experimental studies on post-mortem material. Acta Orthop Scand Suppl. 1960;43:1–104. doi: 10.3109/ort.1960.31.suppl-43.01. [DOI] [PubMed] [Google Scholar]
- 74.Nachemson A, Lewin T, Maroudas A, Freeman MA. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop Scand. 1970;41(6):589–607. doi: 10.3109/17453677008991550. [DOI] [PubMed] [Google Scholar]
- 75.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 76.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25(8):1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 77.Nomura T, Mochida J, Okuma M, Nishimura K, Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop Relat Res. 2001;389:94–101. doi: 10.1097/00003086-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 78.O’halloran DM, Pandit AS. Tissue-engineering approach to regenerating the intervertebral disc. Tissue Eng. 2007;13(8):1927. doi: 10.1089/ten.2005.0608. [DOI] [PubMed] [Google Scholar]
- 79.Okuda S, Iwasaki M, Miyauchi A, Aono H, Morita M, Yamamoto T. Risk factors for adjacent segment degeneration after PLIF. Spine. 2004;29(14):1535–1540. doi: 10.1097/01.BRS.0000131417.93637.9D. [DOI] [PubMed] [Google Scholar]
- 80.Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res. 2000;18(6):988–997. doi: 10.1002/jor.1100180620. [DOI] [PubMed] [Google Scholar]
- 81.Olson EJ, Hanley EN, Jr, Rudert MJ, Baratz ME. Vertebral column allografts for the treatment of segmental spine defects. An experimental investigation in dogs. Spine. 1991;16(9):1081–1088. doi: 10.1097/00007632-199109000-00013. [DOI] [PubMed] [Google Scholar]
- 82.Pezowicz CA, Robertson PA, Broom ND. The structural basis of interlamellar cohesion in the intervertebral disc wall. J Anat. 2006;208(3):317–330. doi: 10.1111/j.1469-7580.2006.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Putzier M, Schneider SV, Funk JF, Tohtz SW, Perka C. The surgical treatment of the lumbar disc prolapse: nucleotomy with additional transpedicular dynamic stabilization versus nucleotomy alone. Spine. 2005;30(5):E109–E114. doi: 10.1097/01.brs.0000154630.79887.ef. [DOI] [PubMed] [Google Scholar]
- 84.Rajasekaran S, Babu JN, Arun R, Armstrong BR, Shetty AP, Murugan S. ISSLS prize winner: a study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine. 2004;29(23):2654–2667. doi: 10.1097/01.brs.0000148014.15210.64. [DOI] [PubMed] [Google Scholar]
- 85.Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308(3):401–407. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- 86.Rapoport J, Jacobs P, Bell NR, Klarenbach S. Refining the measurement of the economic burden of chronic diseases in Canada. Chronic Dis Can. 2004;24(4):13–21. [PubMed] [Google Scholar]
- 87.Ray CD. The PDN prosthetic disc-nucleus device. Eur Spine J. 2002;11(Suppl 2):S137–S142. doi: 10.1007/s00586-002-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Razaq S, Wilkins RJ, Urban JP. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur Spine J. 2003;12(4):341–349. doi: 10.1007/s00586-003-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Richardson SM, Mobasheri A, Freemont AJ, Hoyland JA. Intervertebral disc biology, degeneration and novel tissue engineering and regenerative medicine therapies. Histol Histopathol. 2007;22(9):1033–1041. doi: 10.14670/HH-22.1033. [DOI] [PubMed] [Google Scholar]
- 90.Richardson SM, Walker RV, Parker S, Rhodes NP, Hunt JA, Freemont AJ, et al. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24(3):707–716. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 91.Risbud MV, Albert TJ, Guttapalli A, Vresilovic EJ, Hillibrand AS, Vaccaro AR, et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine. 2004;29(23):2627–2632. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 92.Roberts S, Evans EH, Kletsas D, Jaffray DC, Eisenstein SM. Senescence in human intervertebral discs. Eur Spine J. 2006;15(Suppl 3):S312–S316. doi: 10.1007/s00586-006-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 94.Roughley P, Hoemann C, DesRosiers E, Mwale F, Antoniou J, Alini M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials. 2006;27(3):388–396. doi: 10.1016/j.biomaterials.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 95.Ruan D, He Q, Ding Y, Hou L, Li J, Luk KD. Intervertebral disc transplantation in the treatment of degenerative spine disease: a preliminary study. Lancet. 2007;369(9566):993–999. doi: 10.1016/S0140-6736(07)60496-6. [DOI] [PubMed] [Google Scholar]
- 96.Sahlman J, Inkinen R, Hirvonen T, Lammi MJ, Lammi PE, Nieminen J, et al. Premature vertebral endplate ossification and mild disc degeneration in mice after inactivation of one allele belonging to the Col2a1 gene for Type II collagen. Spine. 2001;26(23):2558–2565. doi: 10.1097/00007632-200112010-00008. [DOI] [PubMed] [Google Scholar]
- 97.Sakai D, Mochida J, Iwashina T, Hiyama A, Omi H, Imai M, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27(3):335–345. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 98.Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine. 2005;30(21):2379–2387. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 99.Sato M, Asazuma T, Ishihara M, Ishihara M, Kikuchi T, Kikuchi M, et al. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine. 2003;28(6):548–553. doi: 10.1097/00007632-200303150-00007. [DOI] [PubMed] [Google Scholar]
