Abstract
Anthropogenic disturbances intertwined with climatic changes can have a large impact on the upper trophic levels of marine ecosystems, which may cascade down the food web. So far it has been difficult to demonstrate multi-level trophic cascades in pelagic marine environments. Using field data collected during a 33-year period, we show for the first time a four-level community-wide trophic cascade in the open Baltic Sea. The dramatic reduction of the cod (Gadus morhua) population directly affected its main prey, the zooplanktivorous sprat (Sprattus sprattus), and indirectly the summer biomass of zooplankton and phytoplankton (top-down processes). Bottom-up processes and climate–hydrological forces had a weaker influence on sprat and zooplankton, whereas phytoplankton variation was explained solely by top-down mechanisms. Our results suggest that in order to dampen the occasionally harmful algal blooms of the Baltic, effort should be addressed not only to control anthropogenic nutrient inputs but also to preserve structure and functioning of higher trophic levels.
Keywords: Baltic Sea, pelagic marine ecosystem, food web, bottom-up versus top-down control, climate, eutrophication
1. Introduction
The sensitivity of different trophic levels to anthropogenic stress and climate variations has important implications in the functioning of pelagic ecosystems, as it may synchronize or uncouple ecological interactions the consequences of which may propagate through the food web (Edwards & Richardson 2004; Litzow & Ciannelli 2007). This is particularly relevant in the current period of rapid climate change which has resulted in changes in the distribution of top predators such as fishes (Perry et al. 2005) and lower trophic levels (Beaugrand et al. 2002). Community-wide trophic cascades, defined by top-down control by predators and the propagation of indirect mutualism between non-adjacent trophic levels, have been described in a variety of systems (Pace et al. 1999; Shurin et al. 2002). However, it has been difficult to demonstrate trophic cascades in real marine systems, although promising examples exist from manipulated marine mesocosms and field experiments (Micheli 1999; Shurin et al. 2002) as well as benthic communities (Estes et al. 1998; Halpern et al. 2006). On the other hand, in pelagic marine ecosystems, most of the evidence of top-down regulation is based on inverse relationships between two adjacent trophic levels, e.g. between top predators and their prey (Pauly et al. 1998; Worm & Myers 2003) or between planktivorous fish and zooplankton (Micheli 1999; Casini et al. 2006), although indications of three-level trophic cascades have been provided (Shiomoto et al. 1997). To our knowledge only two studies, one in the eastern Scotian Shelf (Frank et al. 2005) and one in the Black Sea (Daskalov et al. 2007), have showed clear evidence of trophic cascades involving more than three trophic levels in marine pelagic ecosystems. Moreover, the investigations that have revealed multi-level trophic cascades in marine systems have often overlooked the potential contemporary effects of bottom-up (resource-mediated) processes and climate–hydrological forces, which have the potential to affect entire food webs (e.g. Beaugrand & Reid 2003; Ware & Thomson 2005). The simultaneous investigation of top-down, bottom-up and hydrological forces would help reveal how resilient food webs are to the combined effect of resource-mediated and predator-mediated forces. This would also provide a deeper understanding of ecosystem functioning and elucidate the causes of ecosystem change (Menge et al. 1997; Winder & Schindler 2004).
Using the information collected during the past three decades (1974–2006), we investigated the potential occurrence in summer of a community-wide trophic cascade in the Baltic Sea pelagic ecosystem, involving four levels of the food web: piscivorous fish (the gadoid cod, Gadus morhua), zooplanktivorous fish (the clupeids: sprat, Sprattus sprattus and herring, Clupea harengus), zooplankton and phytoplankton. The relative importance of both bottom-up processes and climate–hydrological factors was also estimated. We focus on the past three decades because they encompass the period of both cod and clupeid analytical stock assessment estimates (International Council for the Exploration of the Sea (ICES) 2007) as well as of regular field measurement of the lower trophic levels (i.e. zooplankton and phytoplankton). This period is characterized by very low population levels of the piscivorous seals (grey seal Halichoerus grypus, ringed seal Phoca hispida and common seal Phoca vitulina) and harbour porpoises (Phocoena phocoena) which were abundant at the beginning of the 1900s, but decreased drastically afterwards due to human activities (Österblom et al. 2007). Hence, in our study period cod has been, together with man, the dominant top predator in the open Baltic Sea (Harvey et al. 2003). We show evidence that during summer, top-down processes are stronger than both bottom-up and climate–hydrological forces in the Baltic Sea pelagic ecosystem.
2. Material and methods
(a) Data
Time series of cod biomass (age 2+) and biomass and abundance of sprat and herring (age 1+) at the start of the year in the Baltic Sea were calculated using a virtual population analysis and retrieved from the annual stock assessment report (ICES 2007). The Latvian Fish Resources Agency (LATFRA) provided data on the density (abundance per 1 m3) of the major zooplankton species in the Gotland Basin (central Baltic Sea, i.e. the copepods Pseudocalanus spp., Temora longicornis, Acartia spp. and the cladocerans Bosmina coregoni maritima, Evadne nordmanni and Podon spp.). These species represent also the main zooplankton prey for clupeids in the study area (Kornilovs et al. 2001; Möllmann et al. 2004). The biomass of each zooplankton species per 1 m3 was estimated using standard wet weights (Hernroth 1985). Sampling was performed in daytime at several depths from the surface down to a maximum depth of 100 m (or to sea bottom for shallower stations). Further details on sampling procedure and plankton identification are described in Kornilovs et al. (2001). The zooplankton data were constructed averaging the biomass calculated for the deeper (more than 100 m depth) and shallower (50–80 m depth) stations. Chlorophyll a (μg l−1) sampled in the Gotland Basin was extracted from the Helsinki Commission (HELCOM) database stored in the ICES website (www.ices.dk). Chlorophyll a was averaged over 0–20 m depth (samples at surface and at 5 m depth intervals). The profiles of temperature, salinity and nutrients (nitrate and phosphate), collected monthly in the Gotland Basin, were provided by the Swedish Meteorological and Hydrological Institute (SMHI; available at www.smhi.se). Water temperature (°C) and salinity (psu) were averaged over the 0–100 m depth strata (samples at surface and at 10 m depth intervals). Nutrients (μmol l−1) were averaged over 0–50 m depth (samples at surface and at 10 m depth intervals). North Atlantic Oscillation (NAO) winter index (December–March) was used as an indicator of large-scale climate forcing (available at the Climate Analysis Section website www.cgd.ucar.edu/cas/jhurrell/indices.html). NAO in winter is acknowledged to influence spring and summer hydrological and biological features in the Baltic Sea (Dippner et al. 2001; Hänninen et al. 2003; MacKenzie & Köster 2004).
