Abstract
Degenerative instability affecting the functional spinal unit is discussed as a cause of symptoms. The value of imaging signs for assessing the resulting functional impairment is still unclear. To determine the relationship between slight degrees of degeneration and function, we performed a biomechanical study with 18 multisegmental (L2-S2) human lumbar cadaveric specimens. The multidirectional spinal deformation was measured during the continuous application of pure moments of flexion/extension, bilateral bending and rotation in a spine tester. The three flexibility parameters neutral zone, range of motion and neutral zone ratio were evaluated. Different grading systems were used: (1) antero-posterior and lateral radiographs (degenerative disk disease) (2) oblique radiographs (facet joint degeneration) (3) macroscopic and (4) microscopic evaluation. The most reliable correlation was between the grading of microscopic findings and the flexibility parameters; the imaging evaluation was not as informative.
Keywords: Spinal biomechanics, Degenerative disk disease, Flexibility parameters, Radiographic findings, Pathological findings
Introduction
Low back pain related to degenerative disk disease is quite common and the relationship of pain provocation to lumbar disc deterioration was reported. Acute and chronic pain associated with clinical instability would result from a progressive degenerative disk disease and the resulting spinal disorders that lead to pathologic motion, misalignment, progressive deformity and spinal stenosis. Radiological and patho-anatomical data are of great value for planning the surgical approach and selecting the tissues that have to be removed; decompression and fusion are operative methods in widespread use [1].
As a diagnostic tool plain radiographs in a supine position constitute the basis of initial patient evaluation [14, 16]. Signs of degenerative disk disease and osteoarthritis with a diminishing disk height and narrowing of the facet joints are frequent radiographic findings in these patients [5, 10, 20, 26]. Spondylophytes and sclerosis of the upper and lower endplates are accepted signs of established degeneration [11]. Narrowing of the lateral recesses and stenosis of the spinal canal appear visible upon CT, CT-myelographic and MRI, which has far better soft tissue resolution [2, 6, 21, 31]. With discography and MR imaging, radial and concentric tears, cystic spaces and a disruption of the annulus fibrosus can be seen [8]. Low signal intensity on T2-weighted MR imaging reflects disk degeneration, but it is less certain whether it is a reliable indicator of structural degenerative changes [21]. Disc degeneration and a high-signal-intensity zone in MRI were not helpful in the identification of symptomatic disk derangement and when severe endplate abnormalities were considered abnormal, all injected disks caused concordant pain with provocation [26].
A pathologic movement of the intervertebral motion segment is considered to be the origin of symptoms [15, 23, 24, 28]. As defined by Panjabi, lumbar instability would imply that the spine has lost the ability to maintain its pattern of displacement under physiologic loads and thus avoid neurological deficits, incapacitating deformity and intractable pain [27]. From a biomechanical point of view, the instability of a vertebral motion segment is defined as an abnormal reaction to applied loads with an increasing range of motion (ROM). The neutral zone (NZ), the displacement between the neutral position and the initiation point of spinal resistance to physiological motion, is considered to be a better indicator of lumbar instability than the ROM [18, 19].
Kirkaldy-Willis and Farfan [12] have proposed three functional phases of degenerative disk disease: (1) temporary dysfunction without instability (2) unstable phase (3) stabilization following ligament calcification and spondylophyte support. In early degenerative disk disease according to these phases 1 and 2 the hypothesis is stated that increasing microscopical structural deterioration causes increasing functional impairment. In the study, the flexibility parameters of unloaded multisegmental lumbar spine specimen were compared with morphological and radiological criteria.
Materials and methods
This study was an in vitro biomechanical, radiological, microscopic and macroscopic patho-anatomical investigation of 18 human lumbar fresh frozen spine specimens (average age 53.4 years) extending from the second lumbar vertebra to the second sacral vertebra. Specimens displaying changes indicative of spinal diffuse idiopathic skeletal hyperostosis (DISH), osteolysis, trauma or deformity were excluded from the present study. Fat and muscles were removed but the ligaments, the joint capsules, the nucleus pulposus and the annulus fibrosus were all preserved. With the specimen embedded with the segment L2/3 cranial and L5/S1 caudal in the universal spine tester [30] two goniometric linkage systems were fixed in L3/4 and L4/5 measured the load displacement from the 36 motion segments (L3/4 n = 18; L4/5 n = 18) under continuous application of pure moments.
