Abstract
The implantation of lumbar disc prostheses based on different design concepts is widely accepted. This paper reviews currently available literature studies on the biomechanics of TDA in the lumbar spine, and is targeted at the evaluation of possible relationships between the aims of TDA and the geometrical, mechanical and material properties of the various available disc prostheses. Both theoretical and experimental studies were analyzed, by a PUBMED search (performed in February 2007, revised in January 2008), focusing on single level TDA. Both semi-constrained and unconstrained lumbar discs seem to be able to restore nearly physiological IAR locations and ROM values. However, both increased and decreased ROM was stated in some papers, unrelated to the clinical outcome. Segmental lordosis alterations after TDA were reported in most cases, for both constrained and unconstrained disc prostheses. An increase in the load through the facet joints was documented, for both semi-constrained and unconstrained artificial discs, but with some contrasting results. Semi-constrained devices may be able to share a greater part of the load, thus protecting the surrounding biological structure from overloading and possible early degeneration, but may be more susceptible to wear. The next level of development will be the biomechanical integration of compression across the motion segment. All these findings need to be supported by long-term clinical outcome studies.
Keywords: Lumbar, Disc arthroplasty, Biomechanics, Motion preservation, Spinal alignment
Introduction
Total disc arthroplasty (TDA) is currently widely used in Europe and in North America to treat chronic discogenic back pain, and several Investigational Device Exemption studies are currently in progress in the US. Satisfactory clinical outcomes as well as complications have been reported [63, 91, 96], pointing out the need for further investigations. The importance of patient selection was found to be a critical point: a class action lawsuit against the Charité artificial disc (Depuy Acromed Inc., Mountain View, CA, USA) is currently ongoing, due to low success rate and unexpected, potentially life-threatening complications. These problems may be related to overextending the indications and inappropriate patient selection. A number of biomechanical studies about lumbar TDA have been published, but the relationship between the different designs and the resulting biomechanics of the surgically changed spine has not been clearly described.
This paper reviews currently available literature on the biomechanics of TDA in the lumbar spine. The review is structured along the aims of TDA, related to the following biomechanical parameters:
restoration of a physiological kinematics and mobility, avoiding segmental instability
restoration of a correct spinal alignment
protection of the biological structures, such as the adjacent intervertebral discs, the facet joints and the ligaments, from overloading and resulting accelerated degeneration
device stability and wear.
The goal of the present review is to evaluate possible relationships between the aforementioned aims and the geometrical, mechanical and material properties of the various currently available disc prostheses, outlined in studies focusing on single-level disc arthroplasty. For this purpose, a PUBMED search (National Library of Medicine and National Institute of Health, USA) was performed in February 2007 and repeated in January 2008. Only papers written in English were reviewed. Search strings were “lumbar disc arthroplasty”, “lumbar artificial disc”, “lumbar disc replacement”, “lumbar disc prosthesis”. The search offered 668 papers; 77 papers were not published in English. About 498 papers did not contain relevant biomechanical information and have been excluded. Ninety-three papers were reviewed in total.
Restoration of a physiological kinematics and mobility
Many models of lumbar artificial discs are currently available; a summary of the disc prostheses, either commercially available or under clinical trial, which have been referenced in the papers included in the present review is reported in Table 1. The parameters usually employed to describe spine kinematics are the location of the instantaneous axes of rotation (IARs) and range of motion (ROM), both of which are related to the design of the disc prosthesis.
Table 1.
Classification of the lumbar disc prosthesis that have been referenced in biomechanical papers
| Name | Manufacturer | Classification |
|---|---|---|
| Flexicore | Stryker, Kalamazoo, MI, USA | Constrained |
| Maverick | Medtronic Ltd, Memphis, TN, USA | Semi-constrained |
| ProDisc | Synthes Inc., West Chester, PA, USA | Semi-constrained |
| Charité | Depuy Acromed Inc., Mountain View, CA, USA | Unconstrained |
| Acroflex (discontinued) | Depuy Acromed Inc., Mountain View, CA, USA | Unconstrained |
Instantaneous axis of rotation
The locations of the IARs for the lumbar spine in the different planes of motion have been identified in many studies, in both healthy and pathological conditions [70, 81, 95]. Figure 1 illustrates the IAR locations predicted in a recent study by Schmidt et al. [81] in the healthy lumbar spine.
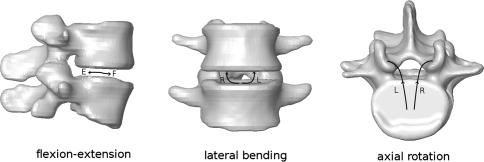
Fig. 1.
Locations of the IARs in the lumbar spine [81]. F and E represent the IAR locations in flexion and extension respectively; L and R are the IAR locations in left lateral bending or left axial rotation (L) and right lateral bending or right axial rotation (R)
A few biomechanical studies regarding the IARs after lumbar arthroplasty have recently been published (Table 2). Cunningham et al. [13] determined the locations of the IAR in flexion-extension, of eight spines implanted with the Charité disc prosthesis at the L4–L5 level. The measured IARs were located in the posterior third of the intervertebral space, similar to the physiological conditions. The same result was obtained by Kotani et al. [42] with a fabric disc prosthesis. Rousseau et al. [78] investigated the IARs of the L5-S1 motion segment after implantation of the semi-constrained prosthesis ProDisc and the unconstrained Charité. Both prostheses had no significant influence on the IAR locations in flexion-extension and lateral bending, despite their different design concepts. The authors demonstrated that the instantaneous axis of rotation does not necessarily correspond with the geometrical center of the spherical coupling for semi-constrained prostheses, in particular for shear and lateral bending loading conditions. These results, which seem counter-intuitive, were explained because of deformation of the articulating surfaces and micromotion at the device–endplate interface. However, the results may also be influenced by measurement errors, which cannot be neglected in the experimental estimation of the IAR location [81].
Table 2.
