Abstract
Reduced reproduction extends lifespan in many experimental animals, but the mechanism by which this occurs is unclear. The disposable soma hypothesis suggests that when reproduction is reduced, more nutrients are allocated to the soma and lifespan is extended. Alternatively, the reproductive tissues or the process of reproduction may have a direct (i.e., non-nutritional) negative effect on lifespan. We used ovariectomized grasshoppers to examine the effects of reduced reproduction throughout the lifespan at the physiological level. We focused on protein, the limiting nutrient for egg production. Ovariectomized females lived significantly longer than sham females. Because both groups ingested similar amounts, the effect was independent of dietary restriction. Despite this, ovariectomized females gained less body mass than sham females. Ovariectomized grasshoppers produced the egg yolk-precursor protein vitellogenin. At the time sham females laid their first clutch, cumulative reproductive protein was similar in ovariectomized and sham females. By advanced ages, however, ovariectomized females had produced about 5-fold less cumulative reproductive protein than sham females. In contrast, old ovariectomized females had at least 2-fold more hemolymph storage protein. These results are consistent with ovariectomy extending lifespan in part via enhanced protein allocation to storage at the expense of reproduction.
Keywords: resource allocation, reduced reproduction, vitellogenin, hexamerin, storage protein, lubber grasshopper
INTRODUCTION
Reduced reproduction extends lifespan in many experimental animals (see examples in Barnes et al. 2006; reviewed in Partridge et al. 2005a), but the mechanism(s) by which reduced reproduction extends lifespan are unclear. Most often, the effects of reproduction on lifespan have been suggested to act via allocation of nutritional resources, as put forward in the disposable soma hypothesis (Kirkwood 1987; 2002). These explanations propose that reducing allocation (at least relative allocation, see O’Brien et al. 2008) of ingested nutrients toward reproduction makes more nutrients available for the maintenance of the soma and results in extended lifespan (e.g., Sgró and Partridge 1999). The alternative to the disposable soma hypothesis is direct effects of reproduction on longevity, which broadly encompasses negative effects of reproduction on lifespan that are not mediated through nutrition (Tatar 2001). Typically, these direct effects are postulated to occur through organismal signaling mechanisms such as hormones and to stimulate cellular mechanisms such as anti-oxidant activity (e.g., Arantes-Oliveira et al. 2002; Dillon et al. 2002; Hsin and Kenyon 1999; Hwangbo et al. 2004; Wood et al. 2004). Testing the disposable soma hypothesis at the physiological level is a vital field within biogerontology.
If reproduction competes with the soma for nutrients, the nutrient that limits reproduction will be subject to the strongest competition. Therefore, we focus on protein, the limiting major nutrient for reproduction in female phytophagous insects. These insects consume large quantities of plant material, which is rich in carbohydrates and energy but poor in protein. Many experiments have shown that reducing dietary protein reduces fecundity (e.g., Chapman 1998; Hatle et al. 2006b). In addition, some work suggests that levels of dietary protein, not energy, are responsible for longevity in fruit flies on dietary restriction (Lee et al. 2008; Mair et al. 2005; Piper et al. 2005). Concentrating on the allocation of a single major nutrient, as opposed to energy, facilitates the physiological measures of reproduction (i.e., vitellogenin) in the present paper.
We use the eastern lubber grasshopper (Romalea microptera; hereafter, grasshoppers) to address the physiology of effects of reproduction on aging. These grasshoppers are easy to rear individually to allow quantification of ingestion, and they are large enough for standard physiological techniques such as surgery and repeated hemolymph collection from single individuals (e.g., Hatle et al. 2003a). When offered Romaine lettuce at 70% of ad libitum, grasshoppers have extended longevity, but normal lifetime fecundity and storage of hexameric proteins in the hemolymph (Hatle et al. 2006b). Hexamerins are a well-conserved family of storage proteins known to provide fuel for metamorphosis and reproduction in a wide variety of insects (Burmester 2001; Hauerland 1996).
The physiology underlying plasticity in the production of the first clutch in lubber grasshoppers is well studied (reviewed in Hatle et al. 2003a). Clutches typically weight ∼15% of the mass of the female, and about half of the clutch is protein. Grasshoppers from Florida, USA are not vitellogenic until the second week of adulthood, suggesting that reproductive allocation starts during adulthood (Fei et al. 2005). Vitellogenesis is controlled primarily by juvenile hormone, which is first detectable in females at about day seven of adulthood (Hatle et al. 2000). In well-fed grasshoppers, vitellogenin levels rise rapidly during the second and third weeks of adulthood, then levels fall until oviposition during the sixth week. Levels of hemolymph storage proteins follow a similar trajectory, suggesting their amino acids contribute to the increased protein demands during egg production (Hatle et al. 2001; 2004).
The role of the ovary in regulating lifespan is of interest, as it appears to influence aging in different ways in a broad range of animals. In worms, ablating germ-line stem cells extends lifespan, but ablating the entire ovary does not (Arantes-Oliveira et al. 2002; Hsin and Kenyon 1999). In flies, the ovo- mutation stops egg production and extends lifespan (Sgró and Partridge 1999), and dietary restriction of ovo- mutants extends lifespan still further (Mair et al. 2004). Two germ cell mutants (germ cell-less and tudor) do not extend lifespan (Barnes et al. 2006) while another germ cell mutant (bag-of-marbles) does extended lifespan (Flatt et al. 2008). In mice, lifespan of females allowed to grow old without an ovary is extended by implantation of ovaries from young donors (Cargill et al. 2003).
Ovariectomized grasshoppers do not require ovarian hormones to produce vitellogenin (Hatle et al. 2003b). Adult females of most insects make ecdysteroids in the ovary. These hormones are required for vitellogenesis in flies, and they promote vitellogenesis in some other insects (for insect in general see Chapman 1998; for locusts see Girardie and Girardie 1996; Girardie et al. 1998). Similarly, the ovarian hormone estrogen is needed for allocation to reproduction in mice (Hadley 1996). However, we have shown that juvenile hormone, which is produced and released in the head, is necessary and sufficient for vitellogenesis in lubber grasshoppers (Hatle et al. 2003b; Fronstin and Hatle 2008), and that ecdysteroids do not alter the progression of vitellogenesis in lubber grasshoppers (Hatle et al. 2003b). Because the ovary is unneeded for allocation of the limiting nutrient to reproduction, the presence of the ovarian tissue and the ability to allocate to reproduction are separable in grasshoppers. In concert with our ability to measure ingestion and collect serial hemolymph samples, this provides an excellent system for studying the effects of reproduction on aging at the physiological level.
