Abstract
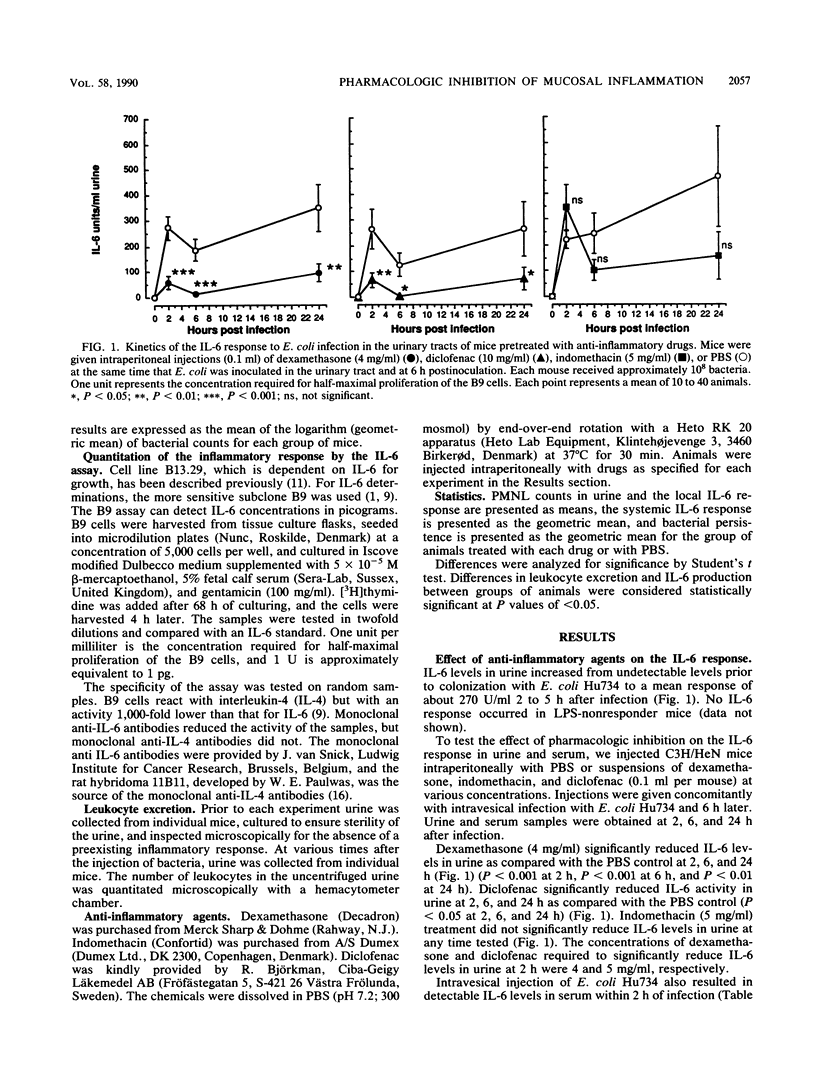
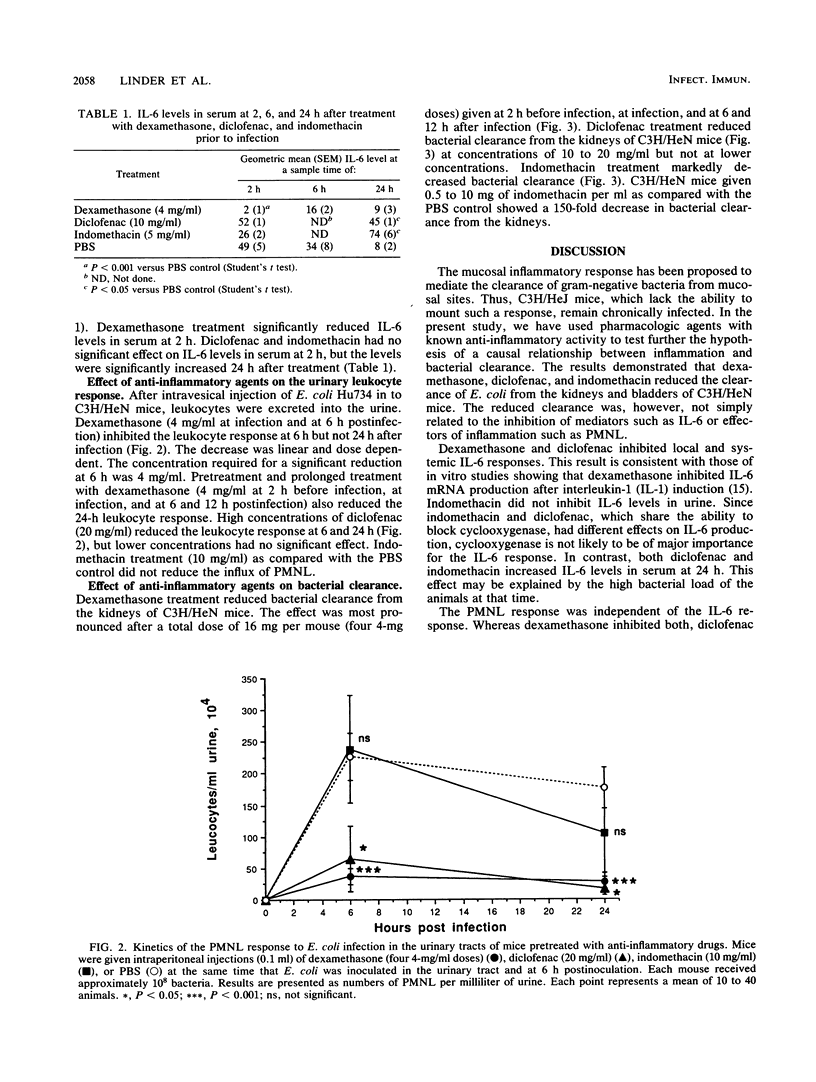
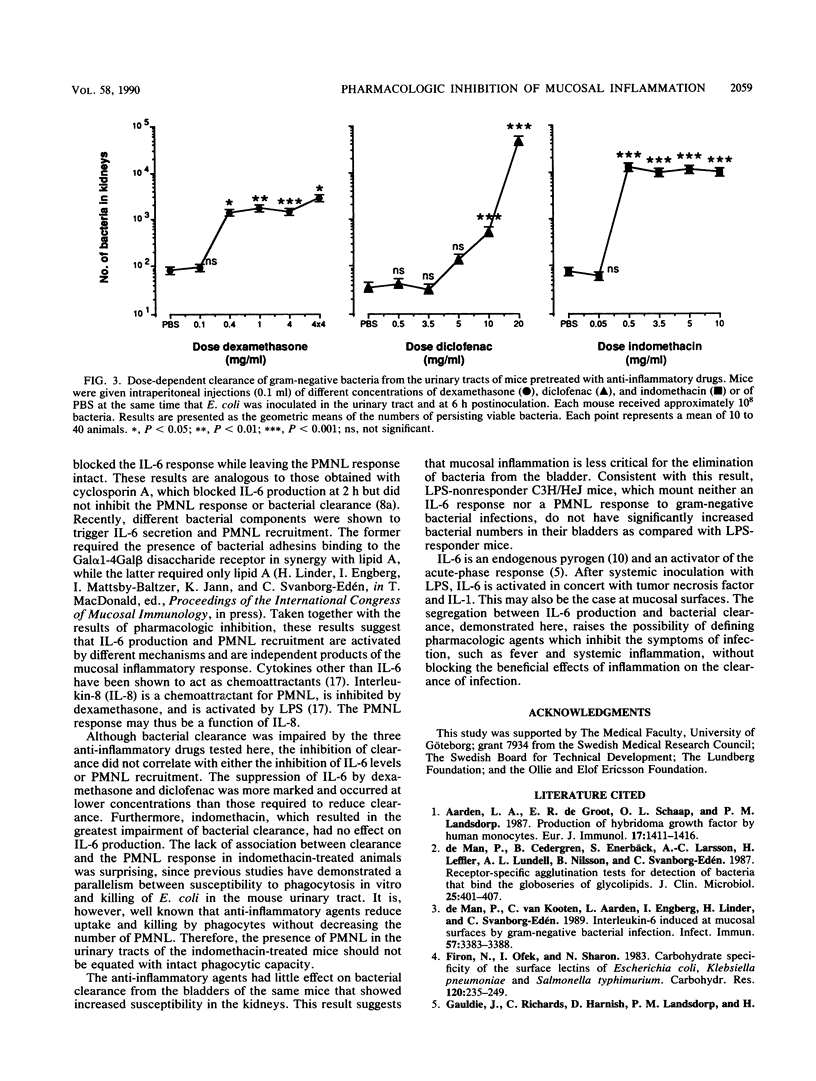
Gram-negative bacterial infections of the urinary tract elicit a mucosal inflammatory response. Interleukin-6 is secreted into the urine, and polymorphonuclear leukocytes (PMNL) are recruited. In the present study we examined the effect of anti-inflammatory agents on these parameters and on bacterial clearance from the kidneys. Dexamethasone reduced interleukin-6 secretion, the PMNL response, and bacterial clearance. Diclofenac abolished the urinary interleukin-6 response but reduced the PMNL response and bacterial clearance only at the highest concentrations. Indomethacin drastically decreased bacterial clearance without the corresponding effect on interleukin-6 production or the PMNL response. The results demonstrate that the inhibition of inflammation impairs bacterial clearance from the kidneys. This is, however, not a direct function of inhibited interleukin-6 production or PMNL recruitment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Freter R., Hagberg L., Hull R., Hull S., Leffler H., Schoolnik G. Inhibition of experimental ascending urinary tract infection by an epithelial cell-surface receptor analogue. Nature. 1982 Aug 5;298(5874):560–562. doi: 10.1038/298560a0. [DOI] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr Res. 1983 Aug 16;120:235–249. doi: 10.1016/0008-6215(83)88019-7. [DOI] [PubMed] [Google Scholar]