An independent observer classified the radiographic degenerative changes in the functional spinal units (FSU) L3/4 and L4/5 using the rating-system I–IV of Mimura et al. [16] with changes in disk height, osteophytes and endplate sclerosis on radiographs anterior/posterior and lateral (Table 1). The presence of facet joint disease was graded 1–4 with joint space narrowing, sclerosis and hypertrophy developed by Pathria et al. [20] on oblique radiographs (Table 2).
Table 1.
Radiographic system for grading disk degeneration on antero-posterior and lateral radiographs according to Mimura et al. [16]
| Lumbar radiographs in anterio-posterior and lateral position | |||||
|---|---|---|---|---|---|
| Grade of disk degeneration | Disk height changes (% of adjacent disc) | Osteophytesa | Endplate sclerosis | ||
| Normal | 0 = 100% | 0 | 0 points | 0: None | |
| I | 0–1 | 1 > 75% | 1 | 1–4 points | 1: Either endplate |
| II | 2–3 | 2 > 50% | 2 | 5–8 points | 2: Both endplates |
| III | 4–6 | 3 > 25% | 3 | 9–12 points | |
| IV | 7–10 | 4 < 25% | 4 | 13–16 points | |
aOsteophytes formation (sum of points on eight edges <3 mm 1 pt, > 3 mm 2 pt
Table 2.
Grading of facet joint disease on oblique radiographs modified according to Pathria et al. [20]
| Lumbar radiographs in oblique position | |
|---|---|
| Grad of facet degeneration | Changes of the facet joint |
| 0 | No changes |
| 1 | Joint space narrowing |
| 2 | Narrowing plus sclerosis or hypertrophy |
| 3 | Severe osteoarthritis with beginning narrowing, sclerosis and osteophytes |
| 4 | Advanced osteoarthritis with hypertrophy, narrowing, sclerosis and osteophytes |
After the biomechanical testing was completed, the L3/4 and L4/5 disks were removed with the cut surfaces parallel to the endplates. Macroscopic disk degeneration was graded A–D (Table 3) according to the criteria developed by Nachemson [17]. For the purpose of microscopic classification, the specimens were arranged in four groups a–d (Table 4) according to the findings of reactive chondrocytes, necrosis and fissures; a modified version of the general criteria put forth by Vernon-Roberts [25] was used here.
Table 3.
Classification of macroscopic patho-anatomic changes associated with disk degeneration according to Nachemson [17]
| Degenerative disk disease assessed by macroscopic inspection | ||
|---|---|---|
| Grade of degeneration | ||
| A | Disks without changes visible to the naked eye. In these cases a gelatinous shiny nucleus pulposus was seen; it was easily delimited from the anulus fibrosus, which was free from macroscopic ruptures | |
| B | Disks which showed macroscopic changes in the nucleus pulposus. The nucleus was somewhat more fibrous, but could be clearly distinguished from the annulus, which was intact | |
| C | Specimens which showed macroscopic changes in both the nucleus pulposus and the annulus fibrosus. The nucleus in these discs was more fibrotic but still soft. The boundary between nucleus and and annulus was no longer so distinct, but could be seen. Changes in the annulus fibrosus consisted of isolated fissures | |
| D | Specimens which showed more severe macroscopic changes. The disk in this group exhibited fissure formation and cavities in both the nucleus and the annulus. Marginal osteophytes were often found in adjoining vertebrae | |
Table 4.