Published studies concerning lumbar kinematics after TDA
| References | Model | Study type | Mechanical variables | Prosthesis | Compared to | Results |
|---|---|---|---|---|---|---|
| Auerbach et al. [1] | Human | Radiographic | Motion | ProDisc (semi-constrained) | Fusion | Preserved motion at implanted and adjacent levels, as compared to fusion |
| Bertagnoli and Kumar [2] | Human | Radiographic | Motion | ProDisc (semi-constrained) | – | Preserved average vertebral motion at the operated level |
| Bertagnoli et al. [4] | Human | Radiographic | Motion | ProDisc (semi-constrained) | – | 3–7 degrees of motion in flexion-extension |
| Bertagnoli et al. [3] | Human | Radiographic | Motion | ProDisc (semi-constrained) | Smokers versus non-smokers | 3–7 degrees of motion in flexion-extension |
| Cakir et al. [6] | Human | Radiographic | Segmental and total lordosis | ProDisc (semi-constrained) | – | Increased segmental lordosis, unchanged total lordosis |
| Cakir et al. [7] | Human | Radiographic | Method for measuring motion | – | – | Choice of different landmarks improves measure reliability |
| Chung et al. [9] | Human | Radiographic | Segmental and total lordosis | ProDisc (semi-constrained) | – | Increased segmental and total lordosis |
| Chung et al. [10] | Human | Radiographic | Motion | ProDisc (semi-constrained) | – | Increased average motion from 9.7 to 12.7 degrees |
| Cinotti et al. [11] | Human | Radiographic | Motion | Charité (unconstrained) | – | Average 9 degrees of motion at the implanted level, 16 degrees at the adjacent levels |
| Cunningham et al. [14] | Non-human primate | In vivo, ex vivo (histopatologic and histomorphometric) | Motion, trabecular ingrowth | Acroflex (unconstrained) | – | Reduced motion; no evidences of pathological changes; heterotopic ossification |
| Cunningham et al. [13] | Human | Ex vivo | Motion, IAR | Charité (unconstrained) | Fusion, instrumented fusion | Preserved motion and IAR |
| Cunningham et al. [12] | Non-human primate | In vivo, ex vivo | Motion, bone ingrowth | Charité, Acroflex (unconstrained) | Intact | Acroflex motion smaller than intact and Charité; excellent bone ingrowth |
| Cunningham [16] | Human, non-human primate | In vivo, ex vivo (histopatologic and histomorphometric) | Motion, IAR, bone ingrowth | Charité, Acroflex (unconstrained) | Intact, fusion | Preserved motion and IAR, excellent bone ingrowth |
| Cunningham et al. [15] | Human | Radiographic | Motion | Charité (unconstrained) | Fusion | Normal motion distribution along the lumbar spine |
| David [17] | Human | Radiographic | Motion | Charitè(unconstrained) | – | Preserved motion |
| Delamarter et al. [19] | Human | Ex vivo | Motion | ProDisc (semi-constrained) | Fusion | 1 operated level: preserved motion and coupling; 2 operated levels: not preserved motion and coupling in 50% |
| Denozière and Ku [20] | Human | Computational | Motion, stability, ligament tensions, facet pressure | Ball and socket (semi-constrained) | Fusion | Greater risk of instability and further degeneration relative fusion |
| Dooris et al. [21] | Human | Computational | Motion, facet loads, intradiscal pressure, shear stresses | Ball and socket (semi-constrained) | – | Modification of the spinal bending stiffness in the sagittal plane |
| Eijkelkamp et al. [23] | – | – | Kinematics, load sharing, stability | – | – | Design guidelines for disc prostheses |
| Enker et al. [24] | Human | Radiographic | Motion, bone ingrowth | Acroflex (unconstrained) | – | Preserved motion, good bone ingrowth |
| Goel et al. [28] | Human | Computational | Motion, facet loads, intradiscal pressure, shear stresses | Charité (unconstrained) | – | Increased motion at the L5-S1 level in flexion-extension; decreased facet loads; higher shear stresses at the TDA L5 endplate relative to those at S1 interface |
| Hedman et al. [29] | – | – | Kinematics, endurance, safety | Generic | – | Design guidelines for disc prostheses |
| Hitchon et al. [30] | Human | Ex vivo | Motion | Maverick (semi-constrained) | – | Preserved motion |
| Huang et al. [34] | – | – | Constraint | Semi-constrained, unconstrained | – | Unconstrained disc prostheses may have a kinematical advantage; semi-constrained disc prostheses may protect the posterior structure in shear |
| Huang et al. [35] | Human | Radiographic | Motion, sagittal alignment | ProDisc (semi-constrained) | – | Preserved motion; improved global and segmental alignment |
| Huang et al. [33] | Human | Radiographic | Motion | ProDisc (semi-constrained) | – | Radiographic follow-up was positively correlated with the clinical outcome |
| Huang et al. [36] | Human | Radiographic | Motion, adjacent level degeneration | ProDisc (semi-constrained) | – | Correlation between motion and adjacent level degeneration |
| Kadoya et al. [37] | Ovine | Ex vivo | Static, viscoelastic and fatigue properties; histological analysis | 3D fabric disc (unconstrained) | – | Mechanical behavior similar to natural sheep disc; no debris detected |
| Kim et al. [39] | Human | Radiographic | Motion | ProDisc (semi-constrained) | – | Reduced motion at L5-S1 implanted level |
| Kosmopoulos et al. [40] | Human | Radiographic | Method for measuring motion | – | – | Patient should be parallel to the plate; the beam should be directed to the disc prosthesis |
| Kotani et al. [42] | Ovine | In vivo, ex vivo | Motion, histological analysis | 3D fabric disc (unconstrained) | 3D fabric disc with internal fixation | Biomechanical properties nearly equivalent to that of the natural disc; excellent fusion capacity |
| Kotani et al. [43] | Ovine | In vivo, ex vivo | Motion, histological analysis | 3D fabric disc (unconstrained) | 3D fabric disc with internal fixation | Reduced motion, excellent fusion |
| Kotani et al. [44] | Human | Ex vivo | Motion, IAR | 3D fabric disc (unconstrained) | Intact, instrumented fusion | Motion and IAR equivalent to that of the intact spine |
| Le Huec et al. [50] | Human | Radiographic | Sagittal alignment | Maverick (semi-constrained) | – | Preserved global and segmental lordosis, decreased lordosis at the above level |
| Leivseth et al. [55] | Human | Radiographic | Motion | ProDisc (semi-constrained) | – | Not preserved normal segmental rotational motion in the sagittal plane |
| Lemaire et al. [56] | Human | Radiographic | Motion | Charité (unconstrained) | – | Preserved motion in flexion-extension and axial rotation |
| Lim et al. [58] | Human | Radiographic | Method for measuring motion | – | – | Using the keels instead of the endplates for measuring the Cobb angles is recommended |
| Lim et al. [59] | Human | Radiographic | Errors in motion measures | – | – | Intraobserver variability: ±4.6 degrees; interobserver variability: ±5.2 degrees |
| McAfee et al. [61] | Human | Radiographic | Motion | Charité (unconstrained) | – | High accuracy during surgery induces better motion |
| McAfee et al. [62] | Human | Radiographic | Motion | Charité (unconstrained) | – | 93.4% patients had motion over 7 degrees |
| Neal et al. [67] | Human | MRI | Motion, motion at the adjacent levels | – | – | Despite artifacts, MRI is a valuable tool for evaluating disc degeneration at the adjacent levels |
| Noailly et al. [68] | Human | Computational | Motion, stiffness | Novel composite disc | – | Higher stiffness than the intact disc |
| O’Leary et al. [69] | Human | Ex vivo | Motion | Charité (unconstrained) | Fusion + Charité | Preserved motion, not preserved motion patterns |
| Panjabi et al. [72] | Human | Ex vivo | Motion | Charité (unconstrained) | Fusion | Preserved motion at implanted and adjacent levels (TDA); affected motion redistribution at adjacent levels (fusion) |
| Panjabi et al. [71] | Human | Ex vivo | Motion | ProDisc (semi-constrained) | Fusion | Preserved motion (TDA) as compared to fusion at all spinal levels |
| Putzier et al. [74] | Human | Radiographic | Motion | Charité (unconstrained) | – | Spontaneous ankylosis in 60% patients after 17 years |
| Rohlmann et al. [77] | Human | Computational | Motion | ProDisc (semi-constrained) | – | TDA height and position, ALL and AF removing affect the segment biomechanics |
| Rousseau et al. [78] | Human | Computational | Motion, IAR, facet forces | Charité (unconstrained) ProDisc (semi-constrained) | – | ProDisc: decreased facet forces, IAR variable; Charité: increased facet forces, IAR less variable |
| SariAli et al. [79] | Human | Computational | Motion | Charité (unconstrained) | Healthy volunteers | Single level TDA: preserved motion and coupling; double level TDA: not preserved motion and coupling in 50% |
| Sasso et al. [80] | Human | Radiographic | Motion | FlexiCore (constrained) | Fusion | Preserved motion in flexion-extension and lateral bending |
| Tortolani et al. [87] | Human | Radiographic | Heterotopic ossification | Charité (unconstrained) | – | Heterotopic ossification in 4.3% patients; all these patients had motion increase after surgery |
| Tournier et al. [88] | Human | Radiographic | Motion, disc height, sagittal balance | Charité (unconstrained) Maverick, ProDisc (semi-constrained) | – | Preserved motion and disc height, preserved sagittal balance, modification of the lumbar curvature |
| Vuono-Hawkins et al. [92] | Canine | In vivo, ex vivo | Motion, bone ingrowth | Elastomeric spacer | – | Increased motion, no significant bone ingrowth after 12 months |
| Zigler et al. [98] | Human | Radiographic | Motion | ProDisc (semi-constrained) | – | Preserved motion (average 7.7 degrees) |
IAR instantaneous axis of rotation, TDA total disc arthroplasty, MRI magnetic resonance imaging, ALL anterior longitudinal ligament, AF annulus fibrosus
Range of motion (ROM)
In many studies, ROM restoration after lumbar disc arthroplasty was investigated by using radiographic measurements, laboratory experiments and computational models. The various disc prostheses were shown to preserve generally the mobility at a nearly physiological level, for both semi-constrained and unconstrained designs (Table 2). However, some dissenting findings are documented in the literature, in particular with reference to the unconstrained Charité disc prosthesis. Goel et al. [28] developed a finite element model of the lumbar spine including the Charité prosthesis at the L5-S1 level. The results showed an increase in ROM at the implanted level in flexion (18.9%) and in extension (43.4%), with consequent decrease in ROM at the adjacent levels. O’Leary et al. [69] conducted an experimental study on the Charité disc prosthesis implanted at the L5-S1 level, and reported an average ROM increase of 5.6 degrees in flexion-extension. However, computer simulations and in vitro experiments can only approximate the complex physiological loading of the human spine, especially compressive forces due to muscle activity. On the contrary, McAfee et al. [61], in a radiographic study on the same prosthesis, found a reduction in the flexion-extension ROM in 82.5% of the operated patients 24 months after the surgery, accompanied with the good clinical results. Nonetheless, the clinical outcome should be considered the principal functional measure.