- Hagberg L., Engberg I., Freter R., Lam J., Olling S., Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983 Apr;40(1):273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., McGhee J. R., Michalek S. M., Svanborg Edén C. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun. 1984 Dec;46(3):839–844. doi: 10.1128/iai.46.3.839-844.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges S., Linder H., de Man P., Svanborg Edén C. Ciclosporin-dependent, nu-independent, mucosal interleukin 6 response to gram-negative bacteria. Scand J Immunol. 1990 Mar;31(3):335–343. doi: 10.1111/j.1365-3083.1990.tb02776.x. [DOI] [PubMed] [Google Scholar]
- Helle M., Boeije L., Aarden L. A. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988 Oct;18(10):1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Helle M., Brakenhoff J. P., De Groot E. R., Aarden L. A. Interleukin 6 is involved in interleukin 1-induced activities. Eur J Immunol. 1988 Jun;18(6):957–959. doi: 10.1002/eji.1830180619. [DOI] [PubMed] [Google Scholar]
- Lansdorp P. M., Aarden L. A., Calafat J., Zeiljemaker W. P. A growth-factor dependent B-cell hybridoma. Curr Top Microbiol Immunol. 1986;132:105–113. doi: 10.1007/978-3-642-71562-4_14. [DOI] [PubMed] [Google Scholar]
