Abstract
A prospective randomised 2-year follow-up study on patients undergoing lumbar disc herniation surgery. The objective was to investigate the relationship between peridural scarring and clinical outcome, the scar development 6 and 24 months postoperatively by using MRI, and if ADCON-L (a bioresorbable carbohydrate polymer gel) has an effect on scar size and/or improve patients’ outcome after lumbar disc herniation surgery. The association between peridural scarring and recurrent pain after lumbar disc herniation surgery is debated. Numerous materials have been used in attempts to prevent or reduce postoperative peridural scarring; however, there are conflicting data regarding the clinical effects. The study included 119 patients whose mean age was 39 years (18–66); 51 (47%) were women. Sixty patients (56%) were perioperatively randomised to receive ADCON-L, and 48 (44%) served as controls. All patients underwent MRI at 6 and 24 months postoperatively, and an independent radiologist graded the size, location and development of the scar, by using a previously described scoring system. Pre- and 2-year postoperatively patients graded their leg pain on a visual analogue scale (VAS). At the 2-year follow-up patients rated their satisfaction with treatment (subjective outcome) and were evaluated by an independent neurologist (objective outcome), using MacNab score. There was no relationship between size or localisation of the scar and any of the clinical outcomes (VAS, subjective and objective outcome). The scar size decreased between 6 and 24 months in 49%, was unchanged in 42% and increased in 9% of the patients. Patients treated with ADCON-L did not demonstrate any adverse effects, nor did they demonstrate less scarring or better clinical outcome than control patients. No significant association between the presence of extensive peridural scar or localisation of scar formation and clinical outcome could be detected in the present study. Further, no positive or negative effects of ADCON-L used in disc herniation surgery could be seen.
Keywords: Disc herniation surgery, Peridural scar, Sciatica, ADCON-L, Clinical outcome
Introduction
Earlier reported results after surgical treatment of disc herniation have shown over 90% success rate, while long-term results have been less positive with 10% of the patients reporting not completely favourable results [2, 17]. One suggested explanation could be peridural fibrosis formed during the healing process after surgical intervention. A relationship between extensive peridural fibrosis, diagnosed by magnetic resonance imaging (MRI), and increasing low back pain and/or recurrent radicular pain has been reported [19, 25]. Furthermore, the presence of peridural fibrosis has been described in as much as 24% of patients with failed back surgery syndrome [5, 28]. However, the clinical significance of the scar size and development is still unclear and debated.
In the normal process of wound healing after lumbar spinal surgery, fibrotic tissue replaces normal epidural fat by fibroblast migration. In contrast to epidural fat, which allows the dura and nerve roots to move without compression or tethering, fibrotic tissue formed peridurally can bind the dura and nerve roots to surrounding structures and thereby cause compression or stretching of the nervous structures [22]; this fibrotic tissue (epidural scar) can be visualised by MRI. The relationship between mechanical compression of spinal nerve roots and experienced pain has been discussed in disc herniation patients, and disc material (nucleus pulposus) has been demonstrated to increase the sensitivity of the nerve root to mechanical influence [21]. In the process of scar development after disc herniation surgery a number of different inflammatory substances can also be expected to be present initially, but little is known about their influence on the nerve structures during the scar development.
Numerous synthetic and natural materials such as, polytetrafluoroethylene, free fat transplantation, protein-based polymer, high-molecular weight hyaluronan, Oxiplex/SP, and DuraGen have been evaluated to prevent or reduce postoperative peridural scar formation in both animal and human studies [12–14, 27, 29, 30].
One of the materials is ADCON-L, a bioresorbable carbohydrate polymer gel, described to form a protective membrane when applied on nervous structures and is suggested to prevent scar formation. The purpose of this material is to cover the dura and nerve root(s) until the fibrosis formation is completed [25]. However, there are conflicting data regarding the clinical effects of ADCON-L where some authors have demonstrated reduced scar formation and improved clinical outcome [7, 25], whereas others have not seen any positive effects [9, 23]. Furthermore, studies have indicated an increased risk of cerebrospinal fluid leakage after the application of ADCON-L in spine patients [11, 15].