Intervertebral disk degeneration classified according to a modified version of the microscopic criteria of Vernon-Roberts [25]
| Intervertebral disc degeneration assessed by microscopic examination | ||||
|---|---|---|---|---|
| Grading of degeneration | Reactive chondrocytes “Brutkapseln” | Fissures, clefts, splints | Areals of necrosis | Damage of annular layers |
| a | Few | Isolated, flat | Isolated, small | 0–1 ring |
| b | Moderate | Ample, flat | Several, focal | 1–2 rings |
| c | Ample | Numerous deep | Multiple, partly confluent | 2–3 rings |
| d | A lot | Numerous very deep | Great, diffuse extended | 3–4 rings |
In the spine tester [30] alternating sequences of flexion/extension (±Mx), lateral bending (±Mz) and axial rotation (±My) moments ±7.5 Nm were applied continuously by stepper motors at a constant rate of 1.7°/s. Three cycles were performed to precondition the specimen and to minimize the visco-elastic effects [18, 19]. Only the data of the third and last cycle were evaluated. The pure moment specification of 7.5 Nm was chosen to stay within the estimated failure limits of the specimens so they could be tested in multiple modes of loading [30]. It also is in the range used in many in vitro experiments on the human spine, making these results comparable with published data from human specimens [30].
The spine tester allowed the specimen to move in all six degrees of freedom while both electrogoniometric linkage systems measured the three-dimensional movements of L3/4 and L4/5 to a degree of accuracy of 0.1 mm and 0.1°. Load displacement curves were plotted in order to evaluate functional alteration and determine the correlation between such alterations and degenerative disk disease [16, 18] (Fig. 1).
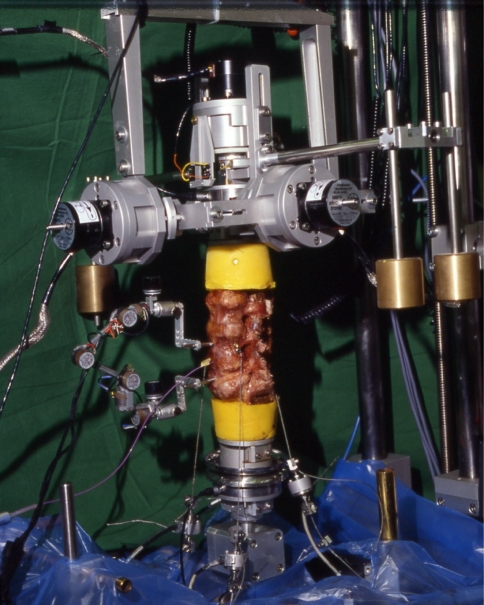
Fig. 1.
Spine tester with human lumbar cadaveric specimen L2-S2. The three-dimensional motion between L3/4 and L4/5 was measured with two electrogoniometric linkage systems. At the top is the gimbal arranged with stepper motor for the application of external moments
We analyzed the ROM, the NZ and the neutral zone ratio (NZR) for each direction of loading. The segmental intervertebral motion is characterized by a nonlinear relationship between maximum load and displacement which corresponds to a hysteresis curve [27, 30]. The NZ is a measure of laxicity for very low loads; it is defined as the difference at zero load between the angular positions corresponding to the loading phases of the test cycle [30]. The quotient of NZ and ROM is defined as the NZR [18].
In addition, the degenerative disk disease was graded according to radiological criteria by an experienced orthopedic surgeon and according to patho-anatomical findings by a neuropathologist without prior knowledge of the flexibility parameters.
Statistical analysis
Since intra- and inter-individual variability was considerable, we computed the representative values (mean value and standard deviation) shown in the Figs. 2 and 3. Panjabi [27], defines this as the representative ROM. To determine whether there were significant differences in the flexibility parameters between different grades of degeneration, we performed a one way ANOVA [13]. A statistically significant result using the Kruskal-Wallis test was followed by a two-tailed Wilcoxon test [29].
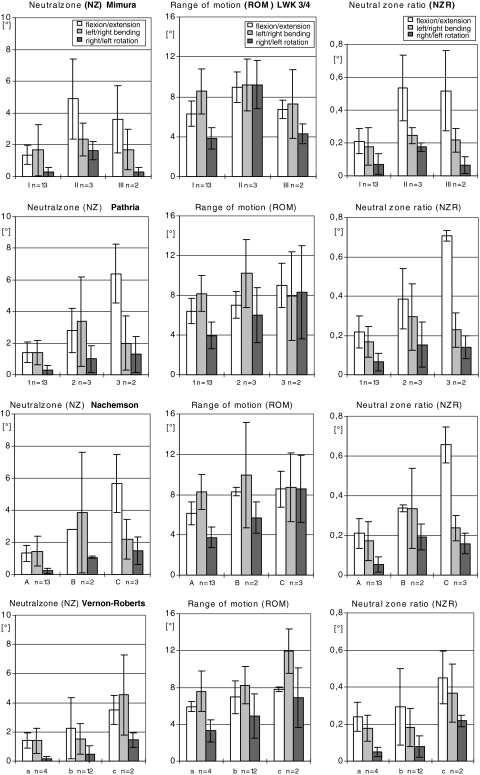
Fig. 2.