- 100.Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E, et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47(8):1436–1446. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 101.Seguin CA, Grynpas MD, Pilliar RM, Waldman SD, Kandel RA. Tissue engineered nucleus pulposus tissue formed on a porous calcium polyphosphate substrate. Spine. 2004;29(12):1299–1306. doi: 10.1097/01.BRS.0000127183.43765.AF. [DOI] [PubMed] [Google Scholar]
- 102.Shahdadfar A, Fronsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23(9):1357–1366. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- 103.Shim CS, Lee SH, Shin HD, Kang HS, Choi WC, Jung B, et al. CHARITE versus ProDisc: a comparative study of a minimum 3-year follow-up. Spine. 2007;32(9):1012–1018. doi: 10.1097/01.brs.0000260795.57798.a0. [DOI] [PubMed] [Google Scholar]
- 104.Simon SR et al (1994) Kinesiology. In: Simon SR (ed) Orthopedic Basic Science. Am Academy of Orthopedic Surgeons, USA, pp 558–68
- 105.Sivan SS, Tsitron E, Wachtel E, Roughley P, Sakkee N, Ham F, et al. Age-related accumulation of pentosidine in aggrecan and collagen from normal and degenerate human intervertebral discs. Biochem J. 2006;399(1):29–35. doi: 10.1042/BJ20060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sivan SS, Wachtel E, Tsitron E, Sakkee N, Ham F, Degroot J, et al. Collagen turnover in normal and degenerate human intervertebral discs as determined by the racemization of aspartic acid. J Biol Chem. 2008;283(14):8796–8801. doi: 10.1074/jbc.M709885200. [DOI] [PubMed] [Google Scholar]
- 107.So K, Takemoto M, Fujibayashi S, Neo M, Kyomoto M, Hayami T, et al. Antidegenerative effects of partial disc replacement in an animal surgery model. Spine. 2007;32(15):1586–1591. doi: 10.1097/BRS.0b013e318074d5d4. [DOI] [PubMed] [Google Scholar]
- 108.Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD (2007) Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J (in press) [DOI] [PubMed]
- 109.Solovieva S, Lohiniva J, Leino-Arjas P, Raininko R, Luoma K, la-Kokko L, et al. COL9A3 gene polymorphism and obesity in intervertebral disc degeneration of the lumbar spine: evidence of gene-environment interaction. Spine. 2002;27(23):2691–2696. doi: 10.1097/00007632-200212010-00008. [DOI] [PubMed] [Google Scholar]
- 110.Stairmand JW, Holm S, Urban JP. Factors influencing oxygen concentration gradients in the intervertebral disc. A theoretical analysis. Spine. 1991;16(4):444–449. doi: 10.1097/00007632-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 111.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23(3):403–411. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 112.Sun Y, Hurtig M, Pilliar RM, Grynpas M, Kandel RA. Characterization of nucleus pulposus-like tissue formed in vitro. J Orthop Res. 2001;19(6):1078–1084. doi: 10.1016/S0736-0266(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 113.Sutton EJ, Henning TD, Pichler BJ, Bremer C, drup-Link HE (2008) Cell tracking with optical imaging. Eur Radiol 18(10):2021–2032 [DOI] [PubMed]
- 114.Trout JJ, Buckwalter JA, Moore KC, Landas SK. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14(2):359–369. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- 115.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29(23):2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 117.Urban MR, Fairbank JC, Bibby SR, Urban JP. Intervertebral disc composition in neuromuscular scoliosis: changes in cell density and glycosaminoglycan concentration at the curve apex. Spine. 2001;26(6):610–617. doi: 10.1097/00007632-200103150-00010. [DOI] [PubMed] [Google Scholar]
- 118.Vadala G, Studer RK, Sowa G, Spiezia F, Iucu C, Denaro V, et al. Coculture of bone marrow mesenchymal stem cells and nucleus pulposus cells modulate gene expression profile without cell fusion. Spine. 2008;33(8):870–876. doi: 10.1097/BRS.0b013e31816b4619. [DOI] [PubMed] [Google Scholar]
- 119.Vernengo J, Fussell GW, Smith NG, Lowman AM (2007) Evaluation of novel injectable hydrogels for nucleus pulposus replacement. J Biomed Mater Res B Appl Biomater 84(1):64–69 [DOI] [PubMed]
- 120.Virtanen IM, Karppinen J, Taimela S, Ott J, Barral S, Kaikkonen K, et al. Occupational and genetic risk factors associated with intervertebral disc disease. Spine. 2007;32(10):1129–1134. doi: 10.1097/01.brs.0000261473.03274.5c. [DOI] [PubMed] [Google Scholar]
- 121.Waldman SD, Grynpas MD, Pilliar RM, Kandel RA. Characterization of cartilagenous tissue formed on calcium polyphosphate substrates in vitro. J Biomed Mater Res. 2002;62(3):323–330. doi: 10.1002/jbm.10235. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y, Huso DL, Harrington J, Kellner J, Jeong DK, Turney J, et al. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7(6):509–519. doi: 10.1080/14653240500363216. [DOI] [PubMed] [Google Scholar]
- 123.Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine. 1999;24(8):755–762. doi: 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- 124.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 125.Zhang YG, Guo X, Xu P, Kang LL, Li J. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop Relat Res. 2005;430:219–226. doi: 10.1097/01.blo.0000146534.31120.cf. [DOI] [PubMed] [Google Scholar]
- 126.Zigler J, Delamarter R, Spivak JM, Linovitz RJ, Danielson GO, III, Haider TT, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine. 2007;32(11):1155–1162. doi: 10.1097/BRS.0b013e318054e377. [DOI] [PubMed] [Google Scholar]
- 127.Zuk PA, Zhu M, Ashjian P, Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]