The aim of the present study was to investigate, (1) if there is any relationship between the size or location of the peridural formed scar and clinical outcomes 2 years after lumbar discectomy, (2) scar development between 6 and 24 months postoperatively evaluated by MRI, and (3) if ADCON-L has an effect on scar size and/or improves patients’ outcome.
Materials and methods
Patients
One hundred twenty-eight patients were recruited for the study. The indication for surgery was a CT or an MRI verified lumbar disc herniation either at L4–L5 or L5–S1 level correlated to the patients’ symptoms, radicular pain and failure of proper conservative therapy. Patients with prior surgery on the herniated disc segment or other spinal disorders were not included. Nine patients were excluded due to new surgical procedures performed within the 2-year follow-up period. Six patients were operated because of recurrent disc herniation and three patients had fusion surgery; 119 patients were included, 11 were lost to follow-up, and of the remaining 103 (95%) completed the MRI examination at 24 months, 99 (92%) filled in the 2-year follow-up questionnaire about satisfaction with treatment, and 102 (94%) were examined by the independent observer.
The study population had a mean age of 39 years (18–66) and 51 (47%) patients were women. Sixty (56%) patients received ADCON-L perioperatively and 48 (44%) patients served as controls. Forty-eight (44%) patients underwent surgery at the L4–L5 level and 60 (56%) at the L5–S1 level.
The Regional Ethical Review Board approved the study and all the patients gave their informed consent for inclusion (Table 1).
Table 1.
The demographics at baseline of the patients included in the study
| Characteristic | ADCON-L n = 60 |
Control n = 48 |
|---|---|---|
| Male | 48% | 58% |
| Female | 52% | 42% |
| Age (mean ± SD) (range) | 38 ± 10 (18–65) | 40 ± 12 (20–66) |
| L4/L5 | 40% | 50% |
| L5/S1 | 60% | 50% |
| VAS leg pain (median) | 57 | 57 |
Surgical procedure
All patients underwent the procedure of partial discectomy. By using a midline approach the paravertebral muscles were dissected down to the laminae and the interlaminar ligaments were resected. A partial laminotomy was performed when necessary. Herniated disc material and loose fragments from the disc were removed to decompress the affected neural structures. The surgery was performed with or without microscope due to the surgeons’ preference. The patients were randomised at the end of the surgery by envelope to receive ADCON-L or not (controls). After the removal of the herniated disc fragment(s), prior to closure, 3 g of ADCON-L was applied to surround the nerve root, thecal sac and posterior longitudinal ligament, up to the lower surface of the lamina. Controls received drain and closure of the wound. Six different spine surgeons performed the surgery at one hospital.
Preoperative and 2-year follow-up assessments
Preoperatively, and at the 2-year follow-up the patients reported radicular pain by using three visual analogue scales ranging from 0 to 100, representing “pain at its worst”, “pain at its least” and “pain at the present time”. The mean value of the three scales was recorded as “VAS leg pain”.
At the 2-year follow-up a self-reported overall outcome was used, where the patients were asked to rate their satisfaction with the treatment as satisfied, partly satisfied, or not satisfied.
Further at the 2-year follow-up an independent observer, a neurologist, blinded to treatment examined the patients and graded their outcome according to Macnabs’ classification [18] (Fig. 1).
Fig. 1.
Description of the Macnab classification, clinical outcome (objective)
MRI evaluation
The subjects were examined by MRI on two occasions postoperatively at 6 and 24 months, respectively.
All MRI scans were obtained at the same University Hospital. Most examinations were made with 0.5-T imagers on Philips Gyroscan T5-NT. A small number of examinations were made with 1.5 T imagers, Siemens Magnetom Vision Plus or Philips Gyroscan Intera T15 due to technical reasons (e.g., upgradings or service).