The figures show the range of motion (ROM), neutral zone (NZ) and neutral zone ratio (NZR) in the form of a bar char showing the mean values and standard deviation executed by L3/4 on flexion/extension, left/right bending and right/left rotation
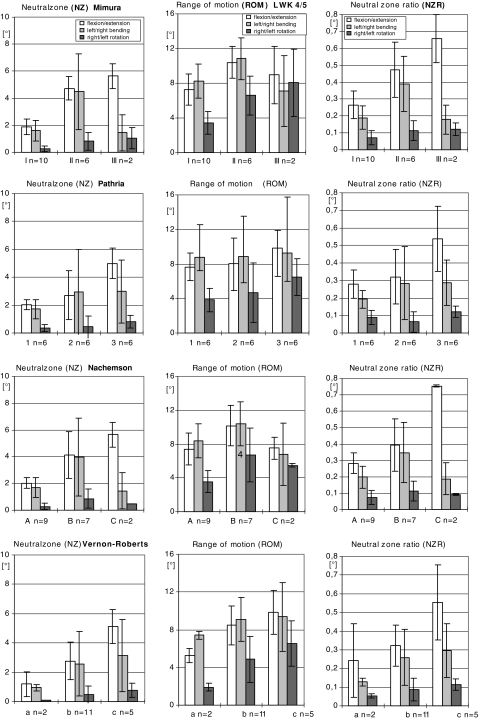
Fig. 3.
Presentation of the three flexiblity parameters of L4/5 and the relationship between a modified grading system on degenerative disk disease based on 1. radiographs, 1.1 AP/lateral position (Mimura-score), 1.2 oblique position (Pathria-score) and 2. patho-anatomic criteria, 2.1 macrsocopic findings (Nachemson-score), 2.2 microscopic findings (Vernon-Roberts-score)
Results
The morphological changes characteristic of progressive disk degeneration determined by microscopic examination were accompanied by reliable functional changes, i.e., increases in all parameters: ROM, NZ and NZR (Figs. 2, 3).
In L4/5 the NZ, which is an indicator for instability, displayed increasing values correlating with advanced disk degeneration (Fig. 3) under the various load conditions: the values measured during flexion/extension where: 1.2° (0.8) in grade a, 2.8° (1.2) in grade b, 5.1° (1.1) in grade c; those measured during axial rotation were: 0.1° (0) in grade a, 0.5° (0.5) in grade b and 0.8° (0.4) in grade c (P < 0.05).
The values obtained for representative ROM increased with progressing disk degeneration (Fig. 3) during flexion/extension and rotation. The values measured during flexion/extension were: 5.2° (0.8) in grade a, 8.4° (2.1) in grade b, 9.8° (2.3) in grade c; those measured during rotation were: 1.9° (0.4) in grade a, 4.8° (2.4) in grade b and 6.5° (2.3) in grade c (P < 0.05).
In disks with higher grades of degenerative disk disease (Fig. 3), the NZR rose during flexion/extension: 0.2° (0.2) in grade a, 0.3° (0.1) in grade b, 0.5° (0.2) in grade c and during rotation: 0.05° (0.01) in grade a, 0.1° (0.06) in grade b and 0.1° (0.03) in grade c (P < 0.05).
For the two lower grades in the four different grading systems (grade I–II, grade 1–2, grade A–B, grade a–b), the application of a given moment (flexion/extension or axial rotation) provoked an increase in the ROM (P < 0.05) and caused a higher value for the NZ (P < 0.05); this correlates positively with the instability of the FSU encountered in cases with more pronounced degeneration (P < 0.05).
Nevertheless, this parallelism was no longer seen in cases with more distinct degeneration visible in radiograms (grade II–III, grade 2–3); in single investigations a decrease was observed in several of the flexibility parameters measured. At higher degrees of degeneration according to the microscopic criteria (grade b–c), however, the values measured for all the flexibility parameters were still increased.