Instead of using annual mean values, we focused our analysis on the summer period, which corresponds to the annual peak of zooplanktivorous fish feeding intensity (Aro 1989; Möllmann et al. 2004) and zooplankton production (Möllmann et al. 2000). This offers the possibility to investigate potential multilevel top-down regulation. In fact, the seasonality characteristic of temperate environments implies the occurrence of season-specific trophic interactions (Worm et al. 2000), which could be masked if annual averages are employed. Also, summer corresponds to the season of occurrence of cyanobacterial blooms (Finni et al. 2001) and to the current main spawning season of cod (Wieland et al. 2000). Data from other seasons, however, were also used in order to investigate the bottom-up and climate-hydrological forces acting on the trophic levels.
(b) Statistical analysis
The potential occurrence of trophic cascades was initially investigated using Pearson correlations and cumulative z-scores (cumulative sum methods, e.g. Molinero et al. 2005). The z-scores are standardized anomalies, calculated by taking the deviations from the mean of the investigated time series and dividing by the standard deviation. Plots of the cumulative z-scores indicate periods with predominantly positive or negative anomalies in the time series (shown by upward or downward trends in the z-scores), and can be used to detect in a simple way the intensity and duration of homogenous periods within the time series (Molinero et al. 2005).
Successively, the relative importance of top-down, bottom-up and climate–hydrological forces on the different trophic levels was investigated by general linear models (GLMs). Firstly, for each trophic level, all the hypothesized predictors (selected a priori on the basis of recognized ecological, biological and physiological mechanisms; table S1 in the electronic supplementary material) were included in the GLM analysis (initial model). Secondly, a backward stepwise model selection based on the Mallows' Cp information criteria (Mallows 1973) was applied to find the best possible subset of predictors (final model).
The Mallows' Cp is computed as
where yp is the predicted value of y from the p predictors; s2 is the residual mean square after regression on the complete set of predictors; and n is the sample size.
Cp, similar to other model selection criteria, accounts simultaneously for the degrees of freedom used and the goodness of fit, and tends to find the best subset that includes only the most important predictors among the hypothesized response variables. Therefore, a model with lower Cp has more explanatory power, and hence is preferred, compared with a model with higher Cp. At each step of the backward stepwise model selection procedure, the models were screened by the ecological criterion, which implies that the sign of the relationship between certain variables cannot be accepted although selected by the GLM owing to the lack of ecological basis (Dippner et al. 2001; Casini et al. 2006). For example, there is no ecological basis for temperature to affect negatively sprat abundance in the Baltic Sea (MacKenzie & Köster 2004). Thus if some of the relationships found in the models were not fulfilling the ecological criterion, the variable was excluded and the backward selection was continued. The final models were, therefore, selected based on the following criteria when fulfilled at the same time: parsimonious principle (the largest amount of deviance explained with the minimum number of predictors, i.e. with the lowest Cp) and meaningful ecological relationships. We calculated the proportion of the total deviance explained by the initial and final models, the probability value of the models and the proportion of the total deviance explained by each predictor. Residuals of the final models were analysed using graphical methods (Cleveleland 1993) to check for departure from the model assumptions or other anomalies in the data. Residuals were also tested for autocorrelation. Before the GLM analyses, missing values were estimated using a linear trend regression (five data points for chlorophyll a, three data points for zooplankton and nutrients and two data points for salinity and temperature) and data standardized (X=X−mean/s.d.). The outcomes of GLMs were also compared with the results of generalized additive models (GAMs) using the same parameters and procedure to evaluate the robustness of the observed patterns to the analytical tool used.
For the lower trophic levels (zooplankton and phytoplankton), the values of the predictors were taken in the same year of the response variables, since we assumed that the high turnover rates of zooplankton and phytoplankton would make them responding promptly (the same year) to changes in the environment. On the other hand, for sprat the use of a different approach was needed due to the co-occurrence in the population of several cohorts persisting in time (years). Therefore, the input data for the GLM analysis for sprat were prepared following two different approaches: (i) we tested the effect of the predictors on sprat population with 1-year lag (sprat biomass and abundance are calculated for the 1 January of each year, therefore the predictors at t−1 can be compared to sprat population). This approach gives a snapshot of the effect of the predictors on the response; (ii) we constructed the predictors' time series as to represent their potential influence on sprat population over the period of existence of a cohort. Since the sprat population is mainly constituted of eight age classes (from 1 to 7, plus the 8+ constituted of all the ages older than 7 years pooled together; ICES 2007), sprat biomass and abundance at time t are the result of forces that have acted over the previous 8 years (i.e. at years t−1, t−2, …, t−8). However, since on average approximately 90% of the sprat population are constituted of 1- to 4-year-old fish (ICES 2007), we used predictor values only from t−1 to t−4 in this analysis, which decreased the influence of age classes scarcely represented in the sprat population and the loss of too many data points for the analysis. The responses (sprat biomass and abundance) at time t, thus, were related to the 4-year mean (at time t−1, …, t−4) of each predictor. The same approach was used by MacKenzie & Köster (2004) in the investigation of temperature effects on Baltic sprat landings 3 years ahead. The Gotland Basin represents the area with the most complete time series in the SMHI database and most extensively covered by zooplankton sampling. Hydrological conditions (but also zooplankton time series) are highly intercorrelated between the main areas of sprat occurrence and recruitment (Möllmann et al. 2000; MacKenzie & Köster 2004). Thus, hydrological and zooplankton data from other areas would most probably have the same effect on the sprat population as those from the Gotland Basin (MacKenzie & Köster 2004). Only sprat was initially included in the analysis because this species is by far the main prey for cod (Horbowy 1996; ICES 2006) and the major zooplantivore (Rudstam et al. 1992; Casini et al. 2004) in the open Baltic Sea. We do not exclude, however, the impact of other pelagic fish on zooplankton. For this reason, we also used herring in the GLMs, both as single predictor and pooled together with sprat (total clupeids).
Statistical analyses were performed using Statistica v. 6, S-PLUS v. 6.1 for Windows, and R.