Here we test whether ovariectomy extends lifespan in grasshoppers and whether lifespan extension is independent of nutrient ingestion. Further, we measure the amounts of vitellogenin, hemolymph storage protein, and total reproductive protein accumulated throughout the lifespan. This allows us to examine whether ovariectomy extends lifespan via dietary restriction or reducing investment in reproductive protein.
METHODS
Experimental animals
Lubber grasshoppers (Romalea microptera) were collected near Miami, FL, USA and shipped to the laboratory in Jacksonville, FL, USA. Juveniles were reared en masse on ad libitum Romaine lettuce, occasional green beans and green onions, on a 14L:10D photoperiod, and at 28 ± 2°C. Upon adult molt, each female was transferred to an environmental chamber on a 14L:10D photoperiod and a corresponding 32C:24C thermocycle. Individuals were reared singly in 500 ml, plastic, ventilated containers. The first individual that molted was assigned to the ovariectomized group, the second individual that molted was assigned to the sham operated group, and so on.
Ovariectomy
Surgeries were performed on day 0, 1, or 2 of adulthood following procedures described in Hatle et al. (2003b). Females were cold anesthetized at 4°C for ≥1 h. For ovariectomized females (n = 36), the entire ovary was removed (both germ-line and somatic ovarian tissues), which also removes some attached fat body and tracheal tissue. For sham operated females (n = 38), the incision was made, the ovary was grasped with forceps and moved into view until it could be identified, some fat body and trachea were removed, and the ovary was returned to the abdomen.
Nutrient ingestion
Each day of adulthood, each female was offered 4.0 wet g of Romaine lettuce and 3 - 5 oatmeal flakes. Grasshoppers rarely consumed the entire meal. The next day, the remaining food was scored, with 5 indicating the entire lettuce meal was consumed, 1 indicating no food was eaten, and intermediate scores in between. Each point on this scale corresponds to approximately 0.8 wet g of lettuce. Testing these raw data for amounts eaten by MANOVA using time as a dependent variable is inappropriate; for this test, empty cells cannot be tolerated, so individuals that died before the experiment was terminated would be dropped. Because calorie restriction is known to rapidly reduce mortality rates in flies (Mair et al. 2003), censoring ingestion data would bias the results. Instead, lifetime amounts eaten were compared statistically by fitting a 5th order polynomial equation (using Microsoft Office Excel 2003, Seattle, USA) to the amount eaten profile for each individual. The coefficients for each curve describe the changes in feeding for that individual throughout its lifetime. Coefficients were subsequently compared using MANOVA. In addition, a simpler, more conservative, but less appropriate statistical analysis of lifetime ingestion was done. The average amount eaten per week was calculated for each individual, and the averages for ovariectomized and sham operated females were compared with a student’s t-test.
Survivorship and mortality rate
Females were reared until death or termination of the experiment at 205 d. Median ages at death were compared using the Kaplan-Meier survival analysis, with significant differences tested using the log-rank test (Hatle et al. 2006b; Lee and Wang 2003). Individuals from both groups that died before the median age of first oviposition (∼42 d for the sham group) were dropped from the experiment; this was ∼15% of the individuals that were operated.
Body mass
Body mass of each individual was measured daily and tested through week 21 by MANOVA, with time as an independent variable (see Hatle et al. 2003b).
Hemolymph collection
About once weekly, we bled 19 ovariectomized and 21 sham females (see Hatle et al. 2000 for bleeding procedure). These same 40 females were bled throughout the experiment, and they were used for determination of accumulation of reproductive proteins. Hemolymph samples (5 μl) were placed in buffer (Hatle et al. 2001) and stored at −20°C until analysis. Adult females have ∼2.25 ml of hemolymph (S. Li and D. Borst, unpublished data), so collecting 5 μl samples does not significantly reduce storage or delay development (cf. Hatle et al. 2002a; 2004).
Vitellogenin analysis
Vitellogenin levels for these females were determined by enzyme-linked immunosorbent assay (ELISA; modified from Borst et al. 2000). We did not use a capture antibody, but instead bound the protein of interest (i.e., vitellogenin in the hemolymph sample) directly to the plate; the accuracy of this procedure was verified across the range of the standard curve. For standards, we used egg yolk proteins. The total protein in the egg yolk homogenate was determined by the Bradford (1976) assay, and this was multiplied by 90% to estimate vitellin. Next, wells were probed with a polyclonal rabbit anti-serum to lubber grasshopper vitellin (gift from DW Borst, Univ. of Central Florida). Hemolymph vitellogenin is operationally defined as female-specific hemolymph protein that binds to the anti-vitellin serum. Next, anti-vitellin antibody was bound with an anti-rabbit IgG antibody conjugated to alkaline phosphatase (Sigma Chemical, St. Louis, USA), and the assay was developed with a p-nitrophenyl phosphate solution (Pierce, Rockford, IL, USA). Standard curves were sigmoidal-shaped and were fit with 3rd order polynomial equations for calculations.
Estimation of total accumulation of reproductive protein
Ovariectomized females produce high levels of vitellogenin as adults (Hatle et al. 2003b). This reproductive protein accumulates in the hemolymph, and there is no other depot for reproductive proteins in ovariectomized females. Hence, for ovariectomized females, for a specific age, we estimated accumulation of reproductive protein as the concentration of hemolymph vitellogenin (in mg/ml) multiplied by the average hemolymph volume of an adult female (2.25 ml). This was done for each female at about 50, 100, 150, and 200 days. In each case, the sample collected at the age closest to each of these four target ages was used. For both ovariectomized and sham females at each of the four ages, the average sample age was always within two days of the target age.
For sham operated females, accumulation of reproductive protein was determined by adding together the protein from vitellin in eggs and vitellogenin in the hemolymph. This was done at ages 50, 100, and 150 days. Egg masses were collected from sham females throughout the study, dried at 55°C at least overnight, and weighed. Eggs of most insects are ∼50% protein (Chapman 1998). In lubber grasshoppers specifically, both anion exchange and size exclusion chromatography have been used to show that ∼90% of the egg protein is vitellin (HD Wood and DW Borst, unpublished data). Hence, reproductive protein in eggs was calculated as total mass of eggs multiplied by 50% and then 90%. Reproductive protein in the hemolymph was determined as for ovariectomized females.