The studies consisted of localiser in three planes, sagittal T2- and T1-weighted images and axial T1- and PD-weighted images. After intravenous Gadolinium, sagittal and axial sequences were repeated.
The T1 sagittal sequences were obtained using parameters of TR 492 ms/TE 10 ms with turbo spin echo (TSE), 35 cm field of view (FOV), a 256 × 189 matrix and 3 mm slice thickness with 0.3 mm spacing, T2 sagittal sequences by 3,224 ms/130 ms (TR/TE) with TSE, a 35 cm FOV, a 256 × 170 matrix, 3.0 mm slice thickness with 0.3 mm spacing. PD axial sequences were acquired in the plane of the disc by a 1,654 ms/40 ms (TR/TE) with TSE, 26 cm FOV, a 256 × 190 matrix and 4.0 mm slice thickness with 0.4 mm spacing.
The slice thickness was 3 and 4 mm for sagittal and axial sequences, respectively, for examinations with Philips Gyroscan T5-NT, and 4 mm for both sagittal and axial sequences for the other two imagers. The PD-weighted axial sequences were stacked slices, including the three most caudal lumbar intervertebral discs through the inferior aspect of S1. Axial T1-weighted sequences before and after Gadolinium were angled according to the lumbar discs L4–L5 and L5–S1.
All studies were read by an experienced independent neuroradiologist, blinded to treatment and clinical findings, familiar with the MRI imagers used. The reader did not know the clinical status of the subjects. Readings of examinations at 6 and 24 months postoperatively were performed at different times without access to the previous reading of the same subject.
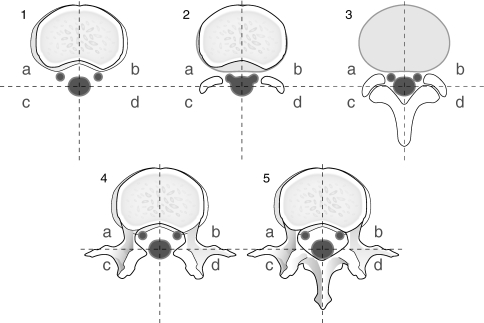
The evaluation included scar size, scar formation around the circumference of the nerve root, scar tissue affecting the nerve and/or dura (dislocation, compression, scar surrounding >2/3 of the nerve root circumference and swelling of the nerve root). The amount of peridural scar was evaluated according to the grading system described by Ross et al. [25], by using a score of 0–4 (0–>75% scar). Scar score were assigned to four spatial quadrants of each MRI slice; there were five slices available for evaluation (two slices above, one at and two below the disc level). Twenty MRI quadrants for each patient were evaluated and the quadrant with the highest number at the Ross score was used for calculation (Fig. 2).
Fig. 2.
The amount and location of peridural scar was evaluated according to the grading system described by Ross et al. by using a score of 0–4. Five axial MRI slices/patient were available for evaluation (1–5). Two slices above the disc (1, 2), one in the disc level (3) and two below the disc (4, 5). Each slice was divided into four quadrants (a–d)
Statistical analysis
Due to the qualitative nature of the outcome variables non-parametrical tests were used. For paired data, i.e., change in scar score at 6 versus 24 months, Wilcoxon signed rank test was used.
For comparisons between two independent groups, Mann–Whitney U test, Kruskal–Wallis or a chi-square test was used. In some cases, due to few observations, categories of a variable have been pooled together; 5% was used as significance level.
Results
The demographics at baseline of the patients in regard to ADCON-L treated or controls are presented in Table 1.