3. Results
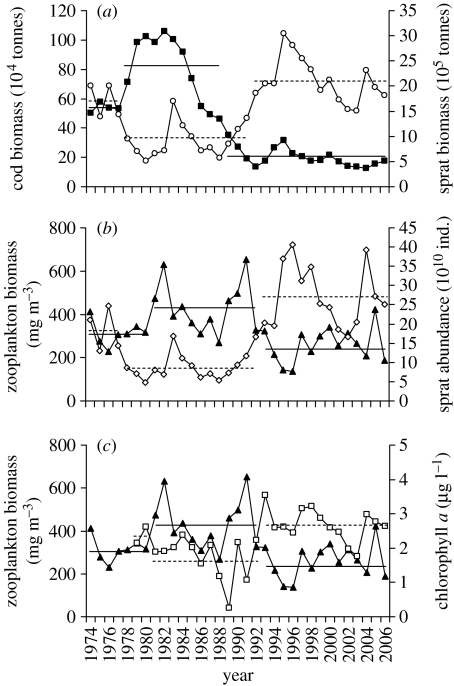
We observed a community-wide trophic cascade in the Baltic Sea (figure 1a–c) caused by the sharp decline in cod biomass which began in the early 1980s. Since the early 1990s, the cod stock has been low and has not shown any tendency to recover (figure 1a). The severe decline of the top piscivorous fish has been followed by a drastic increase in its main prey, the zooplanktivorous sprat (figure 1a,b). Also, during the mid-1970s the sprat population was fairly high coinciding with a relatively low cod biomass. The correlations between cod biomass and sprat biomass and abundance were negative (respectively, r=−0.63 and −0.60, n=33). Since the mid-1990s, the sprat stock has been high, although rather variable (figure 1a,b). During the observed period, total zooplankton biomass first increased and then decreased following inverse sprat population development (sprat biomass–zooplankton biomass, r=−0.53, n=33; sprat abundance–zooplankton biomass, r=−0.59, n=33; figure 1b), whereas phytoplankton biomass (chlorophyll a) showed an inverse pattern to zooplankton biomass (r=−0.47, n=28; figure 1c). The negative relationships between adjacent trophic levels were also identified by the z-scores (figure S1 in the electronic supplementary material).
Figure 1.
Trends of (a) cod biomass (squares) and sprat biomass (circles); (b) sprat abundance (diamonds) and zooplankton biomass (triangles) (ind., individuals); and (c) zooplankton biomass (triangles) and chlorophyll a (squares). The horizontal lines indicate periods of different average levels in the biological time series as detected by the cumulative z-scores (see text and figure S1 in the electronic supplementary material).
Cross-correlation function and z-scores demonstrated that the highest correlation between cod biomass and sprat biomass occurred at a lag of +3 years (r=−0.71, n=33) and between cod biomass and sprat abundance at a lag of +5 years (r=−0.73, n=33). The delay can be explained not only by the cumulative direct effect of cod feeding on the same sprat cohort during several successive years but also by the indirect effects of cod predation on sprat reproductive output which will have a delayed effect on the sprat population. On the other hand, zooplankton and phytoplankton responded promptly (the same year) to the increase in sprat abundance. Non-adjacent trophic levels were positively correlated (cod–zooplankton at a lag of +3 years, r=0.46, n=33; sprat biomass–phytoplankton, r=0.62, n=28; sprat abundance–phytoplankton r=0.63, n=28), suggesting the existence of indirect mutualism between non-adjacent trophic levels and reinforcing the evidence of trophic cascades in the Baltic Sea.
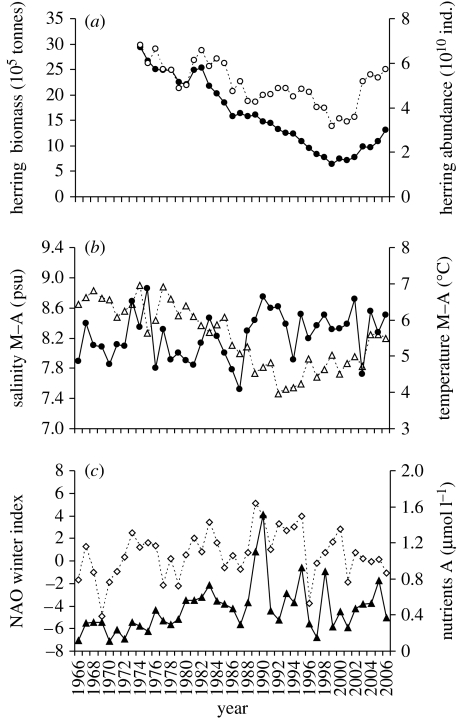
The stock of the other main prey for cod in the Baltic Sea, the herring, decreased until the beginning of 2000 when a slight increase started to occur (figure 2a). Temperature showed a general albeit weak increasing trend, whereas salinity strongly dropped up to the early 1990s and increased afterwards (figure 2b). The NAO winter index (December–March) presented strong inter-annual oscillations, with a slight increase up to the early 1990s followed by a decrease. Total summer nutrients concentration rose from the 1960s up to the early 1990s and levelled off afterwards (figure 2c).
Figure 2.
(a) Trends of herring biomass (closed circles) and abundance (open circles; ind., individuals; ICES 2007); (b) trends in temperature (circles) and salinity (triangles; average of May and August (M–A) data integrated over 0–100 m depth); and (c) trends in NAO winter index (diamonds; December–March) and August (A) nutrients (triangles; nitrate+phosphate integrated over 0–50 m depth). Data from 1966 are illustrated in order to better show the long-time trends of the abiotic factors.