Under the load component of bending, the statistical tests employed did not reveal significant differences in the flexibility parameters for the different degeneration grades in every case.
Obvious more structural changes qualitative and quantitative correspond with higher degrees of degeneration found during microscopic examination (grade b, n = 12; grade c, n = 2) than during radiographic (grade II, n = 3; grade III, n = 2) or macroscopic (grade B, n = 2; grade C, n = 3) evaluation in L3/4 (Fig. 2). In all the scoring systems less degeneration was detected in the L3/4 segment than in the L4/5 segment (Fig. 3).
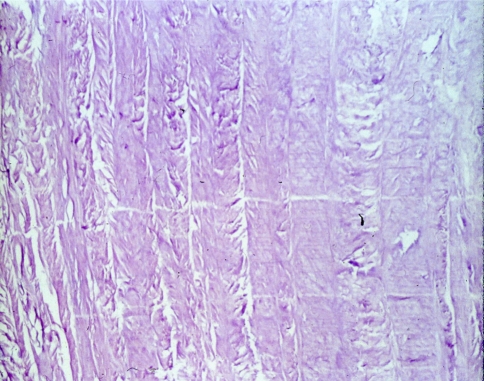
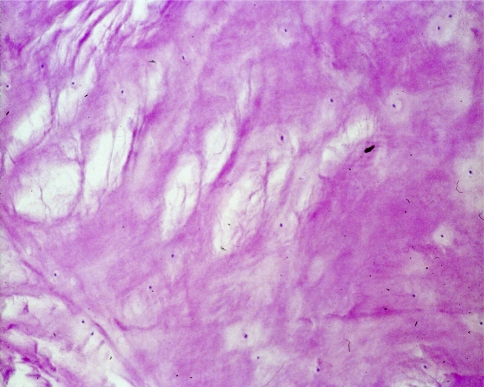
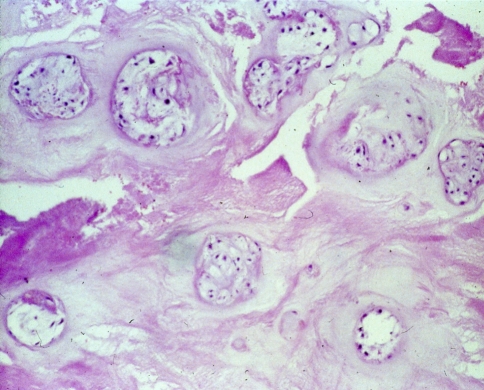
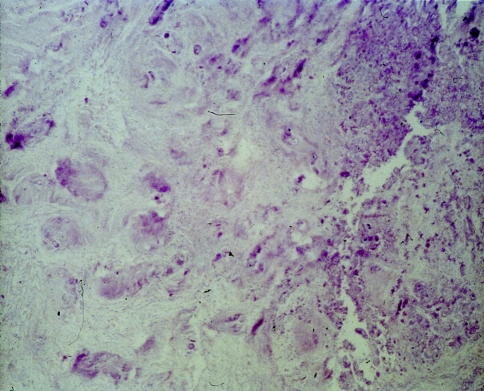
In the degeneration seen in our study, the nucleus loses its gel-like consistency, splits and clefts are frequently seen parallel to the endplates (Fig. 4). Furthermore, a fragmentation of the nucleus is encountered in some cases, with clefts and fissures radiating from the center of the disk towards the periphery and extending into the annulus (Fig. 5). Radial clefts were seen in the posterior part of the annulus; annular tears seemed to extend circumferentially. The clefts and new surfaces seen in the nucleus pulposus are the result of new cell layer formation (endothelization) on the surface of the granular tissue. Occasionally, a vascular ingrowth was detected around the margins; this was interpreted as a repair reaction. Focal chondrocytic proliferations (so called “Brutkapseln”) and areas of cystic degeneration associated with vascular granulation tissue were frequently seen (Fig. 6). Some areas of necrosis were visible with cell proliferation at the borders of the necrosis (Fig. 7).
Fig. 4.