Leivseth et al. [55] observed ROM values between 27% (L5-S1 level) and 64% (L2-L3) of the normal ROM, in 41 patients 24 months after implantation of the semi-constrained ProDisc. The authors postulated that the main cause of this phenomenon would be the soft tissue adaptation during the preoperative period. These changes, induced by restricted spine motion due to symptomatic periods of low back pain, are considered to be irreversible. This assertion appeared to be confirmed by the observed ROM decrease also at the adjacent levels.
Discussion
Most papers state that both semi-constrained and unconstrained disc prostheses are able to restore correct spinal kinematics, in terms of IAR and ROM. However, some dissenting findings have been reported, thus indicating that some aspects, namely the possible increase or reduction of the ROM, still need to be clarified. The reported difference between the geometrical center and the IAR location of semi-constrained disc prostheses need to be verified, in particular with reference to the possible measurement error in the estimation of the IAR position. Both ROM increase and decrease were reported in different papers; a clinical investigation about the actual incidence of these alterations in vivo is required. At present, we cannot state that the monitored ROM alterations are predictors for the clinical outcome.
Spinal alignment
Segmental lordosis is increased after lumbar TDA in most cases, for both semi-constrained [6] and unconstrained disc prostheses [31, 69]. Globally, preoperative insufficient segmental lordosis was found to be restored to a normal lordosis angle [57], while excessive segmental lordosis was observed in cases of normal lordosis in the preoperative stage [6]. However, the overall lumbar lordosis was preserved [6].
McAfee et al. [62] found iatrogenic lumbar scoliosis in an alarming number of patients subjected to lumbar TDA, with both semi-constrained and unconstrained disc prostheses. The actual incidence of this complication was not reported in the paper. The authors related this phenomenon to the rotational instability of the lumbar spine after “radical” preparation for TDA (discectomy, resection of the anterior longitudinal ligament, stretching of resection of the posterior longitudinal ligament, intervertebral distraction).
Discussion
The increase of the segmental lordosis is a rather frequent consequence of lumbar TDA, described in many papers, with possible clinical consequences. The clinical relevance of such a significant lordosis alteration needs to be evaluated in long-term follow-up studies. Up to now, no relation between the postoperative sagittal alignment and the prosthesis design has been demonstrated.
Iatrogenic lumbar scoliosis is documented in both patients with or without minor preoperative scoliosis, though only in a single case-report paper. A pre-existing minor scoliosis should be defined as an absolute contraindication for TDA; however, exclusion criteria for lumbar TDA generally admit scoliosis up to 11° Cobb angle [62]. Semi-constrained devices may help in the restoration of the rotational stiffness of the functional unit, as suggested by McAfee et al. [62].
Protection of the biological structures
Several papers addressing the estimation of loads and stresses acting in the lumbar spinal structures after TDA are currently available (Table 3). These studies are generally targeted to the investigation of the possible alteration of the forces transmitted through the facet joints and of the intradiscal stresses at the adjacent levels [76]. In fact, one of the premises of TDA is preventing the overloading of the surrounding biological structures, the main drawback of spinal fusion. However, some authors suggested that the increase in the biomechanical stresses after fusion may not have a real clinical significance [93], thus questioning the importance of motion preservation technologies in the protection of the biological structures.
Table 3.
Published studies concerning loads, stresses and sagittal balance after TDA
| References | Model | Study type | Mechanical variables | Prosthesis | Compared to | Results |
|---|---|---|---|---|---|---|
| Cakir et al. [6] | Human | Radiographic | Sagittal balance | ProDisc (semi-constrained) | – | Increased segmental lordosis, overall lordosis preserved |
| Chung et al. [9] | Human | Radiographic | Sagittal balance | ProDisc (semi-constrained) | – | Increased segmental and overall lordosis |
| Denozière and Ku [20] | Human | Computational | Motion,stability, ligament tensions, facet pressure | Ball and socket (semi-constrained) | Fusion | Greater risk of instability and further degeneration relative fusion |
| Dooris et al. [21] | Human | Computational | Motion, facet loads, intradiscal pressure, shear stresses | Ball and socket (semi-constrained) | – | Modification of the spinal bending stiffness in the sagittal plane |
| Goel et al. [28] | Human | Computational | Motion, facet loads, intradiscal pressure, shear stresses | Charité(unconstrained) | – | Increased motion at the L5-S1 level in flexion/extension; decreased facet loads; higher shear stresses at the TDA L5 endplate relative to those at S1 interface |
| Huang et al. [34] | – | – | Constraint | Semi-constrained, unconstrained | – | Unconstrained disc prostheses may have a kinematical advantage; semi-constrained disc prostheses may protect the posterior structure in shear |
| Kadoya et al. [37] | Ovine | Experimental | Static, viscoelastic and fatigue properties; histological analysis | 3D fabric disc (unconstrained) | – | Mechanical behavior similar to natural sheep disc; no debris detected |
| Ledet et al. [53] | Non-human primate | In vivo | In vivo interbody force | Instrumented interbody spacer | – | The baboon may be an appropriate animal model of the human lumbar spine |
| Le Huec et al. [52] | Human | Radiographic | Sagittal balance | Maverick (semi-constrained) | – | Preserved sagittal balance |
| Le Huec et al. [51] | Human | Radiographic | Sagittal balance, facet loads | Maverick (semi-constrained) | – | Facet loads may be not increased after implantation of a semi-constrained disc prosthesis |
| Lemaire et al. [56] | Human | Radiographic | Sagittal balance | Charité (unconstrained) | – | 87% patients had a restoration of lumbar sagittal balance |
| Mathew et al. [60] | Human | – | – | ProDisc (semi-constrained) | – | Complication: bilateral pedicle fracture, probably related to the lordosis angle distribution of the prosthesis design |
| McAfee et al. [62] | Human | Ex vivo | Motion | Charité (unconstrained) | – | TDA accentuates scoliotic tendencies in the lumbar spine |
| Moumene and Geisler [66] | Human | Computational | Loading on the facet joints, stress on the polyethylene core | Unconstrained, semi-constrained | – | Unconstrained TDA unloads facet joints and presents decreased core stress as compared to fixed-core (semi-constrained) TDA |
| Rohlmann et al. [77] | Human | Computational | Alignment in standing position and flexion | ProDisc (semi-constrained) | – | Implant position strongly influences intersegmental rotation in standing and flexion |
| Rousseau et al. [78] | Human | Experimental | Motion, facet forces | Charité (unconstrained) ProDisc (semi-constrained) | – | ProDisc: decreased facet forces, IAR variable; Charité: increased facet forces, IAR less variable |
| Shim et al. [84] | Human | – | – | ProDisc (semi-constrained) | – | Case report of two split fractures of the vertebral body due to the keel design |
| Tournier et al. [88] | Human | Radiographic | Motion, disc height, sagittal balance | Charité (unconstrained) Maverick, ProDisc (semi-constrained) | – | Preserved motion and disc height, preserved sagittal balance, modification of the lumbar curvature |
| Trouillier et al. [89] | Human | Radiographic | Facet joint integrity | Charité (unconstrained) | – | Implantation of the disc prosthesis was not associated to increased loading in the facet joints |
| Van Ooij et al. [91] | Human | Radiographic | Degeneration, stability | Charité (unconstrained) | – | Observed complications: degeneration of adjacent discs, facet joint arthrosis at the implanted and other levels, subsidence |
| Wenzel and Sheperd [94] | Human | Computational | Contact stresses on the articulating surfaces | Ball-and-socket | – | Stresses below the fatigue strength of the employed materials |
TDA total disc arthroplasty, IAR instantaneous axis of rotation
Some papers describing numerical models of the lumbar spine after TDA are available in the literature. An increase in the ROM at the treated level was observed in some studies [20, 21, 28]. The hypermobility was related to a major increase of the forces in the facet joints, for semi-constrained disc prosthesis [20], with pressure values exceeding the ultimate strength of the articular cartilage. A strong sensitivity of the facet forces to the anteroposterior positioning of the disc prosthesis was also observed [20, 21]. The authors suggested the preservation of as much annulus as possible during surgery (in contrast to the mentioned radical preparation leading to iatrogenic scoliosis), as a good practice rule, in order to avoid instability and non-physiological loading on facet joints and ligaments [20]. Moumene and Geisler [66] estimated the effect of lumbar semi-constrained and unconstrained (mobile-core) artificial disc design and placement on the loading of the facet joints, using a 3D non-linear finite element model of the L4-L5 motion segment. Results showed that an unconstrained artificial disc design is less sensitive to placement and unloads facet joints, as compared to a semi-constrained design. Trouillier et al. [89] observed no significant increase in subchondral bone density 6 months after implantation of the unconstrained Charité disc prosthesis, and a significant decrease in 10/13 patients. These findings might indicate a reduced loading in facet joints, if compared to the preoperative conditions. Furthermore, the unconstrained design has been found to induce only marginal changes in the intradiscal pressures at the adjacent levels [28].