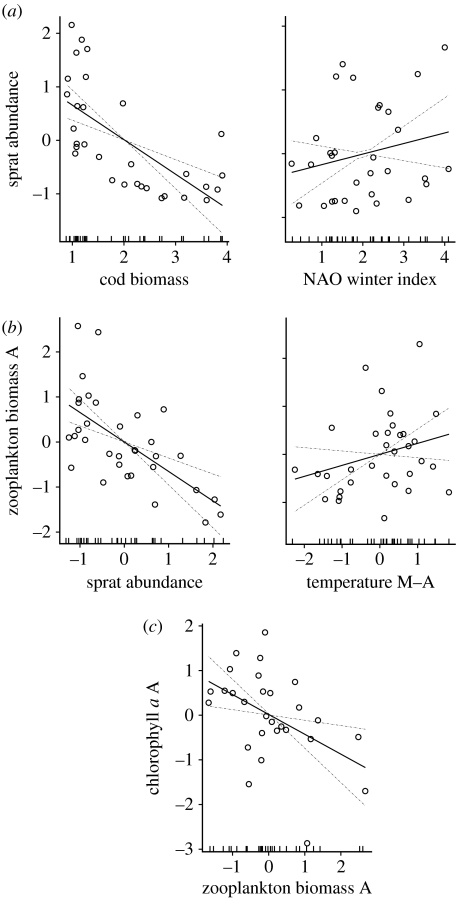
The results of the GLM modelling showed that top-down forces played the most important role in shaping each trophic level (tables 1 and 2; figure 3a–c). Concerning the sprat models, cod biomass explained the largest proportion of models' deviance irrespective of the approach used to construct the predictors' time series. We show here the outcome of the sprat model approach (ii) (the results of the approach (i) are presented in the electronic supplementary material, table S2). Accordingly, the predictors included in the sprat biomass initial model were cod biomass, larval sprat prey biomass in May (Acartia spp. and T. longicornis) and NAO winter index (initial model, 59.2% of the deviance explained), since temperature and summer zooplankton biomass did not fulfil the ecological criterion (table 1). Cod biomass and NAO were also included in sprat final model (56.6% of the deviance explained), since the elimination of either of those would have increased the Cp of the model. Cod and NAO explained, respectively, 76.7 and 23.3% of the deviance of the sprat biomass final model (table 2). Also in the sprat abundance model, only cod biomass and NAO were the predictors present in the final model (48.6% of the deviance explained by the model), with cod explaining almost all the deviance (table 2; figure 3a). The results of the GLM using the 4-year mean of the predictors (i.e. averaged at t−1, …, t−4, see §2) were very similar to those using either 3- and 5-year means (predictors averaged, respectively, at t−1, …, t−3 and t−1, …, t−5). Sprat fishing mortality was not included as a predictor in the sprat models because it followed a pattern similar to sprat stock development (figure S2 in the electronic supplementary material); this probably indicates that during the past three decades, the sprat stock level has influenced sprat fishing mortality (a higher population level usually allows the fishing quotas to raise) and not the opposite (ICES 2007).
Table 1.
Results of the GLM analyses, initial models. (Predictors, proportion of the deviance explained by the models, Cp and probability of the models are indicated. The proportion of the model deviance explained by each predictor (PED (%)) is also indicated. The empty cells indicate that the corresponding predictor did not fulfil the ecological criterion and, thus, was discarded from the analysis. J, January, M, May; and A, August. The sign of the relationships between the responses and the predictors and the number of observations (n) are also indicated.)
| initial models | predictors | d.f. | deviance explained (%) | Cp | Pr | PED (%) | sign | n |
|---|---|---|---|---|---|---|---|---|
| sprat biomassa (approach (ii)) | cod biomass | 54.9 | − | 31 | ||||
| zooplankton A | ||||||||
| preys for larvae M | 4.4 | + | 31 | |||||
| temperature J–M | ||||||||
| NAO winter index | 40.7 | + | 31 | |||||
| model | 3 | 59.2 | 16.76 | <0.0001 | ||||
| sprat abundancea (approach (ii)) | cod biomass | 90.2 | − | 31 | ||||
| zooplankton A | ||||||||
| preys for larvae M | 4.9 | + | 31 | |||||
| temperature J–M | ||||||||
| NAO winter index | 4.9 | + | 31 | |||||
| model | 3 | 51.1 | 20.03 | <0.0001 | ||||
| zooplankton biomassb | sprat biomass | 70.5 | − | 33 | ||||
| chlorophyll a M–A | ||||||||
| temperature M–A | 7.7 | + | 33 | |||||
| salinity M–A | 1.5 | + | 33 | |||||
| NAO winter index | 20.3 | + | 33 | |||||
| model | 4 | 40.3 | 25.92 | 0.002 | ||||
| zooplankton biomassb | sprat abundance | 82.7 | − | 33 | ||||
| chlorophyll a M–A | ||||||||
| temperature M–A | 11.3 | + | 33 | |||||
| salinity M–A | 2.4 | + | 33 | |||||
| NAO winter index | 3.6 | + | 33 | |||||
| model | 4 | 41.6 | 25.35 | 0.001 | ||||
| chlorophyll ac | zooplankton A | 91.0 | − | 28 | ||||
| nutrients A | ||||||||
| temperature A | 7.8 | + | 28 | |||||
| salinity A | 1.2 | + | 28 | |||||
| model | 3 | 24.4 | 27.25 | 0.05 |
Temperature before and during spawning (winter and spring) and NAO winter index can affect sprat recruitment via acting on adult growth, gonadal maturation, fecundity and on the survival of eggs and larvae (MacKenzie & Köster 2004; Nissling 2004). Temperature in winter and spring are highly correlated (r=0.63; Pr<0.0001; n=33). Prey for sprat larvae are constituted of Acartia spp. and T. longicornis (Grauman et al. 1986; Voss et al. 2003). Zooplankton in August is a proxy of the energy which can be accumulated by sprat before the overwintering period (Casini et al. 2006).
All the major zooplankton species included in the analysis reach their annual peak in biomass during summer, although some species reproduce mainly in spring and others in summer (Möllmann et al. 2000). Thus, an average of both temperature and salinity between May and August was taken, although they are correlated (r=0.74 for temperature and r=0.81 for salinity; Pr<0.0001 in both cases; n=33).
Only the data of August predictors were used, since in summer there is a secondary peak of chlorophyll a (Fleming & Kaitala 2006), the intensity of which is not related to the spring peak (this study, see text).
Table 2.
