Abstract
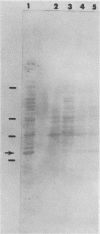
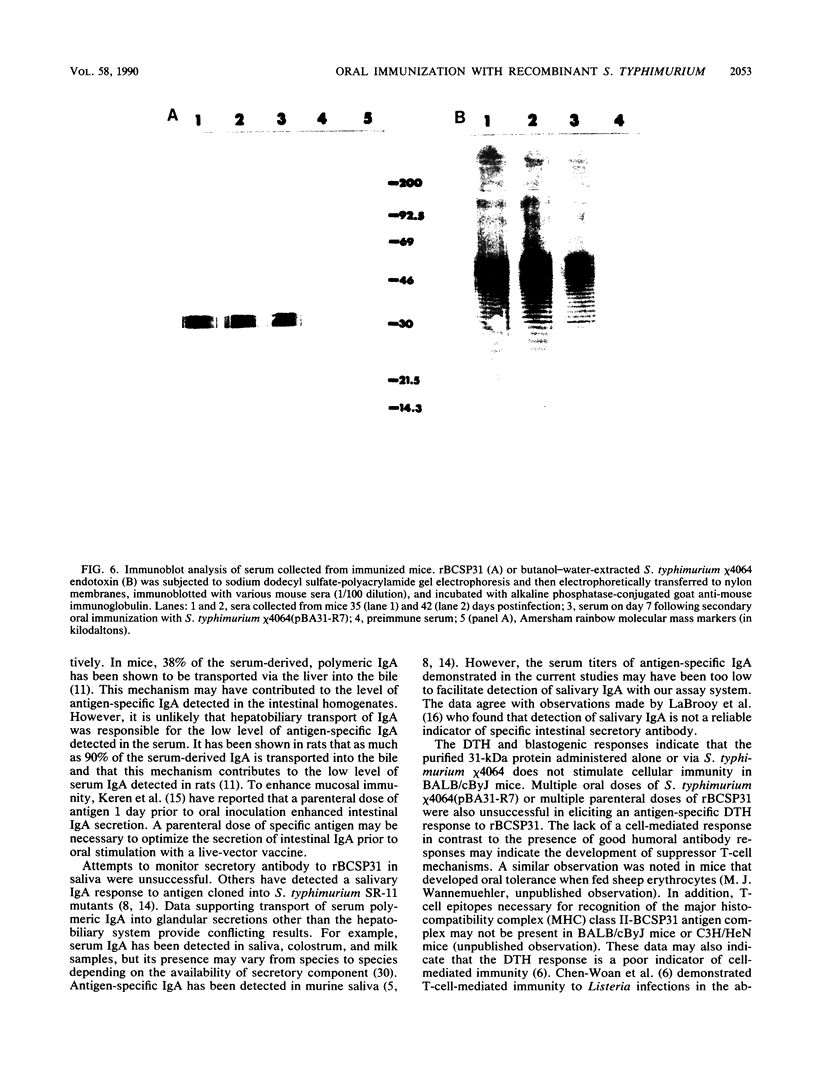
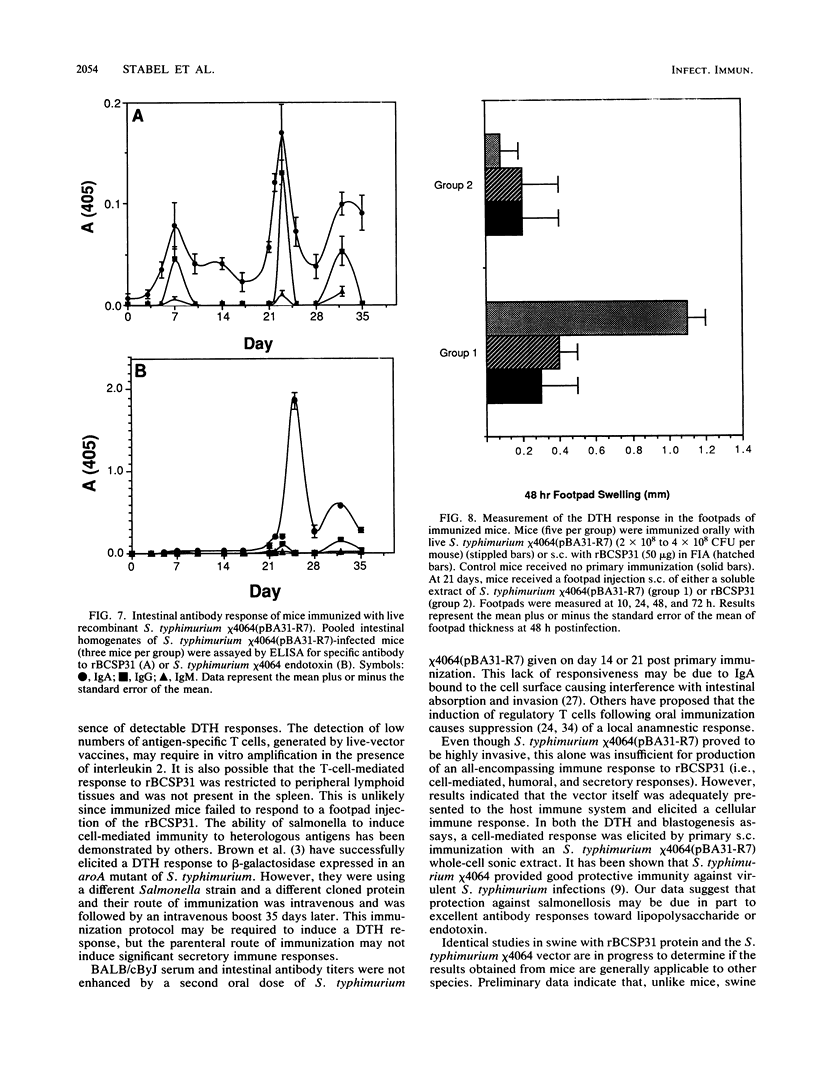
Salmonella typhimurium chi 4064, an attenuated delta cya delta crp mutant of S. typhimurium SR-11, was used as a carrier for the plasmid pBA31-R7. This plasmid codes for the expression of a 31-kilodalton (kDa) protein from Brucella abortus (BCSP31). Recombinant S. typhimurium chi 4064(pBA31-R7) expressed BCSP31 in vitro as shown by Western blot (immunoblot) analysis. The plasmid was stable in vitro and in vivo and did not affect the ability of the mutant to invade and colonize the small intestine, mesenteric lymph nodes, liver, or spleen of BALB/cByJ mice. Animals orally immunized with S. typhimurium chi 4064(pBA31-R7) developed serum and intestinal antibody responses to the B. abortus 31-kDa protein and to salmonella endotoxin as measured by enzyme-linked immunosorbent assay. Mice orally immunized with S. typhimurium chi 4064pBA31-R7 did not develop a delayed-type hypersensitivity following a footpad injection with recombinant BCSP31. Antigen-specific blastogenic data also support these in vivo results. All data indicate that this route of antigen delivery is effective for stimulating antibody-mediated immunity but that the B. abortus 31-kDa protein is a poor immunogen for inducing a cell-mediated immune response in BALB/cByJ mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker B. J., Tabatabai L. B., Deyoe B. L., Mayfield J. E. Conservation of antigenicity in a 31-kDa Brucella protein. Vet Microbiol. 1988 Dec;18(3-4):313–325. doi: 10.1016/0378-1135(88)90096-x. [DOI] [PubMed] [Google Scholar]
- Brown A., Hormaeche C. E., Demarco de Hormaeche R., Winther M., Dougan G., Maskell D. J., Stocker B. A. An attenuated aroA Salmonella typhimurium vaccine elicits humoral and cellular immunity to cloned beta-galactosidase in mice. J Infect Dis. 1987 Jan;155(1):86–92. doi: 10.1093/infdis/155.1.86. [DOI] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challacombe S. J. Salivary antibodies and systemic tolerance in mice after oral immunization with bacterial antigens. Ann N Y Acad Sci. 1983 Jun 30;409:177–193. doi: 10.1111/j.1749-6632.1983.tb26868.x. [DOI] [PubMed] [Google Scholar]
