Abstract
The use of biological technologies for the treatment of degenerative spinal diseases has undergone rapid clinical and scientific development. BMP strategies have gained wide support for an inherent potential to improve the ossification process. It has been extensively studied in combination with various techniques for spinal stabilisation from both anterior and posterior approach. We studied the fusion process after implantation of rhBMP-2 in 17 patients with degenerative lumbar spine diseases in combination with dorsal fixation with pedicle screws and poly-ether-ether-ketone (PEEK) interbody cages. We used 12 mg rhBMP-2 carried by collagen sponge, 6 mg in every cage. Patient follow up consisted of pre-operative radiographic and clinical evaluation. Similar post-operative evaluations were performed at 3 and 6 months. Clinical assessment demonstrated clear improvement in all patients despite evidence of vertebral endplate osteoclastic activity in the 3-month radiographs. The 6-month radiograph, however, confirmed evidence of fusion, and no untoward results or outcomes were noted. While previous studies have shown exclusively positive results in both fusion rates and process, our study demonstrated an intermediate morphology at 3 months during the ossification process using Induct Os in combination with peek-cages using a PLIF-technique. The transient resorption of bone surrounding the peek cage did not result in subsidence, pain or complication, and fusion was reached in all cases within a 6-month-controlled evaluation. Although there was no negative influence on clinical outcome, the potential for osteoclastic or metabolic resorption bears watching during the post-surgical follow up.
Keywords: Degenerative disc disease, Bone morphogenetic protein 2, Lumbar spine, Posterior lumbar interbody fusion, Osteoclastic activity
Introduction
Spinal fusion has been developed as a final course of progressive intents, which are designed to stabilise spine movement, reduce pain, and to moderate further degenerative change. The principal challenge inherent to the successful outcome of the surgical procedure is the requirement of converting a fibrocartilaginous articulation into a rigid and continuous segment of bone. This conversion has been fraught with challenges intrinsic to sustain osteogenic transformation within the dynamics of motion, inflammation, and at the same time accommodating patient comfort while reducing symptomatic pain. Surgical intervention in itself augments the inflammatory process, and establishes new margins for the intended biological transition, compromises the healing properties of the tissue itself. To gain inertia in the conversion of cartilage to bone, mechanical strategies of rigid constraint have been coupled with autologous tissue to initiate osteogenic activity. The culmination of this strategy for spinal fusion surgery involves harvesting bone from the iliac crest. Limitations of this approach include donor site morbidity, increased operative time and increased blood loss, not to mention the economic derivative summed in the separate limitations [3, 13].
In an effort to circumvent the issues of morbidity and improve efficiency, the past decade has shown a keen interest and subsequent rapid evolution in the development of adjunct biological technologies for the treatment of degenerative disc changes in the lumbar spine. In particular, the use of bone morphogenetic proteins (BMPs) to improve the ossification process has gained wide support and clinical focus. Improvement of ossification in and of itself has been met with collateral development of several different types of fusion cages to stabilise the spine during the osteogenic transformation [8].
The use of bone morphogenetic proteins in combination with various techniques for spinal stabilisation using anterior and posterior approaches has been studied in many different animal models and humans in an effort to optimise efficacy and to better define an acceptable population who will benefit from the intervention [2–4, 6, 9, 12, 14].
These initial investigations marked the path to regulatory approval and resulted in widespread clinical application. Among the accepted applications is the use of recombinant human bone morphogenetic protein type 2 (rhBMP-2) with an LT-CAGE lumbar tapered fusion device (InductOs® 12 mg Kit for implant; Wyeth Europa Ltd, Taplow, UK) for single-level (L4–S1) anterior lumbar spine fusion. Such use is warranted as a substitute for autogenous bone graft in adults with degenerative disc disease who have had at least 6 months of non-operative treatment for this condition (InductOs® 12 mg Kit for implant Summary of Product Characteristics. March 2006). In the USA, recombinant human bone morphogenetic protein type 7 (rhBMP-7; rhOP-1) is available as OP-1® Putty (Stryker Biotech), and has been approved under the Humanitarian Device Exemption by the FDA as an alternative to autograft in revision posterolateral lumbar spinal fusion in compromised patients.