Results of the GLM analyses, final models. (Predictors, proportion of the deviance explained by the models, Cp and probability of the models are indicated. The proportion of the model deviance explained by each predictor (PED (%)) is also indicated. M, May and A, August. The sign of the relationships between the responses and the predictors and the number of observations (n) are also indicated.)
| final models | predictors | d.f. | deviance explained (%) | Cp | Pr | PED (%) | sign | n |
|---|---|---|---|---|---|---|---|---|
| sprat biomass (approach (ii)) | cod biomass | 76.7 | − | 31 | ||||
| NAO winter index | 23.3 | + | 31 | |||||
| model | 2 | 56.6 | 16.54 | <0.0001 | ||||
| sprat abundance (approach (ii)) | cod biomass | 94.9 | − | 31 | ||||
| NAO winter index | 5.1 | + | 31 | |||||
| model | 2 | 48.6 | 19.71 | <0.0001 | ||||
| zooplankton biomass | sprat biomass | 77.6 | − | 33 | ||||
| NAO winter index | 22.4 | + | 33 | |||||
| model | 2 | 36.6 | 24.37 | <0.001 | ||||
| zooplankton biomass | sprat abundance | 88.0 | − | 33 | ||||
| temperature M–A | 12.0 | + | 33 | |||||
| model | 2 | 39.1 | 23.51 | <0.001 | ||||
| chlorophyll a | zooplankton A | 100.0 | − | 28 | ||||
| model | 1 | 22.2 | 24.39 | 0.008 |
Figure 3.
Results of the GLM final models for (a) sprat abundance, (b) zooplankton biomass A (August) and (c) chlorophyll a A (August). The effect of each selected predictor on the response variables is shown. In the zooplankton model, we chose to show sprat abundance rather than biomass as top-down force due to the strong density-dependent body growth of Baltic sprat (Casini et al. 2006). The statistics of the GLM analyses are presented in table 2.
The parameters included in the zooplankton biomass initial model were sprat biomass, temperature and salinity (average May–August) and NAO winter index (40.3% of the deviance explained by the initial model), whereas chlorophyll a (average May–August) was excluded from the analysis since it did not fulfil the ecological criterion (because it was negatively correlated to zooplankton, contradicting the expectation of bottom-up regulation; table 1). Only sprat biomass and NAO were present in the final model (36.6% of the deviance explained), with sprat biomass explaining the largest fraction of the deviance (table 2). The use of sprat abundance as a top-down predictor improved both the initial and final zooplankton models (41.6 and 39.1 of the deviance explained, respectively; tables 1 and 2; figure 3b). The same results were obtained when using chlorophyll a in May and August separated as single predictors. When herring abundance or biomass was introduced in the zooplankton GLM as a predictor, they were excluded because they did not fulfil the ecological criterion (their relation to zooplankton had slightly positive signs). Moreover, using total clupeid (sprat+herring) biomass or abundance as a top-down force, the deviance explained by both the initial and final models was lower than that in the models with only sprat (table S3 in the electronic supplementary material). Zooplankton biomasses in spring and summer were not correlated (r=−0.04, Pr=0.84, n=33).
The phytoplankton (i.e. chlorophyll a) initial model included zooplankton biomass, temperature and salinity (which together explained 24.4% of the deviance), with zooplankton biomass explaining almost all the model deviance (table 1). Zooplankton biomass was the only predictor present in the final model (22.2% of the deviance explained; table 2; figure 3c). The same final model was obtained when using temperature, salinity and nutrients integrated over the 0–20 m depth interval, as well as with nutrients separated in nitrate and phosphate. Chlorophyll a values in spring and summer were not correlated (r=−0.26, Pr=0.18, n=28).
Residuals of the final models were homogeneous and did not strongly violate normality assumption. Residuals were not autocorrelated, except for the sprat models in which they showed a very weak autocorrelation at lag 1 (figure S3 in the electronic supplementary material). GLM analyses showed results consistent with GAMs, which is a predominant importance of top-down forces on climate-hydrological forces on all the trophic levels.
4. Discussion
Our study provides evidence that changes at the top of the food web can affect the entire ecosystem down to the level of the primary producers in open marine systems. This also highlights the importance of ecosystem-wide top-down control in pelagic marine ecosystems, which are generally considered to be mainly regulated by bottom-up processes and climate variations (e.g. Beaugrand & Reid 2003; Ware & Thomson 2005). The strength of trophic cascades, in fact, is considered generally weak in marine pelagic habitats (Shurin et al. 2002), and multi-level top-down regulation has been very seldom reported from marine open systems (Frank et al. 2005; Daskalov et al. 2007). The detection of a clear trophic cascade in the semi-enclosed Baltic Sea was probably simplified by the relatively low complexity of its ecosystem, characterized by low diversity, simple food web and weak omnivory, factors that make ecosystems particularly prone to top-down regulation (Pace et al. 1999; Bascompte et al. 2005; Frank et al. 2007).
Here we showed for the Baltic Sea that the decrease in the main top predator (the piscivorous cod) cascaded down the food web, directly affecting its main prey (the zooplanktivorous sprat) and indirectly zooplankton and phytoplankton. The cod drop is probably mostly related to high fishing pressure, but was also facilitated by recruitment failure caused by the lack of salt- and oxygen-rich water inflows from the North Sea which reduced the water volume suitable for cod reproduction (ICES 2006, 2007). The explosion of sprat after the cod collapse occurred probably because (i) in the current Baltic Sea ecosystem cod is the most important piscivore (Harvey et al. 2003; ICES 2006) and (ii) sprat is the main prey for cod in the Baltic Sea (Horbowy 1996; ICES 2006). The increase in the sprat population due to predation release has evidently not been hampered by the increased fishing mortality. Our analysis showed that climate changes (i.e. positive phase of the NAO) have contributed to the outburst of sprat population, probably enhancing growth and gonadal maturation of the spawners as well as the production and survival of eggs and larvae (MacKenzie & Köster 2004; Nissling 2004). A positive phase of NAO may also potentially increase the production of the main food items for larval sprat (Alheit et al. 2005; Möllmann et al. 2008), i.e. the copepods Acartia spp. and T. longicornis (Grauman et al. 1986; Voss et al. 2003). The other main prey for Baltic cod, the herring, did not promptly react to the decrease in the top-predator probably owing to the considerably lower predation mortality experienced by herring compared with sprat, irrespective of prey species abundance (ICES 2006). However, other factors could have delayed herring recovery during the last two decades, including high fishing pressure (ICES 2007) and degradation of coastal spawning grounds due to eutrophication (Cederwall & Elmgren 1990).