Peridural scar and clinical outcomes
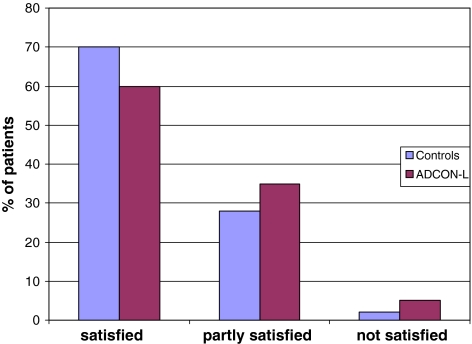
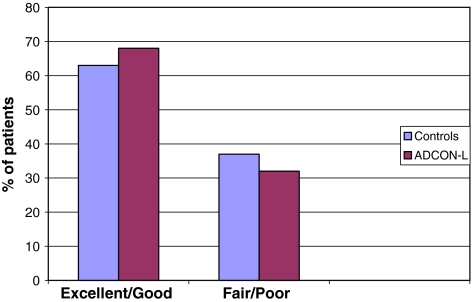
Patients reported clinical outcome (subjective and objective) are presented in Figs. 3 and 4.
Fig. 3.
Patients reported clinical outcome, subjective (satisfied, partly satisfied or not satisfied), 24 months after surgery in control- and ADCON-L treated groups
Fig. 4.
Objective outcome (excellent/good or fair/poor) in control- and ADCON-L treated groups at 24 months follow-up
No relationship between scar size or location and clinical outcome (VAS, subjective and objective outcome) was found.
Further no relationship between scar development over time and clinical outcome (VAS, subjective outcome and objective outcome) was found.
MRI findings
Scar size, location and development
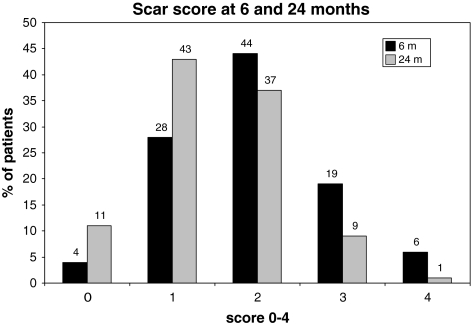
Regarding the scar size, almost half of the patients (44%) had scar score 2 (>25–50% of at least one quadrant filled with scar) at 6 months postoperatively and 43% scar score 1 (size 0–25% of at least one quadrant filled with scar) at 24 months.
Scar scores at 6 and 24 months are presented in Fig. 5.
Fig. 5.
Scar score by Ross et al. in disc herniation patients at 6 and 24 months postoperatively. At 6 months follow-up almost half of the patients had scar score (2) corresponding to >25–50% of at least one of the MRI slices available filled with scar. At the 24 months follow-up most of the patients had lower scar score than at 6 months follow-up
Over 85% of the patients had scar-formation located around the nerve-root (in a, and b area, Fig. 2), and 55% scar influencing dura at 24 months time point.
At 24 months the scar was affecting the nerve root by dislocation, compression or scar surrounding >2/3 of nerve root circumference in 53 of the patients. The types of nerve root influence are presented in Table 2.
Table 2.
Description of scar tissue influence on nerve root in disc herniation patients, visualised by MRI at 24 months postoperatively
| Description of nerve root influence | Numbers | % |
|---|---|---|
| Dislocation | 11 | 21 |
| Compression | 2 | 4 |
| Scar >2/3 | 20 | 38 |
| Dislocation and compression | 2 | 4 |
| Dislocation and scar >2/3 | 8 | 15 |
| Compression and scar >2/3 | 5 | 9 |
| Dislocation, compression and scar >2/3 | 5 | 9 |
The scar score decreased between 6 and 24 months in 49% of the patients, were unchanged in 42% and increased in 9% (P < 0.0001) (Table 3).
Table 3.
Development of scar between 6 and 24 months after disc herniation surgery expressed by scar score
| Scar score | n | % |
|---|---|---|
| Increased 1 step | 9 | 9 |
| Unchanged | 42 | 42 |
| Decreased 1 step | 38 | 38 |
| Decreased 2 step | 10 | 10 |
| Decreased 3 step | 1 | 1 |
P < 0.0001
In the patients with increasing scar scores the increase was from score 1 to 2 in 7/9 patients and from score 2 to 3 in 2/9 patients.