Microscopic grading of disk degeneration a: few focal chondrocytic proliferation, isolated flat fissures, clefts, splints; isolated small areas of necrosis. Here (HE, 20×): four normal layers of annulus fibrosus
Fig. 5.
Microscopic grading of disk degeneration b: moderate reactive chondrocytes, ample flat fissures, clefts, splints; several focal areas of necrosis and three rings of intact annular layers. Here (HE, 100×): microcysts and ample flat fissures
Fig. 6.
Microscopic grading of disk degeneration b: moderate reactive chondrocytes; ample flat fissures, clefts, splints; several focal areas of necrosis and two rings of intact annular layers. Here (HE, 100×): moderate reactive chondrocytes
Fig. 7.
Microscopic grading of disk degeneration c: ample chondrocyte proliferation; numerous deep fissures, clefts, splints; multiple, partly confluent areas of necrosis and 1ring of intact annular layers. Here (HE, 80×): multiple focal and diffus partly confluent necrosis
Discussion
In our study microscopic findings in degenerative disk disease were correlated with a biomechanical functional impairment of the FSU. The ROM, the NZ and the NZR increased as the degenerative disk disease progressed. Under the load components flexion/extension and axial rotation, all statistical tests were conducted at the 95% level of significance. However, these changes were not reliably shown in radiograms. For a comparison of data from different investigations this is additional to the standardized nomenclature founded by radiological imaging in the assessment of disc abnormalities. According to the literature the prevalence of intervertebral disk calcification increases with age and extent of disk space loss. This was evident with radiographically spinal degenerative changes in cadavers by Chanchairujira et al. [4]. Another histological study from Gries et al. [7] showed no correlation between early histological changes in lumbar disks and the associated facet joints (Fig. 8).
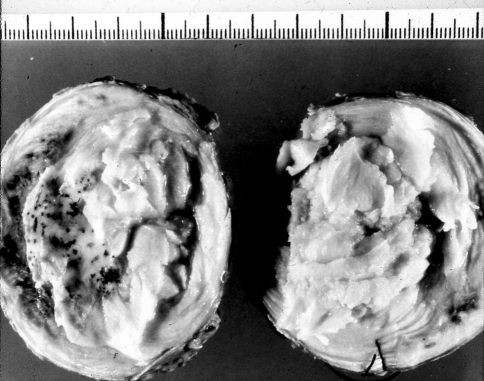
Fig. 8.
Macroscopic grading of disk degeneration. Lumbar disk L3/4 left and L4/5 right, Nachemson grade c: loosening of gel-like consistent and radial clefts
Our functional analysis showed that the grading of degeneration in the facet joints (in L4/5 n = 6 grade 2 and n = 6 grade 3) seemed to be a more accurate method for distinguishing between functional changes than it was on AP and lateral films (in L4/5 n = 3 grade II and n = 2 grade III) of the motion segment. In comparison we found more disks with higher degeneration according to microscopic criteria (in L4/5 n = 11 in grade b and n = 5 in grade c) than according to radiographic criteria (Fig. 3). The dependence of microscopic findings and functional parameters seemed preferable to point out early degenerative disk disease better than radiographic based grading systems.
The application of the classification system to cadaveric and surgical specimens demonstrated a significant correlation with age and macroscopic grade of degeneration by Boss et al. [3]. In our microscopical evaluation as well degenerative disk disease increased with age and from cranial to caudal, L3/4 to L4/5. The average age of our specimens was 53.4 years. After middle life, the nuclei pulposi are solid, no larger turgescent, but dry, feel granular at palpation and are merged with the annulus. Sometimes it was not possible to ascertain where the annulus ended and the nucleus began; there was apparently an increase in collagen in the nucleus and only a small proportion of the nuclear cells were healthy. A comparison of the grading systems based on imaging criteria undertaken in our study has shown that the microscopic pathoanatomical findings are evidently the best measure of degeneration, provided that the degree of degeneration is related to and can be expressed in terms of increasing flexibility parameters.