The strong relationship between sprat and zooplankton is largely explained by the strict zooplanktivorous nature of sprat (Rudstam et al. 1992; Casini et al. 2004) and is mechanistically supported by the strong density-dependent fluctuations in sprat growth due to feeding competition in the Baltic Sea (Casini et al. 2006). This appears to have had cascading effects even on piscivorous seabirds (Österblom et al. 2006). Möllmann et al. (2008) showed that sprat can exert significant control on the population of the copepod Pseudocalanus spp. during spring, when this plankter alone constitutes nearly the entire zooplankton biomass. During summer, on the other hand, the zooplankton diversity largely increases in the Baltic Sea and sprat feed intensively on all the main zooplankton species (Kornilovs et al. 2001; Möllmann et al. 2004). Summer corresponds also to the most important period in shaping sprat body condition before overwintering (Casini et al. 2006). Overall, these facts emphasize the strong sprat–zooplankton community interaction in summer showed here. According to the literature (e.g. Möllmann et al. 2000; Hänninen et al. 2003), important factors regulating zooplankton in the Baltic are also salinity, temperature and climate in general, with the different species having specific abiotic preferences. However, our study showed that zooplankton biomass at the community level is mainly regulated by sprat predation. Particularly interesting is the inability of herring to regulate summer zooplankton in the open sea even in periods of high herring population level, as in the 1970s and early 1980s which corresponded to a period of relatively low sprat stock (ICES 2007). Herring is an obligate zooplanktivore only during its early life stages that are confined to coastal areas (Aro 1989), and switch to nektobenthic preys as a function of size and age (Rudstam et al. 1992; Casini et al. 2004), this probably weakens the magnitude of the interaction strength between herring and zooplankton in the open Baltic. Evidently, the different spatial and ontogenetic patterns in the feeding habits of herring and sprat do not permit herring to fill the functional gap as zooplankton regulator in the open areas of the Baltic Sea during periods of low sprat abundance. This reinforces the general view that in the open Baltic Sea sprat is a keystone species, not only as the main prey for cod but also as major regulator of the lower trophic levels.
Sprat, in turn, via regulating zooplankton dynamics, also indirectly affect summer phytoplankton biomass. These results suggest that top-down forces acting on plant biomass in pelagic habitats may be more important than commonly thought, and contradict the common view that eutrophication and climate changes have been the main causes of the recent increase in summer phytoplankton production in the Baltic Sea (e.g. Finni et al. 2001). Previous studies have shown that the spring phytoplankton biomass (chlorophyll a) also increased during the last decades in the central Baltic (Wasmund et al. 1998). However, while the intensity of the spring bloom is mainly affected by the winter nutrient level (Wasmund et al. 1998; Fleming & Kaitala 2006), we propose that the summer bloom is mostly top-down driven. The absence of relationship between spring and summer chlorophyll a also suggests that spring and summer blooms are not directly coupled but are possibly driven by different mechanisms. Micheli (1999) showed that the presence of zooplanktivores can control herbivores in open pelagic ecosystems, but mesozooplankton commonly has no effect on the phytoplankton. On the other hand, mesocosm experiments indicated that the indirect effect of zooplanktivores on phytoplankton may become significant when nutrients are added (Micheli 1999), results that are also corroborated in some benthic habitats (e.g. Deegan et al. 2007). Thus, the clear positive effect of increased sprat on the summer phytoplankton in the Baltic Sea might have been aided by the high level of eutrophication. This interpretation is supported by simple food chain models (Oksanen et al. 1981), although other experimental studies have shown no consistent effect of system productivity on the magnitude of the herbivore effect on plant (e.g. Borer et al. 2005). In the Baltic Sea, the summer phytoplankton community is largely constituted of cyanobacteria whose intense blooms have before been attributed to eutrophication and high temperature (Finni et al. 2001). However, cyanobacterial blooms can be directly suppressed before the build up process of the filamentous cover when there is sufficient amount of grazing zooplankton in the system (e.g. Chan et al. (2006) and references therein). In the Baltic Sea, in particular, zooplankton is acknowledged to feed also on the toxic species of cyanobacteria (see Karjalainen et al. (2007) and references therein). Hence, it can be suggested that the current sprat predation-induced low biomass of summer zooplankton may have increased the probability for cyanobacterial blooms to occur in the Baltic Sea.
5. Conclusions
Our results emphasize the importance of preserving ecosystem structure and functioning. We showed that a large decrease of the cod population has cascaded down the food web influencing the whole open Baltic Sea ecosystem, from planktivorous fish to primary producers. These findings highlight the fact that, in order to dampen the summer blooms of phytoplankton, often characterized by potentially toxic species (Karjalainen et al. 2007), effort should be made not only to control anthropogenic nutrient inputs but also to prevent large changes at the higher levels of the food web. This is especially urgent in naturally low-diversity ecosystems, as the Baltic Sea, where there is no or lower species compensation. A relevant question to ask, given that trophic cascades can be viewed as a major perturbation of the systems, is whether the observed changes are easily reversible or not. In fact, in the current Baltic Sea ecosystem, the zooplanktivorous cod larvae may suffer food competition with the high sprat population which can, in turn, undermine both cod and ecosystem recovery. There is, therefore, the need for linking the complexity underlying food web dynamics to ecological resilience (Carpenter & Folke 2006) for the Baltic Sea both in space and time. However, the population development of other organisms (e.g. jellyfish and invasive ctenophores; Barz et al. 2006; Haslob et al. 2007) should be monitored and their impact on the Baltic food web must be carefully evaluated, especially in scenarios of rapid climate change.
Acknowledgments
We are very grateful to the Latvian Fish Resources Agency that furnished the zooplankton raw data. Profiles of temperature, salinity and nutrients were kindly provided by the Swedish Meteorological and Hydrological Institute (SMHI), SHARK database. We also thank the Helsinki Commission and the International Council for the Exploration of the Sea (ICES) for the chlorophyll a profiles. We are grateful to F. Vitale and A. Belgrano for their valuable comments on previous drafts of the manuscript, and V. Bartolino for statistical assistance with the GLMs. The constructive criticisms of three anonymous reviewers considerably improved the manuscript. Mic. Cas. was partially funded by Oscar och Lili Lamms Minne Stiftelsen. J.-C.M. is funded by the priority programme AQUASHIFT (the impact of climate variability on aquatic ecosystems; IFM-GEOMAR).