ADCON-L, scar size and outcome
There were no significant baseline differences between the control- and ADCON-L treated groups.
There were no reports of CSF leakage in any of the patients.
No significant differences were seen in scar score between control group and ADCON-L treated group (Table 4).
Table 4.
Differences in scar score compared with VAS score, in control- and ADCON-L treated groups at 24 months
| Scar score | 0 | 1 | 2 | 3–4 | |
|---|---|---|---|---|---|
| ADCON-L | 6.9% (4) | 48.3% (28) | 37.9% (22) | 6.9% (4) | 100% (58) |
| VAS leg pain (median, range) | 34 (0–68) | 19 (0–79) | 22 (0–95) | 17 (9–65) | |
| Controls | 15.6% (7) | 35.6% (16) | 35.6% (16) | 13.3% (6) | 100% (45) |
| VAS leg pain (median, range) | 21 (0–41) | 14 (0–52) | 25 (0–90) | 37 (10–79) |
VAS leg pain at the 2-year follow-up did not significantly differ between the two groups 25 (0–90) in the control group and 19 (0–95) in the ADCON-L treated group.
In the control group 70% of the patients reported that they were satisfied at the 2-year follow-up, 28% partly satisfied and 2% not satisfied. In the ADCON-L group 60% were satisfied, 35% partly satisfied and 5% not satisfied.
At the 2-year follow-up 63% were assessed as “excellent/good” and 37% as “fair/poor” in the control group, and in the ADCON-L group similar results were seen (68 and 32%, respectively).
Discussion
The present study demonstrated no association between postoperative scar formation (size or localisation) and development on MRI and clinical outcome 2 years after discectomy for lumbar disc herniation. There was a reduction in the presence and amount of scar tissue between MRI at 6 months compared to 24 months. No significant effect of ADCON-L on scar size or clinical outcome was found.
Peridural scar after lumbar disc herniation surgery is normally seen to some extent. The postoperative period can be divided into two stages, early (0–6 months) and late (>6 months) [3]. In early stages epidural soft-tissue, due to oedema and haemorrhage, can be seen at MRI in as much as 80% of patients undergoing lumbar surgery. This tissue decreases to about 50% already after 2 months [8]. Early detected epidural tissue on MRI can be due to postoperative haematoma and may develop to epidural scar tissue [4]. Because of the normal changes before 6 months after surgery there is risk of misinterpretations [31–33].
This fact has lead to recommendations to avoid MRI evaluations 0–6 months after surgery.
In the late stage, between 6 and 12 months, studies have shown that only a few patients demonstrate changes in the amount of peridural scar [16, 24]. Further, in a study by Grane et al. [10], more extensive epidural scarring before 12 months than after was reported.
In the present study the scores of the scar differ at 6 and 24 months and in most patients the difference was a decrease of the scar over time.
However, in 42% the size of the scar was unchanged and in nine patients the size increased between the two follow-ups. This increase was moderate but it suggests that the scar probably is not fully developed at 6 months after surgery in some patients.
Only 6% of the disc herniation patients demonstrated extensive scar at 6 months MRI compared to 42% in the study by Ross et al. The difference in extensive scarring would have been even larger if the exactly same criteria for “extensive scarring” had been used since we included scar in all five levels whereas Ross et al., only included the scar in level 3–5 (see Fig. 2). At the 24-month follow-up the patients with extensive scaring at MRI in the present study had decreased further to 1%.
The epidural scar seen on MRI is the fibrotic tissue replacing the normal epidural fat and the removed ligamentum flavum. However, the relationship between the fibrotic tissues, affecting the epidural structures (thecal sac and nerve roots) and clinical symptoms are debated. Some studies suggest that scar tissue is responsible for unfavourable outcome after spinal surgery [19, 25]. In a study by Samy Abdou and Hardy [26], the incidence of patients with “failed back surgery symptoms (FBSS)” caused by epidural scar due to post-discectomy, would be as high as 8–14%. However, in the present study no correlation between scar formation and clinical outcome after lumbar discectomy was detected. This is in agreement with the findings of Nygaard et al. [20], where no associations between the amounts of postoperative peridural scar formation or nerve root displacement and outcome 1 year after microdiscectomy for lumbar disc herniation was seen.