For the 200 day samples, it was possible to also include vitellin in growing oocytes still inside the female. Reproductive protein contained in oocytes at the time of death was estimated from oocyte counts and lengths. For each female, the oocyte length was used to estimate oocyte volume, using the approximation that each oocyte was a cylinder thrice as long as wide. This volume was divided by the volume of a typical egg, which is 10 mm long (Moehrlin and Juliano 1998). This ratio of volumes [(oocyte volume / egg volume)] represents the portion of egg growth that had been completed. From this ratio, the total mass of vitellin in oocytes was calculated. Eggs of wild-caught lubber grasshoppers from Miami weigh ∼15.5 mg, regardless of diet (Moehrlin and Juliano 1998). Therefore, the ratio was multiplied by 15.5 mg / egg to calculate the weight of one unlaid oocyte for that female. Oocytes grow synchronously, so this weight of one unlaid oocyte was multiplied by the total number of oocytes in the female to estimate the total weight of the developing clutch. Last, as for eggs, this weight of the developing clutch was multiplied by 50% and then 90% to estimate the reproductive protein in unlaid oocytes at the time of death. These counts only included terminal oocytes, but the size of subsequent oocytes is negligible compared to the much larger terminal oocytes.
Analysis of hemolymph storage proteins
The levels of hemolymph storage proteins were estimated by measuring total hemolymph protein and subtracting vitellogenin. Three hexameric storage proteins make up 80% of total non-vitellogenin protein in the hemolymph of females throughout the first clutch (see Hatle et al. 2001), so total non-vitellogenin protein from hemolymph serves as a good estimate of the combined hexameric storage proteins. Data were analyzed by MANOVA, with time as a dependent variable.
RESULTS
Nutrient ingestion
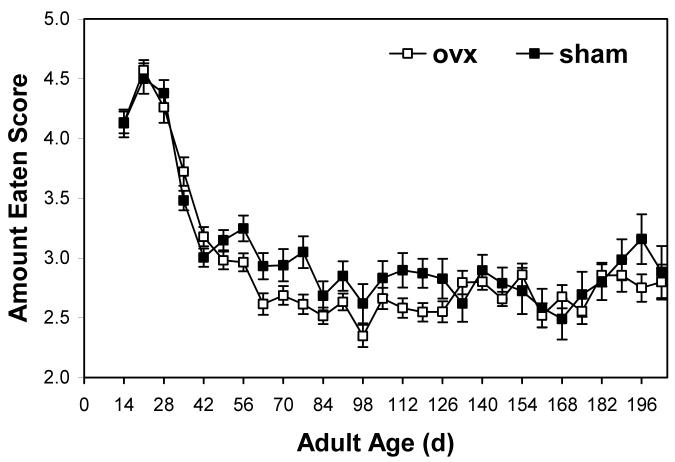
Surgery (i.e., ovariectomy vs. sham) did not significantly effect amounts eaten throughout the lifespan (Fig. 1; MANOVA; Pillai’s Trace F6,67 = 0.41; P = 0.870). Each individual’s profile of amount eaten was fit with a 5th order polynomial equation. The coefficients of these polynomials were not significantly affected by surgery (Table 1). On average, the polynomials fit to lifetime feeding profiles explained ∼70% of the variation in the individual’s feeding data. This result was corroborated by a two-tailed t-test of mean amounts eaten per week (mean ± SE; ovx = 2.95 ± 0.05; sham = 3.10 ± 0.07; df = 1; t = 5.84; P = 0.108).
Figure 1.
When offered Romaine lettuce ad libitum, ovariectomized (ovx) females and sham operated controls of the lubber grasshopper consumed similar amounts (mean ± SE). Surgeries were performed during the first three days of adulthood. Grasshoppers were reared individually and offered 4.0 wet g of lettuce daily; the complete meal was rarely consumed. The next day, the amount of remaining, uneaten lettuce was scored to estimate the amount eaten. The pattern of lifetime feeding closely matches previous data in which the amounts remaining uneaten were determined by drying and then weighing uneaten food (Hatle et al. 2006b). Hence, ovariectomy does not appear to cause dietary restriction.
Table 1.
When offered Romaine lettuce ad libitum, ovariectomized females and sham operated controls of the lubber grasshopper consumed similar amounts. Surgeries were performed during the first three days of adulthood. Individual lifetime profiles of amount eaten (see Fig. 1 for group profiles) were fit with 5th order polynomial equations. These equations explain ∼70% of the variation in the data set. The coefficients of these equations are shown in the table and were compared using MANOVA. In the table, F refers to the MANOVA test statistic and P is the probability that these results could be produced by chance. None of the coefficients were significantly affected by surgery.
| OV- | Sham | F | P | |
|---|---|---|---|---|
| x5 | 0.0000±0.0001 | 0.0000±0.0000 | 0.04 | 0.848 |
| x4 | 0.0003±0.0022 | 0.0012±0.0019 | 0.27 | 0.608 |
| x3 | 0.0136±0.0264 | 0.0098±0.0253 | 0.41 | 0.523 |
| x2 | 0.1384±0.1533 | 0.0369±0.1624 | 0.61 | 0.436 |
| x | 0.2519±0.4582 | 0.3295±0.4986 | 0.73 | 0.395 |
| b | 4.4701±0.5566 | 5.0225±0.5563 | 0.49 | 0.485 |
| r2 | 0.7329±0.0208 | 0.6756±0.0288 |
The lifetime pattern of ingestion closely matches previous data from our lab (Hatle et al. 2006b). In this previous experiment, uneaten lettuce was collected, dried completely and weighed daily. Hence, the similarity in results between these two experiments supports the accuracy of our scoring of the amounts eaten.
Survivorship
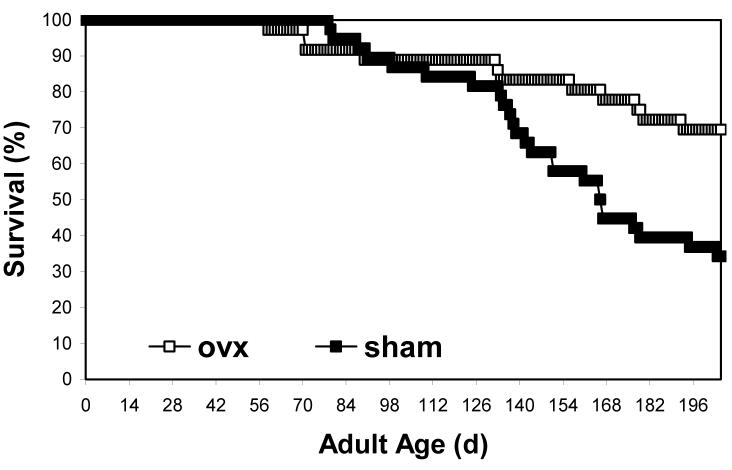
Median age of death was greater in ovariectomized females (Fig. 2; log-rank test; χ2 = 2752; P << 0.001; ovariectomized > 205 d; sham = 167.0 d). During the final 40 d of the experiment, survival rates in ovariectomized females were ∼30% higher than in sham females.