Contrarily, Rousseau et al. [78] described no significant alteration of the facet loads after implantation of the semi-constrained ProDisc device, in an ex vivo study employing thin pressure sensors. However, the unconstrained Charité disc prosthesis increased the facet load, in particular in lateral bending. According to the authors, this phenomenon is due to the contralateral movement of the core, which reduces the intervertebral height at the ipsilateral side, thus closing the corresponding facet joint.
Discussion
In general, most of the reviewed papers described an increase of the facet loads, for both semi-constrained and unconstrained artificial discs, but with some contrasting results jeopardizing a clear-cut statement. Oddly, only two papers addressed the quantification of the stresses in the adjacent levels [21, 28], despite the prevention of adjacent degeneration being probably the most important aim of TDA. In order to avoid spinal instability and excessive loads in the facet joints and the ligaments McAfee et al. [62] analyzed the possibility to introduce constraints in the prosthesis design. A more constrained design should be able to share a greater portion of load, thus decreasing the loads through the facet joints and in the ligaments, possibly allowing the restoration of a correct load sharing pattern. However, further studies, which directly determine of the influence of the prosthesis design on the segmental internal stress condition are required to demonstrate the validity of this assertion.
Device stability and wear
Device wear and stability are potentially problematic in lumbar TDA as in all tribological pairings, since the stresses inside the device and at the interface between the artificial disc and the endplates may reach high values in the lumbar spine (Table 4). A correct choice of biomaterials is a crucial determinant in the prosthesis performance. Commonly used material couplings are cobalt–chrome alloy/UHMWPE (Charité, ProDisc) and cobalt-chrome alloy/cobalt-chrome alloy (Maverick, Flexicore), both acknowledged to have good wear resistance.
Table 4.
Published studies concerning biomaterials, wear and osseointegration
| References | Model | Study type | Observed variables | Prosthesis | Compared to | Results |
|---|---|---|---|---|---|---|
| Büttner-Janz et al. [5] | Human | Experimental | Static and dynamic strength | Charité (unconstrained) | – | Sufficient strength |
| Chang et al. [8] | Leporine | In vivo | Response to wear debris | Titanium particles | – | Minimal biological response |
| Cunningham et al. [14] | Non-human primate | Experimental, in vivo (histopatologic and histomorphometric) | Motion, trabecular ingrowth | Acroflex (unconstrained) | – | Reduced motion; no evidences of pathological changes; heterotopic ossification |
| Cunningham et al. [12] | Non-human primate | In vivo, ex vivo | Motion, bone ingrowth | Charité, Acroflex (unconstrained) | Intact | Acroflex motion smaller than intact and Charité; good bone ingrowth |
| Cunningham [16] | Human, non-human primate | In vivo, ex vivo (histopatologic and histomorphometric) | Motion, IAR, bone ingrowth | Charité, Acroflex (unconstrained) | Intact, fusion | Preserved motion and IAR, excellent bone ingrowth |
| David [18] | Human | Retrieval study, 1 patient | Wear | Charité (unconstrained) | – | Fractured polyethylene core, no wear debris observed |
| Edeland [22] | – | – | – | Composite | – | Proposal for material and design of a new disc prothesis |
| François et al. [25] | Human | Retrieval study, 1 patient | – | Maverick (semi-constrained) | – | Gross metallosis around the articulation of the prosthesis |
| Fraser et al. [26] | – | – | Shock absorption | Acroflex (unconstrained) | – | The design of the prosthesis is aimed to optimize the shock absorption capacity |
| Gloria et al. [27] | – | Experimental | Compressive stiffness, viscoelasticity | Composite polymer | – | Adequate static and dynamic mechanical properties |
| Hou et al. [32] | Non-human primate | In vivo, experimental | Biocompatibility, fatigue | Silicone | – | Good biomechanics, applicability and biocompatibility |
| Kadoya et al. [37] | Ovine | Experimental, in vivo | Static, viscoelastic and fatigue properties | 3D fabric disc (unconstrained) | – | Mechanical behavior similar to natural sheep disc; no debris detected |
| Kostuik [41] | – | Experimental | Wear | Spring-based | – | Low wear if compared to hip prostheses |
| Kotani et al. [43] | Ovine | In vivo, ex vivo | Motion, histological analysis | 3D fabric disc (unconstrained) | 3D fabric disc with internal fixation | Reduced motion, excellent fusion |
| Kotani et al. [44] | Ovine | Experimental | Motion, histological analysis | 3D fabric disc (unconstrained) | 3D fabric disc with internal fixation | Biomechanical properties nearly equivalent to that of the natural disc; excellent fusion capacity |
| Kurtz et al. [45] | Human | Retrieval study, 1 patient | Wear, cracks | Charité (unconstrained) | – | Cracks in the polyethylene core, damage around the periphery of the core |
| Kurtz et al. [46] | Human | Retrieval study, 21 patients | Surface damage | Charité (unconstrained) | – | Observed surface damage |
| Langrana et al. [47] | – | Computational | – | Fiber-reinforced composite | – | Pioneering model of an artificial disc, including realistic material properties |
| Langrana et al. [48] | – | Experimental | Compression and torsion stiffness | Fiber-reinforced composite | – | Adequate stiffness can be achieved with the use of a composite material |
| Lee et al. [54] | – | Experimental | Mechanical properties of the disc | Fiber-reinforced composite | – | Manufacturing and testing of composite disc prostheses |
| Le Huec et al. [49] | – | Experimental | Shock absorption capacity | ProDisc (semi-constrained), Maverick (unconstrained) | – | The two devices have identical shock and vibration transmission properties |
| Mizuno et al. [64] | Murine | In vivo | Biochemical analysis | Tissue-engineered disc | – | Morphology and histology resembled those of the native intervertebral disc |
| Moore et al. [65] | Murine | In vivo | Response to wear debris | Polyolefin rubber | – | Rubber particles induce a localized tissue response consistent to a normal foreign body reaction |
| Revell et al. [75] | Porcine | In vivo | – | Tissue engineered disc | – | Engineered disc histology similar to a native intervertebral disc |
| Schmiedberg et al. [82] | – | Experimental | Wear | Spring-based | – | Analyses of wear debris with a new method |
| Shaheen and Sheperd [83] | – | Computational | Lubrication regimes | Generic ball-and-socket | – | Metal-metal and metal-polymer couplings are likely to generate wear debris; ceramic-ceramic coupling may reduce wear |
| Takahata et al. [86] | Ovine | In vivo | Bone ingrowth | 3D fabric disc (unconstrained) | – | Excellent bone ingrowth |
| Van Ooij et al. [90] | Human | Retrieval study, 4 patients | Wear | Charité (unconstrained) | – | Wear present in all devices, with different extent and severity |
| Vuono-Hawkins et al. [92] | Canine | In vivo, ex vivo | Motion, bone ingrowth | Elastomeric spacer | – | Increased motion, no significant bone ingrowth after 12 months |
| Zeh et al. [97] | Human | Ion concentration analysis in serum | Co–Cr ion concentrations | Maverick (semi-constrained) | – | Co–Cr ion concentration significant, similar or exceeding typical concentrations after total hip arthroplasty |
IAR Instantaneous Axis of Rotation
Two in vivo studies conducted on non-human primates showed no signs of local or systemic accumulation of wear debris, no evidence of pathologic changes in tissues surrounding the disc prosthesis, and excellent osteointegration, after implantation of the Acroflex [12, 14] and the Charité [13] disc prostheses. Heterotopic ossification was observed in many specimens implanted with the Acroflex device, and was explained by the authors as due to the reduced ROM observed in flexibility testing. This disc prosthesis was later discontinued, due to a number of cases of minor defects in the polyolefin rubber core after 1 and 2 years [26, 85].