- Chen-Woan M., Sajewski D. H., McGregor D. D. T-cell co-operation in the mediation of acquired resistance to Listeria monocytogenes. Immunology. 1985 Sep;56(1):33–42. [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Lyon F. L., Lowe K. L., Farrand A. L., el-Morshidy S. Oral immunization of mice with attenuated Salmonella enteritidis containing a recombinant plasmid which codes for production of the B subunit of heat-labile Escherichia coli enterotoxin. Infect Immun. 1986 Sep;53(3):685–692. doi: 10.1128/iai.53.3.685-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix D. L., Malburny G. N., Vaerman J. P. Hepatobiliary transport of plasma IgA in the mouse: contribution to clearance of intravascular IgA. Eur J Immunol. 1985 Sep;15(9):893–899. doi: 10.1002/eji.1830150906. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Katz J., Michalek S. M., Curtiss R., 3rd, Harmon C., Richardson G., Mestecky J. Novel oral vaccines: the effectiveness of cloned gene products on inducing secretory immune responses. Adv Exp Med Biol. 1987;216B:1741–1747. [PubMed] [Google Scholar]
- Keren D. F., McDonald R. A., Carey J. L. Combined parenteral and oral immunization results in an enhanced mucosal immunoglobulin A response to Shigella flexneri. Infect Immun. 1988 Apr;56(4):910–915. doi: 10.1128/iai.56.4.910-915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- La Brooy J. T., Davidson G. P., Sherman D. J., Rowley D. The antibody response to bacterial gastroenteritis in serum and secretions. Clin Exp Immunol. 1980 Aug;41(2):290–296. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell D. J., Sweeney K. J., O'Callaghan D., Hormaeche C. E., Liew F. Y., Dougan G. Salmonella typhimurium aroA mutants as carriers of the Escherichia coli heat-labile enterotoxin B subunit to the murine secretory and systemic immune systems. Microb Pathog. 1987 Mar;2(3):211–221. doi: 10.1016/0882-4010(87)90022-2. [DOI] [PubMed] [Google Scholar]