Supplementary Material
Three tables, three figures and references
References
- Alheit J, Möllmann C, Dutz J, Kornilovs G, Loewe P, Mohrholz V, Wasmund N. Synchronous ecological regime shifts in the central Baltic and the North Sea in the late 1960s. ICES J. Mar. Sci. 2005;62:1205–1215. doi:10.1016/j.icesjms.2005.04.024 [Google Scholar]
- Aro E. A review of fish migration patterns in the Baltic. Rapp. P.-v. Réun. Cons. int. Explor. Mer. 1989;190:72–96. [Google Scholar]
- Barz K, Hinrichsen H.-H, Hirche H.-J. Scyphozoa in the Bornholm Basin (central Baltic Sea)—the role of advection. J. Mar. Syst. 2006;60:167–176. doi:10.1016/j.jmarsys.2006.01.002 [Google Scholar]
- Bascompte J, Melián C.J, Sala E. Interaction strength combinations and the overfishing of a marine food web. Proc. Natl Acad. Sci. USA. 2005;102:5443–5447. doi: 10.1073/pnas.0501562102. doi:10.1073/pnas.0501562102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugrand G, Reid P. Long-term changes in phytoplankton, zooplankton and salmon related to climate. Glob. Change Biol. 2003;9:801–817. doi:10.1046/j.1365-2486.2003.00632.x [Google Scholar]
- Beaugrand G, Reid P.C, Ibanez F, Lindley J.A, Edwards M. Reorganization of North Atlantic marine copepod biodiversity and climate. Science. 2002;296:1692–1694. doi: 10.1126/science.1071329. doi:10.1126/science.1071329 [DOI] [PubMed] [Google Scholar]
- Borer E.T, Seabloom E.W, Shurin J.B, Anderson K.E, Blanchette C.A, Broitman B, Cooper S.D, Halpern B.S. What determines the strength of a trophic cascade? Ecology. 2005;86:528–537. doi:10.1890/03-0816 [Google Scholar]
- Carpenter S.T, Folke C. Ecology for transformation. Trends Ecol. Evol. 2006;21:309–315. doi: 10.1016/j.tree.2006.02.007. doi:10.1016/j.tree.2006.02.007 [DOI] [PubMed] [Google Scholar]
- Casini M, Cardinale M, Arrhenius F. Feeding preferences of herring (Clupea harengus) and sprat (Sprattus sprattus) in the southern Baltic Sea. ICES J. Mar. Sci. 2004;61:1267–1277. doi:10.1016/j.icesjms.2003.12.011 [Google Scholar]
- Casini M, Cardinale M, Hjelm J. Inter-annual variation in herring (Clupea harengus) and sprat (Sprattus sprattus) condition in the central Baltic Sea: what gives the tune? Oikos. 2006;112:638–650. doi:10.1111/j.0030-1299.2006.13860.x [Google Scholar]
- Cederwall H, Elmgren R. Eutrophication of the Baltic Sea: biological effects. Ambio. 1990;19:109–112. [Google Scholar]
- Chan F, Marino R.L, Howarth R.W, Pace M.L. Ecological constraints on planktonic nitrogen fixation in saline estuaries. II. Grazing controls on cyanobacterial population dynamics. Mar. Ecol. Prog. Ser. 2006;309:41–53. doi:10.3354/meps309041 [Google Scholar]
- Cleveleland W.S. Hobart Press; Summit, NY: 1993. Visualizing data. [Google Scholar]
- Daskalov G.M, Grishin A.N, Rodionov S, Mihneva V. Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc. Natl Acad. Sci. USA. 2007;104:10 518–10 523. doi: 10.1073/pnas.0701100104. doi:10.1073/pnas.0701100104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deegan L.A, et al. Susceptibility of salt marshes to nutrient enrichment and predator removal. Ecol. Appl. 2007;17(Suppl.):42–63. doi:10.1890/06-0452.1 [Google Scholar]
- Dippner J.W, Hänninen J, Kuosa H, Vuorinen I. The influence of climate variability on zooplankton abundance in the Northern Baltic Archipelago Sea (SW Finland) ICES J. Mar. Sci. 2001;58:569–578. doi:10.1006/jmsc.2001.1048 [Google Scholar]
- Edwards M, Richardson A.J. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430:881–884. doi: 10.1038/nature02808. doi:10.1038/nature02808 [DOI] [PubMed] [Google Scholar]
- Estes J.A, Tinker M.T, Williams T.M, Doak D.F. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science. 1998;282:473–476. doi: 10.1126/science.282.5388.473. doi:10.1126/science.282.5388.473 [DOI] [PubMed] [Google Scholar]
- Finni T, Kononen K, Olsonen R, Wallström K. The history of cyanobacterial blooms in the Baltic Sea. Ambio. 2001;30:172–178. doi:10.1639/0044-7447(2001)030[0172:THOCBI]2.0.CO;2 [PubMed] [Google Scholar]
- Fleming V, Kaitala S. Phytoplankton spring bloom intensity index for the Baltic Sea estimated for the years 1992 to 2004. Hydrobiologia. 2006;554:57–65. doi:10.1007/s10750-005-1006-7 [Google Scholar]
- Frank K.T, Petrie B, Choi J.S, Legget W.C. Trophic cascades in a formerly cod-dominated ecosystem. Science. 2005;308:1621–1623. doi: 10.1126/science.1113075. doi:10.1126/science.1113075 [DOI] [PubMed] [Google Scholar]
- Frank K.T, Petrie B, Shackell N.L. The ups and downs of trophic control in continental shelf ecosystems. Trends Ecol. Evol. 2007;22:236–242. doi: 10.1016/j.tree.2007.03.002. doi:10.1016/j.tree.2007.03.002 [DOI] [PubMed] [Google Scholar]
- Grauman, G., Line, R. & Sidrevits, L. 1986 On the factors determining abundance of Baltic sprat. ICES CM 1986/J:8.