The Telamon™ poly-ethyl-ether-ketone (PEEK) cage (Medtronic Sofamor Danek, Memphis, TN) was developed to optimise stabilisation of the lumbar spine and to promote intervertebral fusion following surgical correction of disorders of the spine. The broad objective of this study was to evaluate the radiological and clinical outcomes in a population of patients treated with rhBMP-2 on an absorbable collagen sponge carrier (Wyeth Europa Ltd, Taplow, UK) in combination with a Telamon™ (PEEK) cage (Medtronic Sofamor Danek, Memphis, TN) for posterior lumbar interbody fusion (PLIF). More specific goals within the context of the evaluation were to assess dose relationship, carrier stability, efficacy, an ability to achieve delivery by minimally invasive technologies, and interim biological conditions associated with clinical progress.
Methods
Participants
Between January 2004 and June 2004, 17 patients with degenerative disc disease were enrolled in this prospective, non-comparative, non-randomised study and followed clinically over the course of 24 months. All patients had spinal stenosis, intervertebral instability, and degenerative disc changes that had not responded to non-operative intervention. None of the patients experienced disabling lower back or leg pain for any duration. The mean age of the 17 patients (8 males, 9 females) was 67 years (range 47–79) when they were enrolled in the study. Sixteen patients had single-level degenerative disc disease and although two patients had multi-level involvement, only one patient was operated on at two levels. Written consent was obtained from the patients prior admitting to participate in the study. Ethical approval was not required based on the separate approved status of both the Telamon Cage and the BMP protein.
Surgical procedure
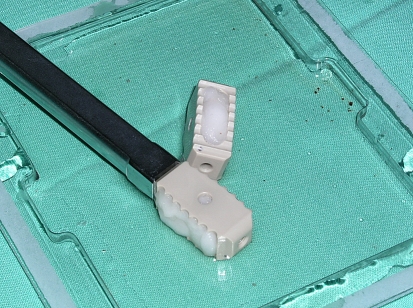
Patients underwent a PLIF procedure using standard surgical protocol, which included laminotomy with extended interlaminar fenestration. Pedicular screws were used for dorsal fixation; fixation systems employed were the Tenor® (Medtronic Sofamor Danek, Memphis, TN) and TSRH-3D® (Medtronic Sofamor Danek, Memphis, TN). The dimensions of the Telamon™ (PEEK) cages (Medtronic Sofamor Danek, Memphis, TN) cages were: 26 mm/10 mm/3°; 26 mm/8 mm/3°; 22 mm/8 mm/3° (Fig. 1).
Fig. 1.
The dimensions of the Telamon Cage, sizing, angulation, etc
Human recombinant BMP-2 was reconstituted according to manufacturer’s directions using sterile water and administered as a single dose of 12 mg/mL in all the study patients applied to a bovine collagen sponge. Prior to placement, the growth factor was allowed to bind to the sponge for 15 min. Although the total dose prepared was consistent at 12 mg per doses, the single patient who had a two-level intervention received a divided allotment, with one-half of the collagen-BMP treatment placed at each of the two levels. The rhBMP-2-soaked sponge then was divided and then placed in the hollow central portions of the PEEK cage before it was placed into the prepared disc space. No additional sponges were placed outside the devices (Table 1).
Table 1.