Figure 2.
Ovariectomized (ovx) females of the lubber grasshopper had greater median lifespan than sham operated controls. Surgeries were performed during the first three days of adulthood (ovx n = 36; sham n = 38).
Vitellogenin levels
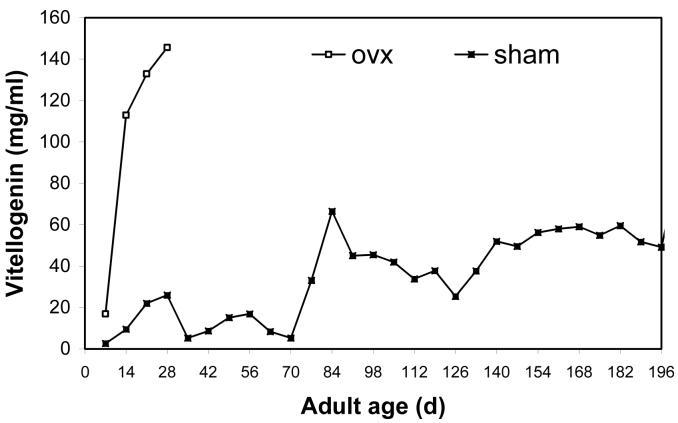
Partial vitellogenin profiles showed that levels in ovariectomized females rose rapidly in the second week of adulthood and remained high until 28 d (Fig. 3). The vitellogenin profile for ovariectomized females is truncated because many samples from 30 d and beyond were initially run at concentrations that exceeded the detection maximum of the assay. Levels in sham females increased to 28 d and then decreased before first oviposition at about 42 d. This is similar to previous results on un-operated females (Hatle et al. 2001; 2004). A similar rise and fall of vitellogenin levels can be seen for a second clutch, with levels peaking around 56 d. Vitellogenin levels in sham females rose sharply at about 77 d and then remained elevated until 200 d.
Figure 3.
Ovariectomized (ovx) females of the lubber grasshopper accumulated much higher levels of hemolymph vitellogenin than sham controls during the first four weeks of adulthood. Surgeries were performed during the first three days of adulthood. Vitellogenin levels were measured by ELISA (Borst et al. 2000). The pattern of vitellogenin levels in sham females during the first clutch (ending at ∼42 d) is similar to previously published profiles (Hatle et al. 2001). Levels of vitellogenin in ovariectomized females over 28 d old were not quantified for each week.
Hemolymph samples were diluted and re-run for quantification of vitellogenin levels at 50, 100, 150, and 200 d. In ovariectomized females, vitellogenin levels were very high at 50 d, then decreased to intermediate levels from 100 to 200 d. For sham females, vitellogenin levels were low at 50 d, then increased steadily to 200 d. This resulted in a significant interaction of surgery and time (Fig. 4; MANOVA; Pillai’s Trace F3,22 = 3.55; P = 0.0342). Specifically, vitellogenin levels were higher in ovariectomized females at 50 d (P = 0.0019), but not at 100 d (P = 0.0775), 150 d (P = 0.9005), or 200 d (P = 0.7212). Analysis by MANOVA requires that there be no missing data for each individual; hence, this analysis only included 23 of the 40 individuals that were bled in this study. Despite this, the qualitative conclusions of this analysis do not appear to be affected by the large number of individuals that were omitted. Eliminating 200 d from the analysis allowed for 29 or 40 individuals to be included in the statistical test, yet yielded nearly identical qualitative results. Similarly, including data from all individuals and then making comparisons within ages with serial t-tests (Bonferroni corrected α = 0.0125) also resulted in the same qualitative results.
Figure 4.
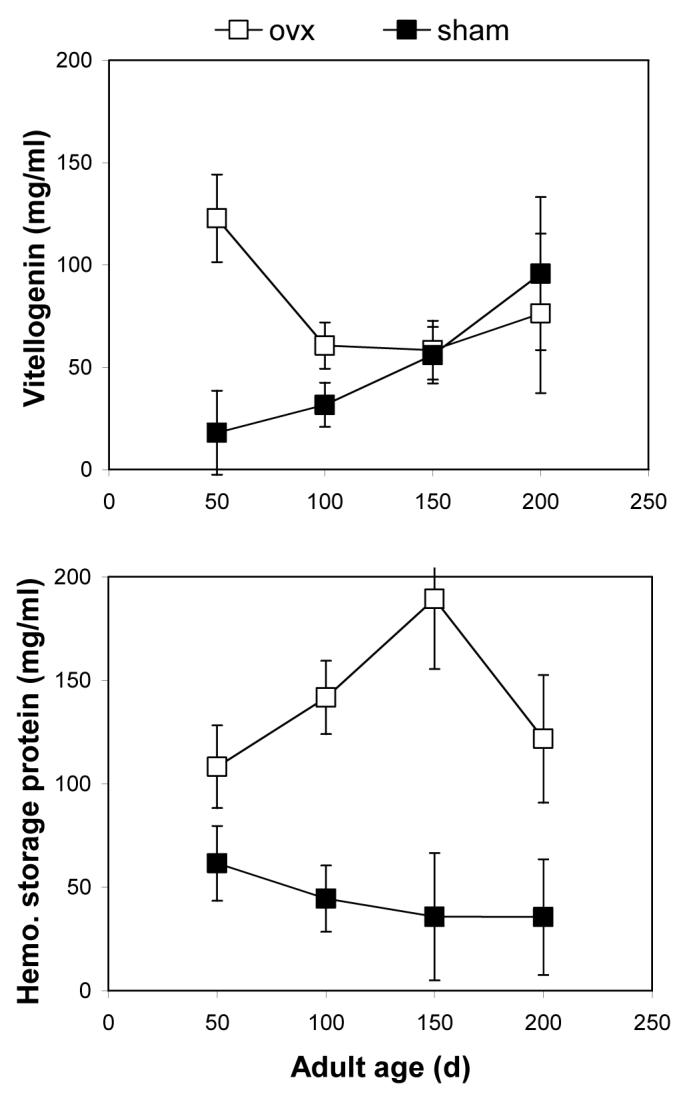
Ovariectomized (ovx) and sham females of the lubber grasshopper had converging levels of vitellogenin from 50 d to 200 d. Ovariectomized females had higher levels (LSmeans ± SE) of vitellogenin than did sham females at age 50 d, shortly after the median age of first oviposition in the sham females. Vitellogenin levels at age 100, 150, and 200 d were statistically similar in the two groups. In contrast, ovx and sham females tended to have divergent levels of hemolymph storage proteins from 50 d to 200 d. Storage protein levels in the two groups were similar at 50 d, while ovariectomized females had higher storage protein levels at 100 and 150 d, and marginally higher levels at 200 d (P = 0.054). Surgeries were performed during the first three days of adulthood. Vitellogenin levels were measured by ELISA (Borst et al. 2000).The combined levels of three hemolymph hexamerins (a conserved family of storage proteins) were estimated by measuring total hemolymph protein and subtracting vitellogenin (see Hatle et al. 2001).