On the contrary, significant wear in lumbar disc prostheses was observed in other studies. Signs of wear were found in Charité disc prostheses retrieved from patients who underwent revision TDA surgery and conversion to fusion [46, 90], which showed surface damage as observed previously in both hip and knee replacements [46], with different extent and severity [90]. In 3/4 patients, implant wear was associated with biomechanical issues such as subsidence, migration, undersizing, and adjacent fusion [90]. Significant systemic release of cobalt and chromium ions was proven in the serum of 10 patients after implantation of the semi-constrained Maverick artificial lumbar disc [97]. The ion concentrations were similar to the values measured in total hip arthroplasty metal-on-metal, or exceeded these values.
Discussion
Generally, the published data are not exhaustive, but the problem of wear appears to be of significant importance for lumbar TDA, more than the device stability. Due to the lower stress sustained and the interface with the biological system, unconstrained designs appear to be more suitable than semi-constrained designs. However, as discussed in the previous paragraph, more constrained designs may be advantageous in terms of load sharing, protecting the surrounding biological structures from overloading. Because of the demonstrated potential for osteolysis in the spine [46], clinical problem related to wear may be of importance and need to be investigated with long term follow-up studies.
New ideas
Although most models of lumbar artificial discs can be related to the ball-and-socket design, either constrained, semi-constrained or unconstrained, some innovative ideas are currently emerging. Three new models currently under development or clinical investigation are subsequently described. The TrueDisc PL by Disc Motion Technologies (Boca Raton, FL, USA) is composed of two parts, each one mimicking a half disc, and can be implanted with a posterior access, thus reducing the invasiveness of the surgery [38]. The disc has been designed to be able to preserve the motion even if the two components are not implanted perfectly parallel. The M6 artificial cervical disc by Spinal Kinetics (Sunnyvale, CA, USA) includes a polymeric nucleus and a woven fiber annulus, thus replicating the structure and the biomechanics of a natural disc. The M6 disc is currently available for investigational use in the cervical spine; the lumbar artificial disc is under development. The Physio-L artificial lumbar disc (Nexgen Spine, Whippany, NJ, USA) has a polycarbonate polyurethane core connected to two porous titanium plates. The nucleus has been designed to closely match the mechanical properties of the healthy intervertebral disc, including the shock-absorption capability. This device is currently under clinical investigation [73]. All these models were presented at the main spine surgery conferences, but have not been documented in the currently available peer-reviewed literature.
Conclusions
Based on the present literature analysis, definitive conclusions cannot be drawn. Both semi-constrained and unconstrained lumbar disc seems to be able to restore nearly physiological IAR locations and ROM values; however, both increased and decreased ROM were observed. Segmental lordosis alterations after TDA were reported in most cases, for both constrained and unconstrained disc prostheses. An increase in the load through the facet joints was documented, for both semi-constrained and unconstrained artificial discs, but with some contrasting results. Semi-constrained devices may be able to share a greater part of the load, but may be more subjected to wear. All these findings need to be supported by long term clinical studies.
References
- 1.Auerbach JD, Wills BP, McIntosh TC, Balderston RA. Evaluation of spinal kinematics following lumbar total disc replacement and circumferential fusion using in vivo fluoroscopy. Spine. 2007;32(5):527–536. doi: 10.1097/01.brs.0000256915.90236.17. [DOI] [PubMed] [Google Scholar]
- 2.Bertagnoli R, Kumar S. Indications for full prosthetic disc arthroplasty: a correlation of clinical outcome against a variety of indications. Eur Spine J. 2002;11(Suppl 2):S131–S136. doi: 10.1007/s00586-002-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertagnoli R, Yue JJ, Kershaw T, Shah RV, Pfeiffer F, Fenk-Mayer A, et al. Lumbar total disc arthroplasty utilizing the ProDisc prosthesis in smokers versus nonsmokers: a prospective study with 2-year minimum follow-up. Spine. 2006;31(9):992–997. doi: 10.1097/01.brs.0000214970.07626.68. [DOI] [PubMed] [Google Scholar]
- 4.Bertagnoli R, Yue JJ, Shah RV, Nanieva R, Pfeiffer F, Fenk-Mayer A, et al. The treatment of disabling single-level lumbar discogenic low back pain with total disc arthroplasty utilizing the Prodisc prosthesis: a prospective study with 2-year minimum follow-up. Spine. 2005;30(19):2230–2236. doi: 10.1097/01.brs.0000182217.87660.40. [DOI] [PubMed] [Google Scholar]
- 5.Büttner-Janz K, Schellnack K, Zippel H. Biomechanics of the SB Charité lumbar intervertebral disc endoprosthesis. Int Orthop. 1989;13(3):173–176. doi: 10.1007/BF00268042. [DOI] [PubMed] [Google Scholar]
- 6.Cakir B, Richter M, Kafer W, Puhl W, Schmidt R. The impact of total lumbar disc replacement on segmental and total lumbar lordosis. Clin Biomech (Bristol, Avon) 2005;20(4):357–364. doi: 10.1016/j.clinbiomech.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Cakir B, Richter M, Puhl W, Schmidt R. Reliability of motion measurements after total disc replacement: the spike and the fin method. Eur Spine J. 2006;15(2):165–173. doi: 10.1007/s00586-005-0942-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang BS, Brown PR, Sieber A, Valdevit A, Tateno K, Kostuik JP. Evaluation of the biological response of wear debris. Spine J. 2004;4(6(Suppl)):239S–244S. doi: 10.1016/j.spinee.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Chung SS, Lee CS, Kang CS, Kim SH. The effect of lumbar total disc replacement on the spinopelvic alignment and range of motion of the lumbar spine. J Spinal Disord Tech. 2006;19(5):307–311. doi: 10.1097/01.bsd.0000208255.14329.1e. [DOI] [PubMed] [Google Scholar]
- 10.Chung SS, Lee CS, Kang CS. Lumbar total disc replacement using ProDisc II: a prospective study with a 2-year minimum follow-up. J Spinal Disord Tech. 2006;19(6):411–415. doi: 10.1097/00024720-200608000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Cinotti G, David T, Postacchini F. Results of disc prosthesis after a minimum follow-up period of 2 years. Spine. 1996;21(8):995–1000. doi: 10.1097/00007632-199604150-00015. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham BW, Dmitriev AE, Hu N, McAfee PC. General principles of total disc replacement arthroplasty: seventeen cases in a nonhuman primate model. Spine. 2003;28(20):S118–S124. doi: 10.1097/00007632-200310151-00005. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham BW, Gordon JD, Dmitriev AE, Hu N, McAfee PC. Biomechanical evaluation of total disc replacement arthroplasty: an in vitro human cadaveric model. Spine. 2003;28(20):S110–S117. doi: 10.1097/01.BRS.0000092209.27573.90. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham BW, Lowery GL, Serhan HA, Dmitriev AE, Orbegoso CM, McAfee PC, et al. Total disc replacement arthroplasty using the AcroFlex lumbar disc: a non-human primate model. Eur Spine J. 2002;11(Suppl 2):S115–S123. doi: 10.1007/s00586-002-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham BW, McAfee PC, Geisler FH, Holsapple G, Adams K, Blumenthal SL, et al. Distribution of in vivo and in vitro range of motion following 1-level arthroplasty with the CHARITE artificial disc compared with fusion. J Neurosurg Spine. 2008;8(1):7–12. doi: 10.3171/SPI-08/01/007. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham BW (2004) Basic scientific considerations in total disc arthroplasty. Spine 4(6)(Suppl):219S–230S (Review). doi:10.1016/j.spinee.2004.07.015 [DOI] [PubMed]