Dallas Score
| Baseline | Follow-up | P value mean change: baseline—final ex | |
|---|---|---|---|
| Pain and Intensity | |||
| N | 13 | 16 | 0.035 |
| Mean ± SD | 4.7 ± 1.9 | 3.0 ± 2.0 | 1.7 |
| p5, p25, p75, p95 | 1.0, 4.0, 6.0, 6.0 | 1.0, 1.0, 5.0, 6.0 | – |
| Median | 6.0 | 2.5 | – |
| Min, Max | 1.0, 6.0 | 1.0, 6.0 | – |
| Personal care | |||
| N | 13 | 15 | 0.051 |
| Mean ± SD | 3.8 ± 1.2 | 2.7 ± 1.3 | 1.1 |
| p5, p25, p75, p95 | 2.0, 3.0, 5.0, 6.0 | 1.0, 1.0, 4.0, 5.0 | – |
| Median | 4.0 | 3.0 | – |
| Min, Max | 2.0, 6.0 | 1.0, 5.0 | – |
| Lifting | |||
| N | 13 | 15 | 0.044 |
| Mean ± SD | 5.1 ± 1.5 | 3.9 ± 1.7 | 1.2 |
| p5, p25, p75, p95 | 1.0, 4.0, 6.0, 6.0 | 1.0, 3.0, 5.0, 6.0 | – |
| Median | 6.0 | 4.0 | – |
| Min, Max | 1.0, 6.0 | 1.0, 6.0 | – |
| Walking | |||
| N | 13 | 16 | 0.003 |
| Mean ± SD | 5.0 ± 1.5 | 3.3 ± 1.5 | 1.7 |
| p5, p25, p75, p95 | 1.0, 4.0, 6.0, 6.0 | 1.0, 2.0, 4.5, 5.0 | – |
| Median | 6.0 | 4.0 | – |
| Min, Max | 1.0, 6.0 | 1.0, 5.0 | – |
| Sitting | |||
| N | 13 | 16 | 0.314 |
| Mean ± SD | 3.7 ± 1.9 | 2.9 ± 1.7 | 0.8 |
| p5, p25, p75, p95 | 1.0, 3.0, 6.0, 6.0 | 1.0, 1.0, 4.5, 5.0 | – |
| Median | 4.0 | 3.0 | – |
| Min, Max | 1.0, 6.0 | 1.0, 5.0 | – |
| Standing | |||
| N | 13 | 16 | 0.275 |
| Mean ± SD | 4.4 ± 1.4 | 3.7 ± 1.4 | 0.7 |
| p5, p25, p75, p95 | 2.0, 3.0, 6.0, 6.0 | 1.0, 3.0, 4.0, 6.0 | – |
| Median | 4.0 | 4.0 | – |
| Min, Max | 2.0, 6.0 | 1.0, 6.0 | – |
| Sleeping | |||
| N | 12 | 16 | 0.299 |
| Mean ± SD | 2.3 ± 1.2 | 1.8 ± 1.1 | 0.5 |
| p5, p25, p75, p95 | 1.0, 1.0, 3.0, 5.0 | 1.0, 1.0, 2.5, 4.0 | – |
| Median | 2.0 | 1.0 | – |
| Min, Max | 1.0, 5.0 | 1.0, 4.0 | – |
| Social life | |||
| N | 12 | 16 | 0.981 |
| Mean ± SD | 4.4 ± 2.8 | 4.3 ± 2.9 | 0.1 |
| p5, p25, p75, p95 | 1.0, 1.0, 7.0, 8.0 | 1.0, 1.0, 7.5, 8.0 | – |
| Median | 4.5 | 4.0 | – |
| Min, Max | 1.0, 8.0 | 1.0, 8.0 | – |
| Travelling | |||
| N | 12 | 16 | 0.022 |
| Mean ± SD | 5.6 ± 1.9 | 3.8 ± 2.0 | 1.8 |
| p5, p25, p75, p95 | 1.0, 4.5, 7.0, 7.0 | 1.0, 2.0, 5.5, 7.0 | – |
| Median | 6.5 | 4.0 | – |
| Min, Max | 1.0, 7.0 | 1.0, 7.0 | – |
| Vocational | |||
| N | 8 | 7 | 0.442 |
| Mean ± SD | 4.1 ± 3.1 | 5.3 ± 2.6 | −1.2 |
| p5, p25, p75, p95 | 1.0, 1.0, 7.5, 8.0 | 1.0, 4.0, 8.0, 8.0 | – |
| Median | 3.5 | 5.0 | – |
| Min, Max | 1.0, 8.0 | 1.0, 8.0 | – |
| Anxiety/Mood | |||
| N | 9 | 14 | 0.006 |
| Mean ± SD | 1.3 ± 0.7 | 3.1 ± 1.5 | −1.8 |
| p5, p25, p75, p95 | 1.0, 1.0, 1.0, 3.0 | 1.0, 2.0, 4.0, 6.0 | – |
| Median | 1.0 | 3.0 | – |
| Min, Max | 1.0, 3.0 | 1.0, 6.0 | – |
| Emotional control | |||
| N | 10 | 14 | 0.022 |
| Mean ± SD | 1.2 ± 0.6 | 2.4 ± 1.5 | −1.2 |
| p5, p25, p75, p95 | 1.0, 1.0, 1.0, 3.0 | 1.0, 1.0, 4.0, 5.0 | – |
| Median | 1.0 | 2.5 | – |
| Min, Max | 1.0, 3.0 | 1.0, 5.0 | – |
| Depression | |||