Levels of hemolymph storage proteins
In ovariectomized females, storage protein levels were relatively high throughout the experiment, and highest at 150 d. For sham females, storage protein levels were relatively low throughout the experiment. The interaction of surgery and time was not significant (Fig. 4; MANOVA; Pillai’s Trace F3,19 = 2.15; P = 0.1344). Nonetheless, storage protein levels were similar at 50 d (P = 0.1018), but higher in ovariectomized females at 100 d (P = 0.0007) and 150 d (P = 0.0036), and marginally higher in ovariectomized females at 200 d (P = 0.0540). Due to the constraints of MANOVA, this analysis only included 20 of the 40 individuals that were bled. To minimized dropped individuals, we re-tested only the first three days. With this analysis, the qualitative results for individual days stayed the same, but the interaction was significant (F2,26 = 3.66; P = 0.0411).
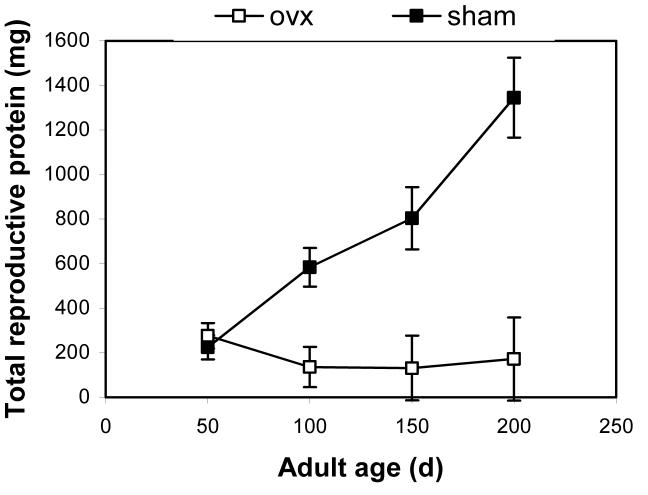
Accumulation of reproductive protein
For ovariectomized females (n = 19), vitellogenin levels are the only reproductive protein, so accumulation of total reproductive protein is simply the vitellogenin level multiplied by the hemolymph volume of 2.25 ml (cf. Figs. 4 and 5). For sham females (n = 21), reproductive protein includes laid eggs, vitellogenin, and at 200 d also includes unlaid oocytes. Total reproductive protein levels for sham females rose throughout the experiment. There was a significant interaction of surgery and time (Fig. 5; MANOVA; Pillai’s Trace F3,22 = 13.86; P < 0.0001). Specifically, total reproductive protein was similar at 50 d (P = 0.5232), but accumulation was higher for sham females at 100 d (P = 0.0018), 150 d (P = 0.0031), and 200 d (P = 0.0002). Similar to the analysis of vitellogenin levels, this statistical test included only 23 of 40 individuals bled in the study, but the veracity of the qualitative conclusions was confirmed using serial t-tests with the Bonferroni correction.
Figure 5.
Ovariectomized (ovx) and sham females of the lubber grasshopper had divergent levels of total reproductive proteins during the study. Levels of total reproductive protein (LSmeans ± SE) were similar at age 50 d, shortly after the median age of oviposition in the sham females. Levels of total reproductive protein at age 100, 150, and 200 d were greater in sham females than in ovariectomized females. Surgeries were performed during the first three days of adulthood. Vitellogenin levels were measured by ELISA (Borst et al. 2000). See text for the operational definition of total reproductive protein.
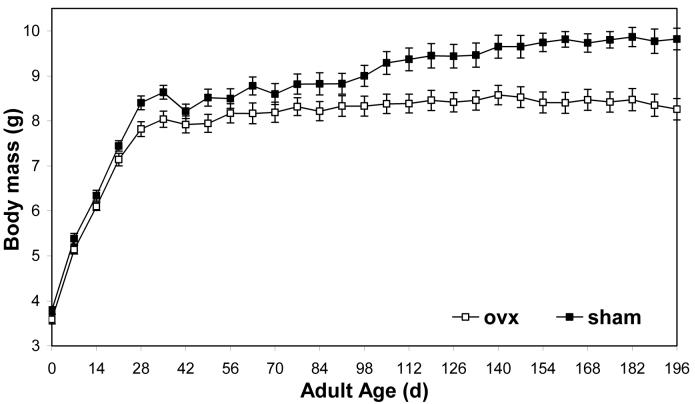
Body mass
Body masses followed a similar trajectory to that observed in previous experiments (e.g., Hatle et al. 2006b), with masses increasing rapidly during the first four weeks of adulthood and then almost attaining a plateau. There was a significant interaction of surgery and time, with sham females growing larger than ovariectomized females through 147 d (Fig. 7; MANOVA; Pillai’s Trace F20,30 = 2.47; P = 0.0123). The mean difference between sham and ovariectomized females increased after 147 d, but this period was not included in the MANOVA to minimize reduction in sample size due to individuals lost to death.
DISCUSSION
Ovariectomized females lived significantly longer than sham females, despite being offered ad libitum food and ingesting similar amounts. These data suggest that the presence of the ovary promotes aging by some means independent of dietary restriction. Hence, our data provides an opportunity to examine nutrient allocation independently of ingestion. Ovariectomized grasshoppers are capable of producing reproductive protein, which accumulates in the hemolymph as vitellogenin. Ovariectomized and sham females had produced similar amounts of reproductive protein shortly after sham females laid their first clutch. However, by advanced ages, ovariectomized females had produced about 5-fold less cumulative reproductive protein than sham females. In contrast, old ovariectomized females had at least 2-fold more hemolymph storage protein than did sham females. This increased storage may provide additional resources for somatic maintenance late in life. These results are consistent with ovariectomy extending lifespan in part via enhanced protein allocation to storage at the expense of reproduction, as predicted by the disposable soma hypothesis.