In a study conducted by Hackney et al. [9] the most common indication for spinal magnetic resonance imaging (MRI) was found to be the evaluation for degenerative disk disease with a focus on the anatomic relationships between the disk, vertebral endplates and facet joints with the subarachnoid space, nerve roots, and spinal cord. Although MRI may be definitive for the imaging of visible findings, our study showed that the relationship established between the disk, vertebral endplates and facet joints Mimura-score [16] displayed a moderate correlation with the observed functional impairment. The correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration was shown by Benneker et al. [2]. They conclude that radiographic parameter were able to distinguish different stages of degeneration, whereas MRI could only detect advanced stages of disk degeneration. In another study Soini et al. [22] assess the relationship between disk degeneration observed in plain radiographs and in discograms. Here instability expressed as abnormal angular movement of lumbar vertebra. They concluded that discography was more sensitive than plain radiography in the diagnosis of disk degeneration. However, no correlation was found between abnormal angular movement and discogram type (Adams’ classification) [22].
The methodology of biomechanical spine studies and the concept of load and deformation have been well established [18, 19, 27]. Nevertheless, the extremely complex and non-linear stiffness properties on which such stability depends may be highly sensitive to the three-dimensional movement of the FSU. One limitation of our study was the unequal number of specimen within the groups formed according to the degree of degeneration. Another was the difficulty of extrapolating the results obtained in vitro to in vivo situations because of the additional physiological factors metabolism and the muscle forces. Although the removal of soft tissue (fat and muscle) and deterioration of cadavers may have altered the mechanical function.
From the viewpoint of a mechanical analysis the comparison of different grading systems using radiographs and pathological anatomical findings showed that the functional impairment in the flexibility parameters (ROM, NZ, NZR) correlated best with a grading system based on microscopic findings. For assessing the severity of degenerative intervertebral disk disease, biomechanical studies based on microscopic grading appear to yield accurate information.
Acknowledgments
This work was partially supported by the Deutsche Forschungsgemeinschaft (DFG CL-77/2-3 D).
References
- 1.Benini A. Lumbar intervertebral disk disease and its sequelae: segmental instability, intervertebral disk displacement, lumbar spinal stenosis-change in a common disease. Schweiz Rundsch Med Prax. 1989;78(31–32):840–850. [PubMed] [Google Scholar]
- 2.Benneker LM, Heini PF, Anderson SE, Alini M, Ito K. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur Spine J. 2005;14(1):27–35. doi: 10.1007/s00586-004-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27(23):2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Chanchairujira K, Chung CB, Kim JY, et al. Intervertebral disc calcification of the spine in an elderly population: radiographic prevalence, location, and distribution and correlation with spinal degeneration. Radiology. 2004;230(2):499–503. doi: 10.1148/radiol.2302011842. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak J, Panjabi M, Chang D, Theiler R, Grob D. Clinical validation of functional flexion-extension roentgenograms of the lumbar spine. Spine. 1991;16:943–950. doi: 10.1097/00007632-199108000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frobin W, Brinckmann P, Kramer M, Hartwig E. Height of lumbar discs measured from radiographs commpared with degeneration and height classified from MR images. Eur Radiol. 2001;11(2):263–269. doi: 10.1007/s003300000556. [DOI] [PubMed] [Google Scholar]
- 7.Gries NC, Berlemann U, Moore RJ, Vernon-Roberts B. Early histologic changes in lower lumbar discs and facet joints and their correlation. Eur Spine J. 2000;9(1):23–29. doi: 10.1007/s005860050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunzburg R, Parkinson R, Moore R, Cantraine F, Hutton W, Vernon Roberts B, Fraser R. A cadaveric study comparing discography, magnetic resonance imaging, histology, and mechanical behavior of the human lumbar disc. Spine. 1992;17(4):417–426. doi: 10.1097/00007632-199204000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Hackney DB. Degenerative disk disease. Top Magn Reson Imaging. 1992;4(2):12–36. [PubMed] [Google Scholar]