| N | 10 | 16 | 0.023 |
| Mean ± SD | 1.5 ± 1.6 | 2.6 ± 2.2 | −1.1 |
| p5, p25, p75, p95 | 1.0, 1.0, 1.0, 6.0 | 1.0, 1.0, 3.0, 8.0 | – |
| Median | 1.0 | 2.0 | – |
| Min, Max | 1.0, 6.0 | 1.0, 8.0 | – |
| Interpersonal relationship | |||
| N | 10 | 16 | 0.120 |
| Mean ± SD | 1.1 ± 0.3 | 1.9 ± 1.5 | −0.8 |
| p5, p25, p75, p95 | 1.0, 1.0, 1.0, 2.0 | 1.0, 1.0, 2.0, 5.0 | – |
| Median | 1.0 | 1.0 | – |
| Min, Max | 1.0, 2.0 | 1.0, 5.0 | – |
| Social support | |||
| N | 10 | 16 | 0.256 |
| Mean ± SD | 1.7 ± 1.2 | 2.2 ± 1.4 | −0.5 |
| p5, p25, p75, p95 | 1.0, 1.0, 3.0, 4.0 | 1.0, 1.0, 2.5, 5.0 | – |
| Median | 1.0 | 2.0 | – |
| Min, Max | 1.0, 4.0 | 1.0, 5.0 | – |
| Punishing response | |||
| N | 10 | 16 | 0.035 |
| Mean ± SD | 1.0 ± 0.0 | 1.7 ± 1.2 | −0.7 |
| p5, p25, p75, p95 | 1.0, 1.0, 1.0, 1.0 | 1.0, 1.0, 2.0, 5.0 | – |
| Median | 1.0 | 1.0 | – |
| Min, Max | 1.0, 1.0 | 1.0, 5.0 | – |
Assessments—clinical and radiographic outcome
Patients were assessed pre- and post-operatively at 3, 6, 12, 24, and 36 months following surgical intervention. Clinical outcomes were evaluated using the Dallas Pain Questionnaire [11]. The Dallas Pain Questionnaire was used to assess the amount of chronic spinal pain that affected aspects of the patients’ lives. This 16-item visual analogue tool assesses the impact of chronic pain on four key aspects of life:
daily activities;
work and leisure activities;
anxiety/depression, and
social interest.
Plain radiographs and thin-cut computed tomographic (CT) scans (1 mm) were used to evaluate the morphology of osteoinduction at similar periods following surgery. The imaging evaluations were scheduled to correlate directly with assessments made of pain and quality of life. Fusion was defined as visible and dense interbody graft with bone continuity between interbody graft and vertebral endplates. Thin-cut CT scans were used to assess new bone formation and bone remodelling within and around the fusion cages. Intervertebral fusion was defined as bone continuity between interbody graft and vertebral endplates on all the sections. Two radiologists independently reviewed the patient’s radiographs and CT films; all patients were available at each time point for the post-operative evaluations.
Results
Plain radiographs
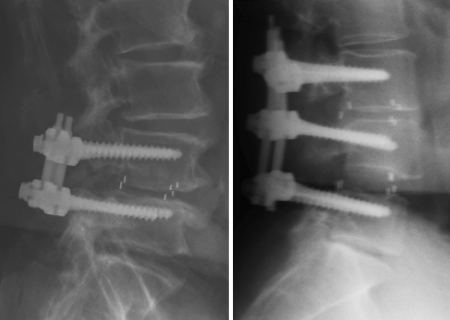
Despite the fact that none of the patients in this study was clinically symptomatic, evidence of vertebral endplate osteoclastic activity was demonstrated radiographically in all 17 patients in 3 months following surgical intervention (Fig. 2). Radiographic evidence of fusion was apparent in all patients at 6 months (Fig. 3). Ossification of the decalcified areas of the vertebrae was evident in all patients at the 6-month assessment and seen at all subsequent examinations.
Fig. 2.