Ovariectomy in grasshoppers does not extend lifespan via dietary restriction
Whether ovariectomy extends lifespan by causing dietary restriction is unclear in other organisms, especially fruit flies. It may be that blocking reproduction reduces feeding, which in effect acts as a dietary restriction that extends lifespan. This would be consistent with the reduced signaling for insulin / insulin-like growth factor that is seen both in some germ cell mutant flies (Flatt et al. 2008; upregulation of FOXO target genes) and in flies on dietary restriction (Tatar 2007). To determine if ovariectomy is extending lifespan via dietary restriction, ingestion must be measured accurately, but the accuracy of feeding measurements for fruit flies has been controversial. There is a contentious literature on how to measure the amount eaten by a fruit fly (Carvalho et al. 2005; Mair et al. 2005; Min et al. 2007; Min and Tatar 2006; Partridge et al. 2005b; Piper et al. 2007), although this may have been resolved recently (Ja et al. 2007; Lee et al. 2008). At the very least, measuring ingestion in individual fruit flies is extremely laborious.
In contrast, precisely measuring the amount eaten by individual grasshoppers is simple (Hatle et al. 2006b; Juliano et al. 2004). Dietary restriction is known to extend lifespan in female lubber grasshoppers, but without reducing lifetime fecundity (Hatle et al. 2006b). At the same time, surgical removal of the source of juvenile hormone (allatectomy) blocks vitellogenesis and reduces feeding by about two-thirds (Fronstin and Hatle 2008). Hence, it is feasible that ovariectomy might also reduce feeding, and this is a reasonable mechanism by which ovariectomy might have extended lifespan in the present experiment. However, when offered free access to food throughout the lifespan, ovariectomized grasshoppers consumed 95% of that consumed by sham females. Life extension by dietary restriction typically requires at least a 15% reduction in feeding. Hence, it is clear that the life extension observed in this experiment is not acting through the same mechanism(s) as dietary restriction.
High levels of reproductive protein are produced by ovariectomized grasshoppers
Protein is the limiting nutrient for egg production in phytophagous insects such as grasshoppers. When only dietary protein is reduced in lubber grasshoppers, vitellogenesis is greatly reduced (Hatle et al. 2006a). In addition, about 90% of egg protein is vitellin. Therefore, while we measured only one component of egg production, it is the most important component.
Ovariectomized grasshoppers produced reproductive protein, which all remained in the hemolymph as vitellogenin. In contrast to grasshoppers, ovariectomy in mammals removes the source of estrogen, which blocks reproductive allocation (Hadley 1996). Ovariectomy in dipteran flies removes ecdysone, which also blocks reproductive allocation (Chapman 1998; Hagedorn et al. 1975 for mosquitoes). Therefore, in two frequently used models for aging research, the ovary is required for allocation of protein to reproduction. In contrast, in grasshoppers we are able to remove this reproductive tissue without halting allocation of the limiting nutrient to reproduction. Indeed, the total amount of vitellogenin in ovariectomized females at 50 d is similar to the vitellin in a typical first clutch of ∼40 eggs (Hatle et al. 2000; Hatle et al. 2002a; Hatle et al. 2006b). In concert, ovariectomy did not significantly effect ingestion in grasshoppers, so the nutrients available are similar in the ovariectomized and sham females. Taken together, this provides a strong approach for testing whether the ovary affects lifespan through nutrient allocation.
Levels of vitellogenin decline and levels of hemolymph storage protein increase after 50 d
Levels of vitellogenin decrease and levels of hemolymph storage proteins increase in ovariectomized females from 50 to 150 d. Our methods do not reveal the exact fate of individual protein molecules. Nonetheless, because we repeatedly sampled individuals, we have a lifetime history of vitellogenin and hemolymph storage proteins for each female. Therefore, it is clear that old ovariectomized females cease accrual of reproductive protein yet storage protein levels remain high. This shift in the allotment of resources is consistent with the disposable soma hypothesis.
The reduction in vitellogenin levels from 50 to 100 d in ovariectomized females may be due to the protein acting as a binding protein for juvenile hormone (Engelmann and Mala 2000), which in turn is required for vitellogenin synthesis. It may be that the extremely high levels of vitellogenin in ovariectomized females at 50 d reduced the available juvenile hormone, so the fat body was no longer fully stimulated to produce additional vitellogenin. However, despite this plausible mechanism, the regulation of total vitellogenin production in lubber grasshoppers has been shown to be due more to total mass of fat body (the tissue that produces vitellogenin) than the rate of mass-specific vitellogenin production (which would be stimulated by juvenile hormone) (Hatle et al. 2006b). Hence, a mechanism that acts via a growth regulator instead of through juvenile hormone may better explain the decline in vitellogenin levels.
The role of vitellogenin as an anti-oxidant linked to longevity in bees is an exciting area of recent research (Amdam and Omholt 2002; Amdam et al. 2004a,b; Guidugli et al. 2005; Nelson et al. 2007; Seehuus et al. 2006). In bees, the caste system of social organization may have allowed the vitellogenin to contribute to longevity of some bees by acting as an anti-oxidant. Queens and hive bees, the most valuable bees in a colony, have higher levels of vitellogenin and enhanced longevity. This does not appear to be the case for lubber grasshoppers, which are gregarious as hatchlings and as adults (Hatle et al. 2002b) but clearly not social.
Reproductive proteins accumulate for old sham but not old ovariectomized females
Accrual of total reproductive protein decreased in old ovariectomized females but continued to increase in old sham females. In terms of the raw material of amino acids needed for reproduction (and not simply the energy needed for reproduction), ovariectomy in grasshoppers caused a change in allocation at older ages. As a result, in sham females allocation to reproductive proteins was greater, and at the same time, lifespan was shorter. These data on allocation of reproductive protein are consistent with the disposable soma hypothesis of aging (Kirkwood 1987; 2002).
We examined resource allocation by directly measuring ingestion to determine that ovariectomized and sham females did not accumulate different levels of nutrition. In addition, nutrient allocation was measured at the physiological level (i.e., vitellogenin in the hemolymph) as well as the organismal level (i.e., egg output). Yet, the actual tracking of ingested nutrients into reproductive proteins is needed to definitively determine whether resource allocation is involved in life extension in ovariectomized grasshoppers. Stable isotopes (Gannes et al. 1997; Min et al. 2006; O’Brien et al. 2008) can be used to label the diet, to allow the animals to label protein made from those ingested nutrients, to explicitly test this hypothesis.
Sham females had greater body masses
Despite ingesting similar amounts, sham females gained more weight than ovariectomized females, reaching a difference of ∼1.3 g (∼15%) late in life. This is surprising, since sham females also deposited cumulative egg masses totaling 1.14 ± 0.26 g. Four possible explanations for this include differences in metabolic rate, excretion of energy, the form of stored energy, or consumption of resources for somatic maintenance. Ovariectomized females may have had a higher metabolic rate than sham females. Second, ovariectomized females may have excreted energy in the feces. Third, ovariectomized females may have stored more energy as fat, which would require less mass than storing energy as carbohydrate or protein. Indeed, ovarian mutants of Drosophila show fat body hypertrophy (Flatt et al. 2005). Fourth, ovariectomy may stimulate somatic maintenance (e.g., activity of anti-oxidant proteins), which consumes energy. Additional data is required to distinguish among these possibilities.