- 17.David T. Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the CHARITE artificial disc in 106 patients. Spine. 2007;32(6):661–666. doi: 10.1097/01.brs.0000257554.67505.45. [DOI] [PubMed] [Google Scholar]
- 18.David T. Revision of a Charité artificial disc 9.5 years in vivo to a new Charité artificial disc: case report and explant analysis. Eur Spine J. 2005;14(5):507–511. doi: 10.1007/s00586-004-0842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delamarter RB, Fribourg DM, Kanim LE, Bae H. ProDisc artificial total lumbar disc replacement: introduction and early results from the United States clinical trial. Spine. 2003;28(20):S167–S175. doi: 10.1097/01.BRS.0000092220.66650.2B. [DOI] [PubMed] [Google Scholar]
- 20.Denoziere G, Ku DN. Biomechanical comparison between fusion of two vertebrae and implantation of an artificial intervertebral disc. J Biomech. 2006;39(4):766–775. doi: 10.1016/j.jbiomech.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Dooris AP, Goel VK, Grosland NM, Gilbertson LG, Wilder DG. Load-sharing between anterior and posterior elements in a lumbar motion segment implanted with an artificial disc. Spine. 2001;26(6):E122–E129. doi: 10.1097/00007632-200103150-00004. [DOI] [PubMed] [Google Scholar]
- 22.Edeland HG. Suggestions for a total elasto-dynamic intervertebral disc prosthesis. Biomater Med Devices Artif Organs. 1981;9(1):65–72. doi: 10.3109/10731198109117602. [DOI] [PubMed] [Google Scholar]
- 23.Eijkelkamp MF, Donkelaar CC, Veldhuizen AG, Horn JR, Huyghe JM, Verkerke GJ. Requirements for an artificial intervertebral disc. Int J Artif Organs. 2001;24(5):311–321. [PubMed] [Google Scholar]
- 24.Enker P, Steffee A, Mcmillin C, Keppler L, Biscup R, Miller S. Artificial disc replacement. Preliminary report with a 3-year minimum follow-up. Spine. 1993;18(8):1061–1070. doi: 10.1097/00007632-199306150-00017. [DOI] [PubMed] [Google Scholar]
- 25.François J, Coessens R, Lauweryns P. Early removal of a Maverick disc prosthesis: surgical findings and morphological changes. Acta Orthop Belg. 2007;73(1):122–127. [PubMed] [Google Scholar]
- 26.Fraser RD, Ross ER, Lowery GL, Freeman BJ, Dolan M (2004) AcroFlex design and results. Spine J 4(6)(Suppl):245S–251S (Review). doi:10.1016/j.spinee.2004.07.020 [DOI] [PubMed]
- 27.Gloria A, Causa F, Santis R, Netti PA, Ambrosio L. Dynamic-mechanical properties of a novel composite intervertebral disc prosthesis. J Mater Sci: Mater Med. 2007;18(11):2159–2165. doi: 10.1007/s10856-007-3003-z. [DOI] [PubMed] [Google Scholar]
- 28.Goel VK, Grauer JN, Patel TC, Biyani A, Sairyo K, Vishnubhotla S, et al. Effects of Charité artificial disc on the implanted and adjacent spinal segments mechanics using a hybrid testing protocol. Spine. 2005;30(24):2755–2764. doi: 10.1097/01.brs.0000195897.17277.67. [DOI] [PubMed] [Google Scholar]
- 29.Hedman TP, Kostuik JP, Fernie GR, Hellier WG. Design of an intervertebral disc prosthesis. Spine. 1991;16(6(suppl)):S256–S260. doi: 10.1097/00007632-199106001-00016. [DOI] [PubMed] [Google Scholar]
- 30.Hitchon PW, Eichholz K, Barry C, Rubenbauer P, Ingalhalikar A, Nakamura S, et al. Biomechanical studies of an artificial disc implant in the human cadaveric spine. J Neurosurg Spine. 2005;2(3):339–343. doi: 10.3171/spi.2005.2.3.0339. [DOI] [PubMed] [Google Scholar]
- 31.Hopf C, Heeckt H, Beske C. Disc replacement with the SB Charite endoprosthesis—experience, preliminary results and comments after 35 prospectively performed operations. Z Orthop Ihre Grenzgeb. 2002;140(5):485–491. doi: 10.1055/s-2002-34000. [DOI] [PubMed] [Google Scholar]
- 32.Hou TS, Tu KY, Xu YK, Li ZB, Cai AH, Wang HC. Lumbar intervertebral disc prosthesis. An experimental study. Chin Med J (Engl) 1991;104(5):381–386. [PubMed] [Google Scholar]
- 33.Huang RC, Girardi FP, Cammisa FP, Jr, Lim MR, Tropiano P, Marnay T. Correlation between range of motion and outcome after lumbar total disc replacement: 8.6-year follow-up. Spine. 2005;30(12):1407–1411. doi: 10.1097/01.brs.0000166528.67425.0e. [DOI] [PubMed] [Google Scholar]
- 34.Huang RC, Girardi FP, Cammisa FP, Jr, Wright TM. The implications of constraint in lumbar total disc replacement. J Spinal Disord Tech. 2003;16(4):412–417. doi: 10.1097/00024720-200308000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Huang RC, Girardi FP, Cammisa FP, Jr, Tropiano P, Marnay T. Long-term flexion-extension range of motion of the prodisc total disc replacement. J Spinal Disord Tech. 2003;16(5):435–440. doi: 10.1097/00024720-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Huang RC, Tropiano P, Marnay T, Girardi FP, Lim MR, Cammisa FP., Jr Range of motion and adjacent level degeneration after lumbar total disc replacement. Spine J. 2006;6(3):242–247. doi: 10.1016/j.spinee.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Kadoya K, Kotani Y, Abumi K, Takada T, Shimamoto N, Shikinami Y, et al. Biomechanical and morphologic evaluation of a three-dimensional fabric sheep artificial intervertebral disc: in vitro and in vivo analysis. Spine. 2001;26(14):1562–1569. doi: 10.1097/00007632-200107150-00012. [DOI] [PubMed] [Google Scholar]
- 38.Kiapour A, Goel VK, Faizan A, Krish M, Friesem T (2007). Lumbar spine kinematics following posterior total disc and artificial facet replacement. SAS 2007, Berlin. (Abstract)
- 39.Kim DH, Ryu KS, Kim MK, Park CK. Factors influencing segmental range of motion after lumbar total disc replacement using the ProDisc II prosthesis. J Neurosurg Spine. 2007;7(2):131–138. doi: 10.3171/SPI-07/08/131. [DOI] [PubMed] [Google Scholar]
- 40.Kosmopoulos V, McManus J, Schizas C. Consequences of patient position in the radiographic measurement of artificial disc replacement angles. Eur Spine J. 2008;17(1):30–35. doi: 10.1007/s00586-007-0486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kostuik JP. Intervertebral disc replacement. Experimental study. Clin Orthop Relat Res. 1997;337:27–41. doi: 10.1097/00003086-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Kotani Y, Abumi K, Shikinami Y, Takada T, Kadoya K, Shimamoto N, et al. Artificial intervertebral disc replacement using bioactive three-dimensional fabric: design, development, and preliminary animal study. Spine. 2002;27(9):929–935. doi: 10.1097/00007632-200205010-00008. [DOI] [PubMed] [Google Scholar]