Radiographical assessment of the 3-month patients; to demonstrate the resorption at the perimeter of the cage and osteclastic activity
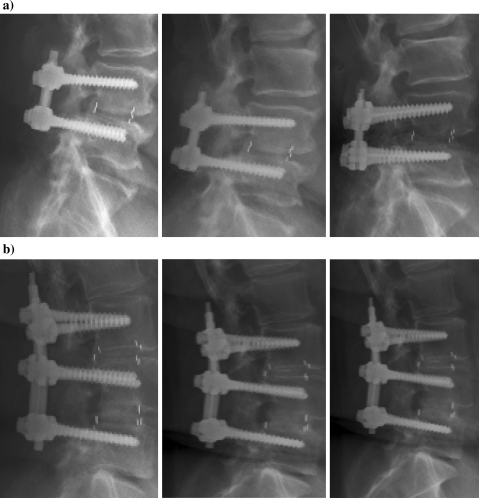
Fig. 3.
Include case reports and X-rays at 6 months for (a) Patient 1 (79-year-old male) and (b) Patient 2 (67-year-old female. Develop figure to show progressive change at each time point that was assessed. Goal of figure is to show continuity in assessment and comparability in the technique of the assessment. a 79-year old male at 6, 12, and 24 b. 67-year-old female at 6, 12, and 24 months
CT scans
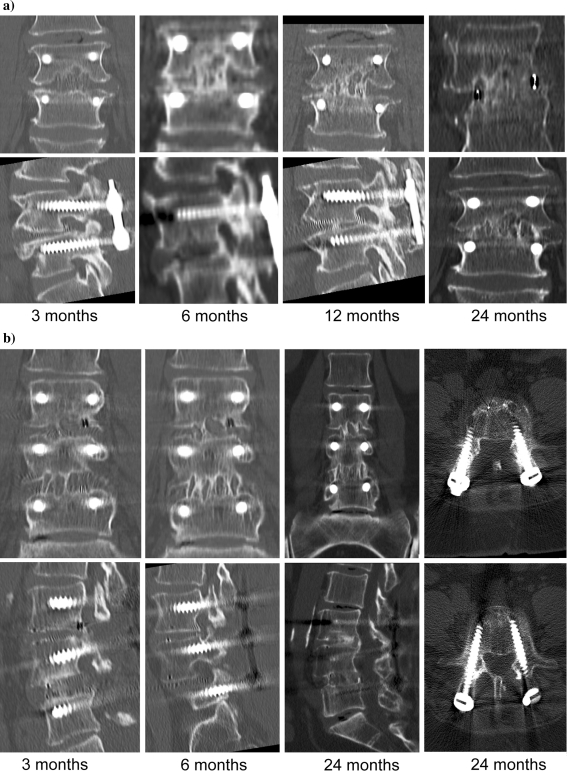
CT scanning confirmed the results seen in the radiographic evaluation (Fig. 4). In all cases, long-term results were reconciled in attaining the clinical objective of fusion. Despite the success of the final outcome, it is important to recognize the transient resorption surrounding the cage 3 months following surgery as a biological phenomenon that was evident at the dosage prescribed for the clinical approval. Whether the interim condition of accelerated resorption is present at other dosages or with other cages is not well understood and may be independent of any relationship
Fig. 4.
CT results graphically depict the changing architecture evaluated at progressive clinical visits. Although the bone surrounding the cage appeared to have lytic changes consistent with osteoclastic resorption at 3 months, clinical follow-up at 6 months demonstrated bone deposition adjacent to the cage that persisted through the 24-month post-surgical evaluation. a 79-year-old male at 3, 6, 12, and 24 months following surgery. b 67-year-old female at 3, 6, 12, and 24 months following surgery
Clinical outcomes
All 17 patients evaluated in this study demonstrated a clinical improvement, with 4 patients showing excellent results, and the remaining 13 patients with good results.
Summarisation of safety
All patients were followed for over of 2 years. Within this group of 17 patients, only 1 patient has demonstrated intracanalar bone formation that could be attributed to rhBMP-2 demonstration (Fig. 5). This patient, however, remains asymptomatic and no additional intervention is anticipated. The remaining patients are similarly asymptomatic, at the 24-month assessment report relief of pain, enhancement of life condition, and related their experience, and quality of life as improved. From the perspective of results obtained in this study, the transient decrease in bone density surrounding the implant that was seen at 3 months ultimately did not affect the intended outcome of the intervention. The strength of the treatment was evident in the lack of stenosis, improvement in VAS scores, and in the lack of affective symptom over the regimen of treatment.