- Mayfield J. E., Bricker B. J., Godfrey H., Crosby R. M., Knight D. J., Halling S. M., Balinsky D., Tabatabai L. B. The cloning, expression, and nucleotide sequence of a gene coding for an immunogenic Brucella abortus protein. Gene. 1988;63(1):1–9. doi: 10.1016/0378-1119(88)90540-9. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., Kiyono H., Wannemuehler M. J., Mosteller L. M., McGhee J. R. Lipopolysaccharide (LPS) regulation of the immune response: LPS influence on oral tolerance induction. J Immunol. 1982 May;128(5):1992–1998. [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Poirier T. P., Kehoe M. A., Beachey E. H. Protective immunity evoked by oral administration of attenuated aroA Salmonella typhimurium expressing cloned streptococcal M protein. J Exp Med. 1988 Jul 1;168(1):25–32. doi: 10.1084/jem.168.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D. Immune responses to enterobacteria presented by various routes. Prog Allergy. 1983;33:159–174. [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Justus C. W., Museteanu C., Simon M. M. Demonstration of antigen-specific T cells and histopathological alterations in mice experimentally inoculated with Borrelia burgdorferi. Infect Immun. 1989 Jan;57(1):41–47. doi: 10.1128/iai.57.1.41-47.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrake R. F., Husband A. J., Watson D. L., Cripps A. W. Selective transport of serum-derived IgA into mucosal secretions. J Immunol. 1984 Jan;132(1):363–368. [PubMed] [Google Scholar]
- Sidberry H., Kaufman B., Wright D. C., Sadoff J. Immunoenzymatic analysis by monoclonal antibodies of bacterial lipopolysaccharides after transfer to nitrocellulose. J Immunol Methods. 1985 Feb 11;76(2):299–305. doi: 10.1016/0022-1759(85)90307-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemuehler M. J., Kiyono H., Babb J. L., Michalek S. M., McGhee J. R. Lipopolysaccharide (LPS) regulation of the immune response: LPS converts germfree mice to sensitivity to oral tolerance induction. J Immunol. 1982 Sep;129(3):959–965. [PubMed] [Google Scholar]