- 10.Hayes M, Howard T, Gruel C. Roentgenographic evaluation of lumbar spine flexion-extension in asymptomatic individuals. Spine. 1989;14:327–331. doi: 10.1097/00007632-198903000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch C. The mechanical response in normal and degenerated lumbar discs. J Bone Joint Surg. 1956;38A:242–243. [Google Scholar]
- 12.Kirkaldy-Willis W, Farfan H. Instability of the lumbar spine. Clin Orthop. 1982;165:110–123. [PubMed] [Google Scholar]
- 13.Kruskal W, Wallis W. Use of ranks on one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. doi: 10.2307/2280779. [DOI] [Google Scholar]
- 14.Madan SS, Rai A, Harley JM. Interobserver error in interpretation of the radiographs for degeneration of the lumbar spine. Iowa Orthop J. 2003;23:51–56. [PMC free article] [PubMed] [Google Scholar]
- 15.McGregor AH, McCarthy I, Hughes S. The effect of presenting symptoms on the motion characteristics of the lumbar spine of low back pain. J Bone Joint Surg. 1996;78-B(Suppl 1):51. [Google Scholar]
- 16.Mimura M, Panjabi M, Oxland T, Crisco J, Yamamoto I, Vasavada A. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine. 1994;19:1371–1380. doi: 10.1097/00007632-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Nachemson A. Lumbar intradiscal pressure. Experimental studies on post-mortem material. Acta Orthop Scand. 1960;43(Suppl):9–104. doi: 10.3109/ort.1960.31.suppl-43.01. [DOI] [PubMed] [Google Scholar]
- 18.Panjabi M. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5(4):390–396. doi: 10.1097/00002517-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Panjabi M, Lydon C, Vasavada A, Grob D, Crisco-3rd JJ, Dvorak J (1994) On the understanding of clinical instability. Spine 19(23):2642–2650 [PubMed]
- 20.Pathria M, Sartoris D, Resnik D. Osteoarthritis of the facet joints: accuracy of oblique radiographic assessment. Radiology. 1987;164:227–230. doi: 10.1148/radiology.164.1.3588910. [DOI] [PubMed] [Google Scholar]
- 21.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic ressonance classification of 1umbar intervertebral disc degeneration. Spine. 2001;26(17):1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 22.Soini J, Antti-Poika I, Tallroth K, Konttinen Y, Honkanen V, Santavirta S. Disc degeneration and angular movement of the lumbar spine: Comparative study using plain and flexion-extension radiography and discography. J Spinal Disord. 1991;4(2):183–187. doi: 10.1097/00002517-199106000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Steffen T, Rubin R, Baramki H, Antoniou J, Marchesi D, Aebi M. A new technique for measuring lumbar segmental motion in vivo. Spine. 1997;22:156–166. doi: 10.1097/00007632-199701150-00006. [DOI] [PubMed] [Google Scholar]
- 24.Strömqvist B, Axelsson P, Johnsson R. Mechanics of the external fixation test in the lumbar spine. A Roentgen Stereo-photogrammetric analysis. J Bone Joint Surg. 1996;78-B(Suppl 1):48. doi: 10.1097/00007632-199602010-00016. [DOI] [PubMed] [Google Scholar]
- 25.Vernon-Roberts B. Pathology of intervertebral discs and apophyseal joints. The lumbar spine and back pain. London: Churchill-Livingstone; 1987. pp. 37–55. [Google Scholar]
- 26.Weishaupt D, Zanetti M, Boos N, Hoodler J. MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal Radiol. 1999;28(4):215–219. doi: 10.1007/s002560050503. [DOI] [PubMed] [Google Scholar]
- 27.White A, Panjabi M (1990) Clinical biomechanics of the spine, 2nd edn. JB Lippincott Company, Philadelphia
- 28.Wiesner L, Rüther W, Fink B. A new method for measurement of segmental motion behavior at the lumbar spine. J Bone Joint Surg. 1995;77-B(Suppl II):161. [Google Scholar]
- 29.Wilcoxon F. Individual comparisons by ranking methods. Biometrics. 1945;1:80–83. doi: 10.2307/3001968. [DOI] [Google Scholar]
- 30.Wilke H-J, Claes LE, Schmitt H, Wolf S. A universal spine tester for in vitro experiments with muscle force simulation. Eur Spine J. 1994;3:91–97. doi: 10.1007/BF02221446. [DOI] [PubMed] [Google Scholar]
- 31.Wybier M. Imaging of lumbar degenerative changes involving structures other than disk space. Radiol Clin North Am. 2001;39(1):101–114. doi: 10.1016/S0033-8389(05)70265-7. [DOI] [PubMed] [Google Scholar]