Fig. 5.
Patient demonstrating intracanalar bone formation 24 month following surgery
Discussion
Use of rhBMP-2 and other bone morphogenetic proteins for enhancing osteoblastic activity and accelerating spinal fusion is well documented [2, 4, 7, 10]. Observations from this study also showed that an improvement in clinical outcome was seen in all patients. The Dallas Pain Score demonstrated that the surgical intervention provided patients with relief from pain and intensity of the previous pathology. To add more, the lack of pain allowed the patients to accentuate and resume key accountability for their personal care at a P value <0.05. Our assessment was further encouraging to the extent that patients retained their mobility; demonstrating a P value <0.003 for walking and P value <0.02 for travelling. Finally, throughout the post-surgical evaluation, the patients in this study maintained a positive emotional outlook as assessed through lack of depression (P value <0.02), emotional control (P value <0.02), and anxiety/mood (P value <0.006) assessments.
The present study adds the understanding of the fusion process by providing an instructive assessment of spinal fusion in the early stages of the healing process. While all patients had achieved fusion by 6 months, the fact that 100% of the patients showed transient osteoclastic resorption at 3 months had not been previously presented in a clinical study evaluating spine fusion. Regulatory approval was gained through systematic development of proofs of principle. During the clinical IDE trials, the safe volume and acceptable local concentration were defined for limited interbody constructs. Based on fusion outcomes that were superior to those observed with other bone graft technologies, approval was granted for clinical use.
Subsequent to approval of the first growth factor offering assurance of biological activity, a large amount of discussion has been given to the osteogenic capacity of the BMP family of growth factors and factors that might modify the response. Much less focus has been offered to the metabolic shift engendered by engaging a dominant regenerate phenotype and sustaining activity directed to a fusion product. Transient peri-implant osteolysis with regard to BMP overfilling and hyper-concentration has been observed in a sheep distal femur model [1]. While that study recognized re-ossification of the voids at 8 weeks, the authors also noted that parity between the distal femur and the central region of the vertebra may not be fully warranted as a clinically relevant spine model.
The effects of BMPs on osteoclast activity and ectopic ossification have not been widely investigated. Vertebral endplate osteoclastic activity observed in the present study was transient and ultimately afforded no negative influence on clinical outcome. The dose of rhBMP-2 used in this study may have been higher than that generally indicated for use in single level lumbar fusion in adults with degenerative disc disease (InductOs® 12 mg Kit for implant Summary of Product Characteristics. March 2006). Taken in light of the common outcome in the single patient who received a split dose for his 2-level intervention, however, the potential for the response to be more general must be considered. Placement of rhBMP-2 can cause initial resorption of trabecular bone as noted earlier, but in the context of spinal fusion, dose-related studies that offer similar insight neither have yet been published nor are the authors are aware of any dose-related reports of excessive bone resorption with rhBMP-2 in Germany. Given the lack of ectopic bone growth despite the higher dose, the metabolic response to osteoblast perfusion seems appropriately coupled as morphogenic balance. Previous studies of BMP-2 implantation have shown that the initiation of a cascade of effects includes vascular, cellular, and even access to a larger pool of mesenchymal and hematopoietic progenitor cells. It is not surprising, therefore, that tissue consolidation as directed in spinal fusion offers inherent modelling capacity integral to sustaining it.
The effect of rhBMP-2 on healing patterns has been investigated in a prospective, non-blinded, multicenter study after anterior lumbar interbody fusion using stand-alone threaded cortical allograft dowels [5]. Transient, localised areas of bone remodelling within the vertebral body adjacent to the allograft dowel were observed in 14% of patients, but no data were collected at 3-months that might be compared to later post-surgical images. Similar to what we have shown, but on a different timeline, all areas were healed by 24 months. Looking at the construct in the broader view, it is likely not the cage that imparts an interface that exaggerates resorption, but the high density of bone that is deposited in response to the osteogenic growth factor. Were the Telamon™ (PEEK) cages, or other interbody fixation devices actually driving the resorption, the expectation of a silhouette parallel to the teeth or the thread would be expected. More likely, interfragmentary strain resulting from high bone deposition accentuates turnover, stimulates modelling, and until biophysical signals are incorporated through the construct. Although migration was seen in some patients, the rigid posterior fixation prevented sufficient slip to be symptomatic.