- 43.Kotani Y, Abumi K, Shikinami Y, Takahata M, Kadoya K, Kadosawa T, Minami A, Kaneda K. Two-year observation of artificial intervertebral disc replacement: results after supplemental ultra-high strength bioresorbable spinal stabilization. J Neurosurg. 2004;100((4 Suppl Spine)):337–342. doi: 10.3171/spi.2004.100.4.0337. [DOI] [PubMed] [Google Scholar]
- 44.Kotani Y, Cunningham BW, Abumi K, Dmitriev AE, Hu N, Ito M, et al. Multidirectional flexibility analysis of anterior and posterior lumbar artificial disc reconstruction: in vitro human cadaveric spine model. Eur Spine J. 2006;15(10):1511–1520. doi: 10.1007/s00586-006-0086-z. [DOI] [PubMed] [Google Scholar]
- 45.Kurtz SM, Peloza J, Siskey R, Villarraga ML. Analysis of a retrieved polyethylene total disc replacement component. Spine J. 2005;5(3):344–350. doi: 10.1016/j.spinee.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Kurtz SM, Ooij A, Ross R, Malefijt J, Peloza J, Ciccarelli L, et al. Polyethylene wear and rim fracture in total disc arthroplasty. Spine J. 2007;7(1):12–21. doi: 10.1016/j.spinee.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Langrana NA, Lee CK, Yang SW. Finite-element modeling of the synthetic intervertebral disc. Spine. 1991;16(6(suppl)):S245–S252. doi: 10.1097/00007632-199106001-00014. [DOI] [PubMed] [Google Scholar]
- 48.Langrana NA, Parsons JR, Lee CK, Vuono-Hawkins M, Yang SW, Alexander H. Materials and design concepts for an intervertebral disc spacer. I. fiber-reinforced composite design. J Appl Biomater. 1994;5(2):125–132. doi: 10.1002/jab.770050205. [DOI] [PubMed] [Google Scholar]
- 49.Le Huec JC, Kiaer T, Friesem T, Mathews H, Liu M, Eisermann L. Shock absorption in lumbar disc prosthesis: a preliminary mechanical study. J Spinal Disord Tech. 2003;16(4):346–351. doi: 10.1097/00024720-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Le Huec J, Basso Y, Mathews H, Mehbod A, Aunoble S, Friesem T, et al. The effect of single-level, total disc arthroplasty on sagittal balance parameters: a prospective study. Eur Spine J. 2005;14(5):480–486. doi: 10.1007/s00586-004-0843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Huec JC, Basso Y, Aunoble S, Friesem T, Bruno MB. Influence of facet and posterior muscle degeneration on clinical results of lumbar total disc replacement: two-year follow-up. J Spinal Disord Tech. 2005;18(3):219–223. [PubMed] [Google Scholar]
- 52.Le Huec JC, Mathews H, Basso Y, Aunoble S, Hoste D, Bley B, et al. Clinical results of Maverick lumbar total disc replacement: two-year prospective follow-up. Orthop Clin North Am. 2005;36(3):315–322. doi: 10.1016/j.ocl.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Ledet EH, Tymeson MP, DiRisio DJ, Cohen B, Uhl RL. Direct real-time measurement of in vivo forces in the lumbar spine. Spine J. 2005;5(1):85–94. doi: 10.1016/j.spinee.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 54.Lee CK, Goel VK (2004) Artificial disc prosthesis: design concepts and criteria. Spine J 4(6)(Suppl):209S–218S (Review). doi:10.1016/j.spinee.2004.07.011 [DOI] [PubMed]
- 55.Leivseth G, Braaten S, Frobin W, Brinckmann P. Mobility of lumbar segments instrumented with a ProDisc II prosthesis: a two-year follow-up study. Spine. 2006;31(15):1726–1733. doi: 10.1097/01.brs.0000224213.45330.68. [DOI] [PubMed] [Google Scholar]
- 56.Lemaire JP, Carrier H, Sariali el H, Skalli W, Lavaste F. Clinical and radiological outcomes with the Charite artificial disc: a 10-year minimum follow-up. J Spinal Disord Tech. 2005;18(4):353–359. doi: 10.1097/01.bsd.0000172361.07479.6b. [DOI] [PubMed] [Google Scholar]
- 57.Lemaire JP, Skalli W, Lavaste F, Templier A, Mendes F, Diop A, et al. (1997) Intervertebral disc prosthesis. Results and prospects for the year 2000. Clin Orthop Relat Res (337):64–76 (Review). doi:10.1097/00003086-199704000-00009 [DOI] [PubMed]
- 58.Lim MR, Girardi FP, Zhang K, Huang RC, Peterson MG, Cammisa FP., Jr Measurement of total disc replacement radiographic range of motion: a comparison of two techniques. J Spinal Disord Tech. 2005;18(3):252–256. [PubMed] [Google Scholar]
- 59.Lim MR, Loder RT, Huang RC, Lyman S, Zhang K, Sama A, et al. Measurement error of lumbar total disc replacement range of motion. Spine. 2006;31(10):E291–E297. doi: 10.1097/01.brs.0000216452.54421.ea. [DOI] [PubMed] [Google Scholar]
- 60.Mathew P, Blackman M, Redla S, Hussein AA. Bilateral pedicle fractures following anterior dislocation of the polyethylene inlay of a ProDisc artificial disc replacement: a case report of an unusual complication. Spine. 2005;30(11):E311–E314. doi: 10.1097/01.brs.0000164135.03844.b6. [DOI] [PubMed] [Google Scholar]
- 61.McAfee PC, Cunningham B, Holsapple G, Adams K, Blumenthal S, Guyer RD et al (2005) A prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part II: evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine 30(14):1576–1583. doi:10.1097/01.brs.0000170561.25636.1c [DOI] [PubMed]
- 62.McAfee PC, Cunningham BW, Hayes V, Sidiqi F, Dabbah M, Sefter JC, et al. Biomechanical analysis of rotational motions after disc arthroplasty implications for patients with adult deformities. Spine. 2006;31(19(suppl)):S152–S160. doi: 10.1097/01.brs.0000234782.89031.03. [DOI] [PubMed] [Google Scholar]
- 63.McAfee PC, Geisler FH, Saiedy SS, Moore SV, Regan JJ, Guyer RD, et al. Revisability of the CHARITE artificial disc replacement: analysis of 688 patients enrolled in the U.S. IDE study of the CHARITE Artificial Disc. Spine. 2006;31(11):1217–1226. doi: 10.1097/01.brs.0000217689.08487.a8. [DOI] [PubMed] [Google Scholar]
- 64.Mizuno H, Roy AK, Vacanti CA, Kojima K, Ueda M, Bonassar LJ. Tissue-engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine. 2004;29(12):1290–1297. doi: 10.1097/01.BRS.0000128264.46510.27. [DOI] [PubMed] [Google Scholar]
- 65.Moore RJ, Fraser RD, Vernon-Roberts B, Finnie JW, Blumbergs PC, Haynes DR, et al. The biologic response to particles from a lumbar disc prosthesis. Spine. 2002;27(19):2088–2094. doi: 10.1097/00007632-200210010-00003. [DOI] [PubMed] [Google Scholar]
- 66.Moumene M, Geisler FH. Comparison of biomechanical function at ideal and varied surgical placement for two lumbar artificial disc implant designs: mobile-core versus fixed-core. Spine. 2007;32(17):1840–1851. doi: 10.1097/BRS.0b013e31811ec29c. [DOI] [PubMed] [Google Scholar]
- 67.Neal CJ, Rosner MK, Kuklo TR. Magnetic resonance imaging evaluation of adjacent segments after disc arthroplasty. J Neurosurg Spine. 2005;3(5):342–347. doi: 10.3171/spi.2005.3.5.0342. [DOI] [PubMed] [Google Scholar]
- 68.Noailly J, Lacroix D, Planell JA. Finite element study of a novel intervertebral disc substitute. Spine. 2005;30(20):2257–2264. doi: 10.1097/01.brs.0000182319.81795.72. [DOI] [PubMed] [Google Scholar]
- 69.O’Leary P, Nicolakis M, Lorenz MA, Voronov LI, Zindrick MR, Ghanayem A, et al. Response of Charite total disc replacement under physiologic loads: prosthesis component motion patterns. Spine J. 2005;5(6):590–599. doi: 10.1016/j.spinee.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 70.Ogston NG, King GJ, Gertzbein SD, Tile M, Kapasouri A, Rubenstein JD. Centrode patterns in the lumbar spine. Baseline studies in normal subjects. Spine. 1986;11(6):591–595. doi: 10.1097/00007632-198607000-00010. [DOI] [PubMed] [Google Scholar]