Ovariectomy and dietary restriction may extend lifespan by different mechanisms
Perhaps the most important conclusion of this paper is the possibility that ovariectomy and dietary restriction may enhance longevity in distinct ways. A common, long-standing supposition has been that reducing diet and reducing reproduction both extend lifespan in the same way, namely by adjusting nutrient allocation. However, in grasshoppers, dietary restriction extends lifespan without reducing lifetime fecundity or hemolymph protein storage (Hatle et al. 2006b), yet ovariectomy extends lifespan by reducing allocation to total reproductive protein while increasing allocation to hemolymph protein storage (this paper). This suggests two distinct mechanisms of lifespan extension. We intend to test whether these mechanisms are truly distinct by manipulating both ingestion and ovarian presence in a full factorial design.
Figure 6.
Ovariectomized (ovx) females of the lubber grasshopper gained less body mass than sham operated controls after 105 d. Both groups were fed ad libitum Romaine lettuce. Surgeries were performed during the first three days of adulthood.
ACKNOWLEDGEMENTS
We thank Marc Tatar for suggesting this line of research, Kevin Brix, Thomas Flatt, Daniel Hahn, Evan Judd, K-J Min, Marc Tatar, and Katharine Wright for critically commenting on the manuscript, Thomas Flatt and Steven Juliano for help with statistics, David Borst for the gift of anti-vitellin serum, Heather Wood for estimating vitellin concentration in eggs and oocytes, Dann Bopp and Chartwell’s food service for a source of Romaine lettuce, Tom Jackson for collecting and shipping grasshoppers, and two anonymous reviewers for their helpful comments. Supported by R15 AG028512-01 from the National Institute on Aging to JDH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amdam GV, Norberg K, Hagen A, Omholt SW. Social exploitation of vitellogenin. Proc. Nat. Acad. Sci. 2004;100:1799–1802. doi: 10.1073/pnas.0333979100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Omholt SW. The regulatory anatomy of honeybee lifespan. J. Theor. Biol. 2002;216:209–228. doi: 10.1006/jtbi.2002.2545. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Simoñes ZLP, Hagen A, Norberg K, Schrøder K, Mikkelsen Ø, Kirkwood TBL, Omholt SW. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp. Geron. 2004;39:767–773. doi: 10.1016/j.exger.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- Barnes AI, Boon JM, Jacobson J, Partridge L, Chapman T. No extension of lifespan by ablation of germ line in Drosophila. Proc. R. Soc. B. 2006;273:939–947. doi: 10.1098/rspb.2005.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst DW, Eskew M, Wagner SJ, Shores KM, Hunter J, Luker L, Hatle JD, Hecht LB. Quantification of juvenile hormone III, vitellogenin, and vitellogenin-mRNA during the oviposition cycle of the lubber grasshopper. Insect Biochem. Mol. Biol. 2000;30:813–819. doi: 10.1016/s0965-1748(00)00053-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burmester T. Evolution and function of the insect hexamerins. Eur. J. Entomol. 2001;96:213–225. [Google Scholar]
- Cargill SL, Carey JR, Muller H-G, Anderson G. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell. 2003;2:185–190. doi: 10.1046/j.1474-9728.2003.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Nat. Acad. Sci. USA. 2007;104:7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Benzer S. Direct quantification of food intake reveals compensatory ingestion upon dietary restriction in Drosophila. Nat. Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. The Insects: Structure and Function. Cambridge University Press; Cambridge: 1998. [Google Scholar]
- Dillin A, Crawford DK, Kenyon C. Timing requirement for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- Engelmann F, Mala J. The interactions between juvenile hormone (JH), lipophorin, vitellogenin, and JH esterases in two cockroach species. Insect Biochem. Mol. Biol. 2000;30:793–804. doi: 10.1016/s0965-1748(00)00051-5. [DOI] [PubMed] [Google Scholar]
- Fei H, Martin TR, Jaskowiak KM, Hatle JD, Whitman DW, Borst DW. Starvation affects vitellogenin production but not vitellogenin mRNA levels in the lubber grasshopper, Romalea microptera. J. Insect Physiol. 2005;51:435–443. doi: 10.1016/j.jinsphys.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Flatt T, Min K-J, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M. Drosophila germ line modulation of insulin signaling and lifespan. Proc. Nat. Acad. Sci. U.S.A. 2008;105:6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T, Tu M-P, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. BioEssays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Fronstin RB, Hatle JD. A cumulative feeding threshold required for vitellogenesis can be obviated with juvenile hormone in lubber grasshoppers. J. Exp. Biol. 2008;211:79–85. doi: 10.1242/jeb.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannes LZ, O’Brien DM, del Rio C. Martinez. Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology. 1997;78:1271–1276. [Google Scholar]
- Girardie J, Girardie A. Lom OMP, a putative ecdysiotropic factor for the ovary in Locusta migratoria. J. Insect Physiol. 1996;42:215–221. [Google Scholar]
- Girardie J, Geoffre S, Delbecque J-P. Arguments for two distinct gonadotropic activities triggered by different domains of the ovary maturating parsin of Locusta migratoria. J. Insect Physiol. 1998;44:1063–1071. doi: 10.1016/s0022-1910(98)00066-3. [DOI] [PubMed] [Google Scholar]
- Guidugli KR, Nascimento AM, Amdam GV, Barchuk AR, Omholt SW, Simoñes ZLP, Hartfelder K. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Letters. 2005;579:4961–4965. doi: 10.1016/j.febslet.2005.07.085. [DOI] [PubMed] [Google Scholar]
- Hadley MC. Endocrinology. Prentice-Hall International; Upper Saddle River, N.J., U.S.A.: 1996. [Google Scholar]