Conclusion
Results from this preliminary study warrant further investigation to evaluate whether rhBMP-2 can be used as an alternative to bone autograft in PLIF. Most important outcome of this study, the transient resorption and potential for cage migration, speaks to a need for careful and controlled study of dosage, carrier stability and to validate that growth factor release offers the expectations of efficacy without additional risk.
Contributor Information
Hans Jörg Meisel, Phone: +49-345-1327404, FAX: +49-345-1327405, Email: meisel@bergmannstrost.com.
Mark Schnöring, Email: mark.schnoering@bergmannstrost.com.
Christian Hohaus, Email: christian.hohaus@bergmannstrost.com.
Yvonne Minkus, Email: yvonne.minkus@bergmannstrost.com.
Andre Beier, Email: beier.andre@web.de.
Timothy Ganey, Email: TIMOTHY.Ganey@tenethealth.com.
Ulrich Mansmann, Email: mansmann@ibe.med.uni-muenchen.de.
References
- 1.Boden SD, Burkus JK, Toth JM, Badura JM, McKay WF (2005) Osteoclastic response to rhBMP-2/absorbable collagen sponges (ACS) in a cancellous bone environment. NASS IRN 2749(066)
- 2.Boden SD, Kangckha PJ, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27:2662–2673. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337–349. doi: 10.1097/00024720-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Burkus JK, Heim SE, Gornet MF, Zdeblick TA. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 2003;16:113–122. doi: 10.1097/00024720-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Burkus JK, Sandhu HS, Gornet MF. Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine. 2006;31:775–781. doi: 10.1097/01.brs.0000206357.88287.5a. [DOI] [PubMed] [Google Scholar]
- 6.Cook DS, Rueger DC. Preclinical models of recombinant BMP induced healing of orthopedic defects. In: Vukicevic S, Sampath KT, editors. Bone morphogenetic proteins from laboratory to clinical practice. Basel: Birkhauser Verlag; 2002. pp. 121–145. [Google Scholar]
- 7.Haid RW, Branch CL, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4:527–539. doi: 10.1016/j.spinee.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Janseen ME, Lam C, Beckham R. Outcomes of allogenic cages in anterior and posterior lumbar interbody fusion. Eur Spine J. 2001;10(Suppl. 2):S158–S168. doi: 10.1007/s005860100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnsson R, Stromqvist B, Aspenberg P. Randomized radiostereometric study comparing osteogenic protein-1 (BMP-7) and autograft bone in human noninstrumented posterolateral lumbar fusion: 2002 Volvo Award in clinical studies. Spine. 2002;27:2654–2661. doi: 10.1097/00007632-200212010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Lanman TH, Hopkins TJ. Lumbar interbody fusion after treatment with recombinant human bone morphogenetic protein-2 added to 70:30 poly (L-lactide-co-D, L-lactide) bioresorbable implant. Neurosurg Focus. 2004;16:3–9. [PubMed] [Google Scholar]
- 11.Lawlis GF, Cuencas R, Selby D, McCoy CE. The development of the Dallas Pain Questionnaire. An assessment of the impact of spinal pain on behaviour. Spine. 1989;14:511–516. doi: 10.1097/00007632-198905000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Sandhu HS. Bone morphogenetic proteins and spinal surgery. Spine. 2003;28(15 Suppl):S64–S73. doi: 10.1097/00007632-200308011-00012. [DOI] [PubMed] [Google Scholar]
- 13.Sasso RC, LeHuec JC, Shaffrey C. Illiac crest bone graft donor site pain after anterior lumbar interbody fusion A prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18(Suppl 1):S77–S81. doi: 10.1097/01.bsd.0000112045.36255.83. [DOI] [PubMed] [Google Scholar]
- 14.Vaccaro AR, Patel T, Fishgrund J, Anderson G, Truumees E, Herkowitz HN, et al. A pilot study evaluating the safety and efficacy of OP-1 putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolosthesis. Spine. 2004;29:1885–1892. doi: 10.1097/01.brs.0000137062.79201.98. [DOI] [PubMed] [Google Scholar]