The conclusion that the extent of peridural scarring defined by MRI is of minor value in patients with recurrent back and leg pain after lumbar microdiscectomy is further agreed upon in a number of recent studies [1, 6, 33].
Since there are diverse opinions about the importance of scar development postoperatively, there has been an interest to investigate if anti-adhesion barriers would make a difference if applied peroperatively in patients undergoing disc herniation surgery. An effect of such a material would indirectly support the theory that the peridural scar causes problems.
In this study we could not find any positive effects of the anti-adhesion gel used, ADCON-L did not reduce the scar size nor did it improve the clinical outcome. Because of earlier reported complications of ADCON-L (chronic leakage of cerebrospinal fluid in combination with intracranial hypotension syndrome) [15] when used in disc herniation patients it should be critically evaluated. No postoperative complications related to administration of ADCON-L were observed in the present study.
Conclusions
The findings in this study on patients subjected to lumbar disc herniation surgery demonstrate no association between peridural scar and clinical outcome; neither could any association between the localisation of scar formation in relation to nervous structures and clinical outcome be detected in this patient group. A change in scar size between 6 and 24 months could be detected in 58% of the patients indicating that the process of scar formation is not fully completed 6 months after surgery.
No positive or negative effects of ADCON-L on the scar size or the clinical outcome could be seen.
A limitation of this study was that the images were taken with a 0.5 T MR scanner, whereas stronger MR scanners are now available. However, if this would change the results remains unclear.
Acknowledgments
The authors thank Gothenburg Medical Society, Doctor Felix Neubergh Foundation for financial support and government grants during LUA agreement. We also thank MD Lena Rutberg, neurologist, for the assessment of clinical outcome 2-year postoperative.
References
- 1.Annertz M, Jonsson B, Stromqvist B, et al. Serial MRI in the early postoperative period after lumbar discectomy. Neuroradiology. 1995;37:177–182. doi: 10.1007/BF01578253. [DOI] [PubMed] [Google Scholar]
- 2.Asch HL, Lewis PJ, Moreland DB, et al. Prospective multiple outcomes study of outpatient lumbar microdiscectomy: should 75 to 80% success rates be the norm? J Neurosurg. 2002;96:34–44. doi: 10.3171/spi.2002.96.1.0034. [DOI] [PubMed] [Google Scholar]
- 3.Babar S, Saifuddin A. MRI of the post-discectomy lumbar spine. Clin Radiol. 2002;57:969–981. doi: 10.1053/crad.2002.1071. [DOI] [PubMed] [Google Scholar]
- 4.Boden SD, Davis DO, Dina TS, et al. Contrast-enhanced MR imaging performed after successful lumbar disk surgery: prospective study. Radiology. 1992;182:59–64. doi: 10.1148/radiology.182.1.1727310. [DOI] [PubMed] [Google Scholar]
- 5.Burton CV, Kirkaldy-Willis WH, Yong-Hing K, et al. Causes of failure of surgery on the lumbar spine. Clin Orthop Relat Res. 1981;157:191–199. [PubMed] [Google Scholar]
- 6.Cervellini P, Curri D, Bernardi L, et al. Computed tomography after lumbar disc surgery: a comparison between symptomatic and asymptomatic patients. Acta Neurochir Suppl (Wien) 1988;43:44–47. doi: 10.1007/978-3-7091-8978-8_11. [DOI] [PubMed] [Google Scholar]