- Hagedorn HH, O’Connor JD, Fuchs MS, Sage B, Schlaeger DA, Bohm MK. The ovary as a source of alpha-ecdysone in an adult mosquito. Proc. Nat. Acad. Sci. U.S.A. 1975;72:3255–3259. doi: 10.1073/pnas.72.8.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatle JD, Andrews AL, Crowley MC, Juliano SA. Interpopulation variation in developmental titers of vitellogenin, but not storage protein, in lubber grasshoppers. Physiol. Biochem. Zool. 2004;77:631–640. doi: 10.1086/420946. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Borst DW, Juliano SA. Plasticity and canalization in the control of reproduction in the lubber grasshopper. Integ. Comp. Biol. 2003a;43:635–645. doi: 10.1093/icb/43.5.635. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Borst DW, Eskew MR, Juliano SA. Maximum titers of vitellogenin and total hemolymph protein occur during the canalized phase of grasshopper egg production. Physiol. Biochem. Zool. 2001;74:885–93. doi: 10.1086/324475. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Crowley MC, Andrews AL, Juliano SA. Geographic variation of reproductive tactics in lubber grasshoppers. Oecologia. 2002a;132:517–523. doi: 10.1007/s00442-002-0994-5. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Juliano SA, Borst DW. Juvenile hormone is a marker of the onset of reproductive canalization in lubber grasshoppers. Insect Biochem. Mol. Biol. 2000;30:821–827. doi: 10.1016/s0965-1748(00)00054-0. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Juliano SA, Borst DW. Hemolymph ecdysteroids do not affect vitellogenesis in the lubber grasshopper. Arch. Insect Biochem. Physiol. 2003b;52:45–57. doi: 10.1002/arch.10067. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Salazar BA, Whitman DW. Survival advantage of sluggish individuals in aggregations of aposematic prey, during encounters with ambush predators. Evol. Ecol. 2002b;16:415–431. [Google Scholar]
- Hatle JD, Waskey T, Jr., Juliano SA. Plasticity of grasshopper vitellogenin production in response to diet is primarily a result of changes in fat body mass. J. Comp. Physiol. B. 2006a;176:27–34. doi: 10.1007/s00360-005-0028-9. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Wells SM, Fuller LE, Allen IC, Gordy LJ, Melnyk S, Quattrochi J. Calorie restriction and late-onset calorie restriction extend lifespan but do not alter protein storage in female grasshoppers. Mech. Ageing Dev. 2006b;127:883–91. doi: 10.1016/j.mad.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- Haunerland NH. Insect storage proteins: gene families and receptors. Insect Biochem. Mol. Biol. 1996;26:755–765. doi: 10.1016/s0965-1748(96)00035-5. [DOI] [PubMed] [Google Scholar]
- Hwangbo DS, Gersham B, Tu M-P, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Pradiology of Drosophila and the CAFE assay. Proc. Nat. Acad. Sci. U.S.A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Olson JR, Murrell EG, Hatle JD. Plasticity and canalization of insect reproduction: testing alternative models of life history transitions. Ecology. 2004;85:2986–2996. [Google Scholar]
- Kirkwood TBL. Immortality of the germ-line versus the disposability of the soma. In: Finch CE, Schneider EL, editors. Handbook of the Biology of Aging. second Van Nostrand Reinhold; New York: 1987. [Google Scholar]
- Kirkwood TBL. Evolution of ageing. Mech. Age. Dev. 2002;123:737–745. doi: 10.1016/s0047-6374(01)00419-5. [DOI] [PubMed] [Google Scholar]
- Lee ET, Wang JW. Statistical Methods for Survival Data Analysis. 3rd Wiley-Interscience; Boulder, CO, U.S.A.: 2003. [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Nat. Acad. Sci. U.S.A. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Ouyang YC, Ostrowski E, Borst DW. Allatotropin regulation of juvenile hormone synthesis by the corpora allata from the lubber grasshopper, Romalea microptera. Peptides. 2005;26:63–72. doi: 10.1016/j.peptides.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Maestro J-L, Danés M-D, Piulachs M-D, Cassier P, Bellés X. Juvenile hormone inhibition in corpora allata from ovariectomized Blattella germanica. Physiol. Entomol. 1994;19:342–348. [Google Scholar]
- Mair W, Goymer P, Fletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Mair W, Sgró CM, Johnson AP, Chapman T, Partridge L. Lifespan extension by dietary restriction in female Drosophila melanogaster is not caused by a reduction in vitellogenesis or ovarian activity. Exp. Geron. 2004;39:1011–1019. doi: 10.1016/j.exger.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MDW, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min K-J, Hogan MF, Tatar M, O’Brien DM. Resource allocation to reproduction and soma in Drosophila: a stable isotope analysis of carbon from dietary sugar. J. Insect Physiol. 2006;52:763–770. doi: 10.1016/j.jinsphys.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Min K-J, Tatar M. Drosophila diet restriction in practice: do flies consume fewer nutrients? Mech. Ageing Dev. 2006;127:93–96. doi: 10.1016/j.mad.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Min K-J, Flatt T, Kulaots I, Tatar M. Counting calories in Drosophila diet restriction. Exp. Gerontol. 2007;42:247–251. doi: 10.1016/j.exger.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehrlin GS, Juliano SA. Plasticity in insect reproduction: testing models of flexible and fixed development in response to different growth rates. Oecologia. 1998;115:492–500. doi: 10.1007/s004420050546. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Ihle KE, Fondrk MK, Page RE, Jr., Amdam GV. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 2007;5:673–677. doi: 10.1371/journal.pbio.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien DM, Min K-J, Larsen T, Tatar M. Use of stable isotopes to examine how dietary restriction extends Drosophila lifespan. Current Biol. 2008;18:R155–R156. doi: 10.1016/j.cub.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005a;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Partridge L, Piper MDW, Mair W. Dietary restriction in Drosophila. Mech. Ageing Dev. 2005b;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Piper M, Mair W, Partridge L. Counting the calories: the role of specific nutrients in extension of life span by food restriction. J. Gerontol. A: Biol. Sci. Med. Sci. 2005;60:549–555. doi: 10.1093/gerona/60.5.549. [DOI] [PubMed] [Google Scholar]
- Piper M, Mair W, Partridge L. Letter to the Editor — reply to Min et al. (2006) Exp. Geron. 2007;42:253–255. doi: 10.1016/j.exger.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Seehuus S-C, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgró CM, Partridge L. A delayed wave of death from reproduction in Drosophila. Science. 1999;286:2521–2524. doi: 10.1126/science.286.5449.2521. [DOI] [PubMed] [Google Scholar]
- Tatar M. Senescence. In: Fox CW, Roff DA, Fairbairn DJ, editors. Evolutionary Ecology: Concepts and Case Studies. Oxford Univ. Press; Oxford: 2001. pp. 128–141. [Google Scholar]
- Tatar M. Diet restriction in Drosophila melanogaster. In: Mobbs CV, Yen K, Hof PR, editors. Mechanisms of Dietary Restriction in Aging and Disease: Interdisciplicinary Topics in Gerontology. Basel, Karger; New York: 2007. pp. 115–136. [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]