- 71.Panjabi M, Henderson G, Abjornson C, Yue J. Multidirectional testing of one- and two-level ProDisc-L versus simulated fusions. Spine. 2007;32(12):1311–1319. doi: 10.1097/BRS.0b013e318059af6f. [DOI] [PubMed] [Google Scholar]
- 72.Panjabi M, Malcolmson G, Teng E, Tominaga Y, Henderson G, Serhan H. Hybrid testing of lumbar CHARITE discs versus fusions. Spine. 2007;32(9):959–966. doi: 10.1097/01.brs.0000260792.13893.88. [DOI] [PubMed] [Google Scholar]
- 73.Pimenta L, Pesantez CA, Lhamby J, Oliveira L, Schaffa T, Coutinho E (2008). Short term clinical results of an elastomeric lumbar disc prosthesis (Physio-L). Eurospine 2008, Geneva (Abstract)
- 74.Putzier M, Funk JF, Schneider SV, Gross C, Tohtz SW, Khodadadyan-Klostermann C, et al. Charité total disc replacement—clinical and radiographical results after an average follow-up of 17 years. Eur Spine J. 2006;15(2):183–195. doi: 10.1007/s00586-005-1022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Revell PA, Damien E, Di Silvio L, Gurav N, Longinotti C, Ambrosio L. Tissue engineered intervertebral disc repair in the pig using injectable polymers. J Mater Sci: Mater Med. 2007;18(2):303–308. doi: 10.1007/s10856-006-0693-6. [DOI] [PubMed] [Google Scholar]
- 76.Rohlmann A, Claes L, Bergmann G, Graichen F, Neef P, Wilke HJ. Comparison of intradiscal pressures and spinal fixator loads for different body positions and exercises. Ergonomics. 2001;44(8):781–794. doi: 10.1080/00140130110047657. [DOI] [PubMed] [Google Scholar]
- 77.Rohlmann A, Zander T, Bergmann G. Effect of total disc replacement with ProDisc on intersegmental rotation of the lumbar spine. Spine. 2005;30(7):738–743. doi: 10.1097/01.brs.0000157413.72276.c4. [DOI] [PubMed] [Google Scholar]
- 78.Rousseau MA, Bradford DS, Bertagnoli R, Hu SS, Lotz JC. Disc arthroplasty design influences intervertebral kinematics and facet forces. Spine J. 2006;6(3):258–266. doi: 10.1016/j.spinee.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 79.SariAli el H, Lemaire JP, Pascal-Mousselard H, Carrier H, Skalli W. In vivo study of the kinematics in axial rotation of the lumbar spine after total intervertebral disc replacement: long-term results: a 10–14 years follow up evaluation. Eur Spine J. 2006;15(10):1501–1510. doi: 10.1007/s00586-005-0016-5. [DOI] [PubMed] [Google Scholar]
- 80.Sasso RC, Foulk DM, Hahn M. Prospective, randomized trial of metal-on-metal artificial lumbar disc replacement: initial results for treatment of discogenic pain. Spine. 2008;33(2):123–131. doi: 10.1097/BRS.0b013e31816043af. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt H, Heuer F, Claes L, Wilke HJ. The relation between the instantaneous center of rotation and facet joint forces - A finite element analysis. Clin Biomech (Bristol, Avon) 2008;23(3):270–278. doi: 10.1016/j.clinbiomech.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 82.Schmiedberg SK, Chang DH, Frondoza CG, Valdevit AD, Kostuik JP. Isolation and characterization of metallic wear debris from a dynamic intervertebral disc prosthesis. J Biomed Mater Res. 1994;28(11):1277–1288. doi: 10.1002/jbm.820281105. [DOI] [PubMed] [Google Scholar]
- 83.Shaheen A, Shepherd DE. Lubrication regimes in lumbar total disc arthroplasty. Proc Inst Mech Eng [H] 2007;221(6):621–627. doi: 10.1243/09544119JEIM204. [DOI] [PubMed] [Google Scholar]
- 84.Shim CS, Lee S, Maeng DH, Lee SH. Vertical split fracture of the vertebral body following total disc replacement using ProDisc: report of two cases. J Spinal Disord Tech. 2005;18(5):465–469. doi: 10.1097/01.bsd.0000159035.35365.df. [DOI] [PubMed] [Google Scholar]
- 85.Szpalski M, Gunzburg R, Mayer M. Spine arthroplasty: a historical review. Eur Spine J. 2002;11(Suppl 2):S65–S84. doi: 10.1007/s00586-002-0474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takahata M, Kotani Y, Abumi K, Shikinami Y, Kadosawa T, Kaneda K, et al. Bone ingrowth fixation of artificial intervertebral disc consisting of bioceramic-coated three-dimensional fabric. Spine. 2003;28(7):637–644. doi: 10.1097/00007632-200304010-00003. [DOI] [PubMed] [Google Scholar]
- 87.Tortolani PJ, Cunningham BW, Eng M, McAfee PC, Holsapple GA, Adams KA. Prevalence of heterotopic ossification following total disc replacement. A prospective, randomized study of two hundred and seventy-six patients. J Bone Joint Surg Am. 2007;89(1):82–88. doi: 10.2106/JBJS.F.00432. [DOI] [PubMed] [Google Scholar]
- 88.Tournier C, Aunoble S, Le Huec JC, Lemaire JP, Tropiano P, Lafage V, et al. Total disc arthroplasty: consequences for sagittal balance and lumbar spine movement. Eur Spine J. 2007;16(3):411–421. doi: 10.1007/s00586-006-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trouillier H, Kern P, Refior HJ, Muller-Gerbl M. A prospective morphological study of facet joint integrity following intervertebral disc replacement with the CHARITE Artificial Disc. Eur Spine J. 2006;15(2):174–182. doi: 10.1007/s00586-005-1010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ooij A, Kurtz SM, Stessels F, Noten H, Rhijn L. Polyethylene wear debris and long-term clinical failure of the Charite disc prosthesis: a study of 4 patients. Spine. 2007;32(2):223–229. doi: 10.1097/01.brs.0000251370.56327.c6. [DOI] [PubMed] [Google Scholar]
- 91.Ooij A, Oner FC, Verbout AJ. Complications of artificial disc replacement: a report of 27 patients with the SB Charite disc. J Spinal Disord Tech. 2003;16(4):369–383. doi: 10.1097/00024720-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 92.Vuono-Hawkins M, Zimmerman MC, Lee CK, Carter FM, Parsons JR, Langrana NA. Mechanical evaluation of a canine intervertebral disc spacer: in situ and in vivo studies. J Orthop Res. 1994;12(1):119–127. doi: 10.1002/jor.1100120115. [DOI] [PubMed] [Google Scholar]
- 93.Wai EK, Santos ER, Morcom RA, Fraser RD. Magnetic resonance imaging 20 years after anterior lumbar interbody fusion. Spine. 2006;31(17):1952–1956. doi: 10.1097/01.brs.0000228849.37321.a8. [DOI] [PubMed] [Google Scholar]
- 94.Wenzel SA, Shepherd DE. Contact stresses in lumbar total disc arthroplasty. Biomed Mater Eng. 2007;17(3):169–173. [PubMed] [Google Scholar]
- 95.White AA, Panjabi MM (1990). Physical properties and functional biomechanics of the spine. In: Clinical biomechanics of the spine 2nd edn, J.B. Lippincott Company, Philadelphia
- 96.Zeegers WS, Bohnen LM, Laaper M, Verhaegen MJ. Artificial disc replacement with the modular type SB Charite III: 2-year results in 50 prospectively studied patients. Eur Spine J. 1999;8(3):210–217. doi: 10.1007/s005860050160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zeh A, Planert M, Siegert G, Lattke P, Held A, Hein W. Release of cobalt and chromium ions into the serum following implantation of the metal-on-metal Maverick-type artificial lumbar disc (Medtronic Sofamor Danek) Spine. 2007;32(3):348–352. doi: 10.1097/01.brs.0000253599.89694.c0. [DOI] [PubMed] [Google Scholar]
- 98.Zigler J, Delamarter R, Spivak JM, Linovitz RJ, Danielson GO, 3rd, Haider TT, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine. 2007;32(11):1155–1162. doi: 10.1097/BRS.0b013e318054e377. [DOI] [PubMed] [Google Scholar]