- 7.Tribolet N, Porchet F, Lutz TW, et al. Clinical assessment of a novel antiadhesion barrier gel: prospective, randomized, multicenter, clinical trial of ADCON-L to inhibit postoperative peridural fibrosis and related symptoms after lumbar discectomy. Am J Orthop. 1998;27:111–120. [PubMed] [Google Scholar]
- 8.Floris R, Spallone A, Aref TY, et al. Early postoperative MRI findings following surgery for herniated lumbar disc. Acta Neurochir (Wien) 1997;139:169–175. doi: 10.1007/BF01844746. [DOI] [PubMed] [Google Scholar]
- 9.Ganzer D, Giese K, Volker L, et al. Two-year results after lumbar microdiscectomy with and without prophylaxis of a peridural fibrosis using Adcon-L. Arch Orthop Trauma Surg. 2003;123:17–21. doi: 10.1007/s00402-002-0455-y. [DOI] [PubMed] [Google Scholar]
- 10.Grane P, Tullberg T, Rydberg J, et al. Postoperative lumbar MR imaging with contrast enhancement. Comparison between symptomatic and asymptomatic patients. Acta Radiol. 1996;37:366–372. doi: 10.3109/02841859609177668. [DOI] [PubMed] [Google Scholar]
- 11.Hieb LD, Stevens DL. Spontaneous postoperative cerebrospinal fluid leaks following application of anti-adhesion barrier gel: case report and review of the literature. Spine. 2001;26:748–751. doi: 10.1097/00007632-200104010-00009. [DOI] [PubMed] [Google Scholar]
- 12.Ivanic GM, Pink PT, Schneider F, et al. Prevention of epidural scarring after microdiscectomy: a randomized clinical trial comparing gel and expanded polytetrafluoroethylene membrane. Eur Spine J. 2006;15:1360–1366. doi: 10.1007/s00586-006-0120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen TT, Asmussen K, Berg-Hansen EM, et al. First-time operation for lumbar disc herniation with or without free fat transplantation. Prospective triple-blind randomized study with reference to clinical factors and enhanced computed tomographic scan 1 year after operation. Spine. 1996;21:1072–1076. doi: 10.1097/00007632-199605010-00016. [DOI] [PubMed] [Google Scholar]
- 14.Kim KD, Wang JC, Robertson DP, et al. Reduction of leg pain and lower-extremity weakness for 1 year with Oxiplex/SP gel following laminectomy, laminotomy, and discectomy. Neurosurg Focus. 2004;17:ECP1. doi: 10.3171/foc.2004.17.1.8. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn J, Hofmann B, Knitelius HO, et al. Bilateral subdural haematomata and lumbar pseudomeningocele due to a chronic leakage of liquor cerebrospinalis after a lumbar discectomy with the application of ADCON-L gel. J Neurol Neurosurg Psychiatry. 2005;76:1031–1033. doi: 10.1136/jnnp.2004.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law JD, Lehman RA, Kirsch WM. Reoperation after lumbar intervertebral disc surgery. J Neurosurg. 1978;48:259–263. doi: 10.3171/jns.1978.48.2.0259. [DOI] [PubMed] [Google Scholar]
- 17.Loupasis GA, Stamos K, Katonis PG, et al. Seven- to 20-year outcome of lumbar discectomy. Spine. 1999;24:2313–2317. doi: 10.1097/00007632-199911150-00005. [DOI] [PubMed] [Google Scholar]
- 18.Macnab I. Chapter 14. Pain and disability in degenerative disc disease. Clin Neurosurg. 1973;20:193–196. doi: 10.1093/neurosurgery/20.cn_suppl_1.193. [DOI] [PubMed] [Google Scholar]
- 19.Maroon JC, Abla A, Bost J. Association between peridural scar and persistent low back pain after lumbar discectomy. Neurol Res. 1999;21(Suppl 1):S43–S46. doi: 10.1080/01616412.1999.11741026. [DOI] [PubMed] [Google Scholar]