- Linder H., Engberg I., Baltzer I. M., Jann K., Svanborg-Edén C. Induction of inflammation by Escherichia coli on the mucosal level: requirement for adherence and endotoxin. Infect Immun. 1988 May;56(5):1309–1313. doi: 10.1128/iai.56.5.1309-1313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T., Nakai S., Kawakami T., Aihara K., Nishino N., Hirai Y. Dexamethasone regulation of the expression of cytokine mRNAs induced by interleukin-1 in the astrocytoma cell line U373MG. FEBS Lett. 1989 Jan 16;243(1):25–29. doi: 10.1016/0014-5793(89)81210-4. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Shahin R. D., Engberg I., Hagberg L., Svanborg Edén C. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J Immunol. 1987 May 15;138(10):3475–3480. [PubMed] [Google Scholar]
- de Man P., Cedergren B., Enerbäck S., Larsson A. C., Leffler H., Lundell A. L., Nilsson B., Svanborg-Edén C. Receptor-specific agglutination tests for detection of bacteria that bind globoseries glycolipids. J Clin Microbiol. 1987 Feb;25(2):401–406. doi: 10.1128/jcm.25.2.401-406.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Man P., van Kooten C., Aarden L., Engberg I., Linder H., Svanborg Edén C. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect Immun. 1989 Nov;57(11):3383–3388. doi: 10.1128/iai.57.11.3383-3388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