- 20.Nygaard OP, Kloster R, Dullerud R, et al. No association between peridural scar and outcome after lumbar microdiscectomy. Acta Neurochir (Wien) 1997;139:1095–1100. doi: 10.1007/BF01410967. [DOI] [PubMed] [Google Scholar]
- 21.Olmarker K, Storkson R, Berge OG. Pathogenesis of sciatic pain: a study of spontaneous behavior in rats exposed to experimental disc herniation. Spine. 2002;27:1312–1317. doi: 10.1097/00007632-200206150-00013. [DOI] [PubMed] [Google Scholar]
- 22.Ozer AF, Oktenoglu T, Sasani M, et al. Preserving the ligamentum flavum in lumbar discectomy: a new technique that prevents scar tissue formation in the first 6 months postsurgery. Neurosurgery. 2006;59:ONS126–ONS133. doi: 10.1227/01.NEU.0000220078.90175.E6. [DOI] [PubMed] [Google Scholar]
- 23.Richter HP, Kast E, Tomczak R, et al. Results of applying ADCON-L gel after lumbar discectomy: the German ADCON-L study. J Neurosurg. 2001;95:179–189. doi: 10.3171/spi.2001.95.2.0179. [DOI] [PubMed] [Google Scholar]
- 24.Ross JS. MR imaging of the postoperative lumbar spine. Magn Reson Imaging Clin N Am. 1999;7:513–524. [PubMed] [Google Scholar]
- 25.Ross JS, Robertson JT, Frederickson RC, et al. Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. ADCON-L European Study Group. Neurosurgery. 1996;38:855–861. doi: 10.1097/00006123-199604000-00053. [DOI] [PubMed] [Google Scholar]
- 26.Samy Abdou M, Hardy RW., Jr Epidural fibrosis and the failed back surgery syndrome: history and physical findings. Neurol Res. 1999;21(Suppl 1):S5–S8. doi: 10.1080/01616412.1999.11758603. [DOI] [PubMed] [Google Scholar]
- 27.Schimizzi AL, Massie JB, Murphy M, et al. High-molecular-weight hyaluronan inhibits macrophage proliferation and cytokine release in the early wound of a preclinical postlaminectomy rat model. Spine J. 2006;6:550–556. doi: 10.1016/j.spinee.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Spangfort EV. The lumbar disc herniation. A computer-aided analysis of 2, 504 operations. Acta Orthop Scand Suppl. 1972;142:1–95. doi: 10.3109/ort.1972.43.suppl-142.01. [DOI] [PubMed] [Google Scholar]
- 29.Tatsui CE, Martinez G, Li X, et al. Evaluation of DuraGen in preventing peridural fibrosis in rabbits. Invited submission from the joint section meeting on disorders of the spine and peripheral nerves, March 2005. J Neurosurg Spine. 2006;4:51–59. doi: 10.3171/spi.2006.4.1.51. [DOI] [PubMed] [Google Scholar]
- 30.Vakis A, Koutentakis D, Karabetsos D, et al. Use of polytetrafluoroethylene dural substitute as adhesion preventive material during craniectomies. Clin Neurol Neurosurg. 2006;108:798–802. doi: 10.1016/j.clineuro.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 31.Kelft EJ, Goethem JW, de La Porte C, et al. Early postoperative gadolinium-DTPA-enhanced MR imaging after successful lumbar discectomy. Br J Neurosurg. 1996;10:41–49. doi: 10.1080/02688699650040520. [DOI] [PubMed] [Google Scholar]
- 32.Goethem JW, Kelft E, Biltjes IG, et al. MRI after successful lumbar discectomy. Neuroradiology. 1996;38(Suppl 1):S90–S96. doi: 10.1007/BF02278130. [DOI] [PubMed] [Google Scholar]
- 33.Vogelsang JP, Finkenstaedt M, Vogelsang M, et al. Recurrent pain after lumbar discectomy: the diagnostic value of peridural scar on MRI. Eur Spine J. 1999;8:475–479. doi: 10.1007/s005860050208. [DOI] [PMC free article] [PubMed] [Google Scholar]