- Halpern B.S, Cottenie K, Broitman B.R. Strong top-down control in southern California kelp forest ecosystem. Science. 2006;312:1230–1232. doi: 10.1126/science.1128613. doi:10.1126/science.1128613 [DOI] [PubMed] [Google Scholar]
- Hänninen J, Vuorinen I, Kornilovs G. Atlantic climatic factors control decadal dynamics of a Baltic Sea copepod Temora longicornis. Ecography. 2003;26:672–678. doi:10.1034/j.1600-0587.2003.03524.x [Google Scholar]
- Harvey C.J, Cox S.P, Essington T.E, Hansson S, Kitchell J.F. An ecosystem model of food web and fisheries interactions in the Baltic Sea. ICES J. Mar. Sci. 2003;60:939–950. doi:10.1016/S1054-3139(03)00098-5 [Google Scholar]
- Haslob H, Clemmesen C, Schaber M, Hinrichsen H.-H, Schmidt J.O, Voss R, Kraus G, Köster F.W. Invading Mnemiopsis leidyi as a potential threat to Baltic fish. Mar. Ecol. Prog. Ser. 2007;349:303–306. doi:10.3354/meps07283 [Google Scholar]
- Hernroth, L. (ed.) 1985 Recommendations on methods for marine biological studies in the Baltic Sea. Mesozooplankton biomass assessment. Baltic Mar. Biol.10, 1–32.
- Horbowy J. The dynamics of Baltic fish stocks on the basis of a multispecies stock-production model. Can. J. Fish. Aquat. Sci. 1996;53:2115–2125. doi:10.1139/cjfas-53-9-2115 [Google Scholar]
- International Council for the Exploration of the Sea 2006 Report of the Study Group on Multispecies Assessment in the Baltic, ICES CM 2006/BCC:07.
- International Council for the Exploration of the Sea 2007 Report of the Baltic Fisheries Assessment Working Group, ICES CM 2007/ACFM:15.
- Karjalainen M, Engström-Öst J, Korpinen S, Peltonen H, Pääkkönen J.-P, Rönkkönen S, Suikkanen S, Viitasalo M. Ecosystem consequences of cyanobacteria in the Northern Baltic Sea. Ambio. 2007;36:195–202. doi: 10.1579/0044-7447(2007)36[195:ecocit]2.0.co;2. doi:10.1579/0044-7447(2007)36[195:ECOCIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kornilovs G, Sidrevics L, Dippner J.W. Fish and zooplankton interaction in the Central Baltic Sea. ICES J. Mar. Sci. 2001;58:579–588. doi:10.1006/jmsc.2001.1062 [Google Scholar]
- Litzow M.A, Ciannelli L. Oscillating trophic control induces community reorganization in a marine ecosystem. Ecol. Lett. 2007;10:1124–1134. doi: 10.1111/j.1461-0248.2007.01111.x. doi:10.1111/j.1461-0248.2007.01111.x [DOI] [PubMed] [Google Scholar]
- MacKenzie B.R, Köster F.W. Fish production and climate: sprat in the Baltic Sea. Ecology. 2004;85:784–794. doi:10.1890/02-0780 [Google Scholar]
- Mallows C.L. Some comments on Cp. Technometrics. 1973;15:661–675. doi:10.2307/1267380 [Google Scholar]
- Menge B.A, Daley B.A, Wheeler P.A, Dahlhoff E, Sanford E, Strub P.T. Benthic–pelagic links and rocky intertidal communities: bottom-up effects on top-down control? Proc. Natl Acad. Sci. USA. 1997;94:14 530–14 535. doi: 10.1073/pnas.94.26.14530. doi:10.1073/pnas.94.26.14530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli F. Eutrophication, fisheries, and consumer–resource dynamics in marine pelagic ecosystems. Science. 1999;285:1396–1398. doi: 10.1126/science.285.5432.1396. doi:10.1126/science.285.5432.1396 [DOI] [PubMed] [Google Scholar]
- Molinero J.-C, Ibanez F, Souissi S, Chifflet M, Nival P. Phenological changes in the northwestern Mediterranean copepods Centropages typicus and Temora stylifera linked to climate forcing. Oecologia. 2005;145:640–649. doi: 10.1007/s00442-005-0130-4. doi:10.1007/s00442-005-0130-4 [DOI] [PubMed] [Google Scholar]
- Möllmann C, Kornilovs G, Sidrevics L. Long-term dynamics of main mesozooplankton species in the central Baltic Sea. J. Plankton Res. 2000;22:2015–2038. doi:10.1093/plankt/22.11.2015 [Google Scholar]
- Möllmann C, Kornilovs G, Fetter M, Köster F.W. Feeding ecology of central Baltic Sea herring and sprat. J. Fish Biol. 2004;65:1563–1581. doi:10.1111/j.0022-1112.2004.00566.x [Google Scholar]
- Möllmann C, Müller-Karulis B, Kornilovs G, St John M.A. Effects of climate and overfishing on zooplankton dynamics and ecosystem structure: regime shifts, trophic cascade, and feedback loops in a simple ecosystem. ICES J. Mar. Sci. 2008;65:302–310. doi:10.1093/icesjms/fsm197 [Google Scholar]
- Nissling A. Effects of temperature on egg and larval survival of cod (Gadus morhua) and sprat (Sprattus sprattus) in the Baltic Sea—implications for stock development. Hydrobiologia. 2004;514:115–123. doi:10.1023/B:hydr.0000018212.88053.aa [Google Scholar]
- Oksanen L, Fretwell S.D, Arruda J, Niemela P. Exploitation ecosystems in gradients of primary productivity. Am. Nat. 1981;118:240–261. doi:10.1086/283817 [Google Scholar]
- Österblom H, Casini M, Olsson O, Bignert A. Fish, seabirds and trophic cascades in the Baltic Sea. Mar. Ecol. Prog. Ser. 2006;323:233–238. doi:10.3354/meps323233 [Google Scholar]
- Österblom H, Hansson S, Larsson U, Hjerne O, Wulff F, Elmgren R, Folke C. Human-induced trophic cascades and ecological regime shifts in the Baltic Sea. Ecosystems. 2007;10:877–889. doi:10.1007/s10021-007-9069-0 [Google Scholar]
- Pace M.L, Cole J.J, Carpenter S.R, Kitchell J.F. Trophic cascades revealed in diverse ecosystems. Trends Ecol. Evol. 1999;14:483–488. doi: 10.1016/s0169-5347(99)01723-1. doi:10.1016/S0169-5347(99)01723-1 [DOI] [PubMed] [Google Scholar]
- Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F., Jr Fishing down marine food webs. Science. 1998;279:860–863. doi: 10.1126/science.279.5352.860. doi:10.1126/science.279.5352.860 [DOI] [PubMed] [Google Scholar]
- Perry A.L, Low P.J, Ellis J.R, Reynolds J.D. Climate change and distribution shifts in marine fishes. Science. 2005;308:1912–1915. doi: 10.1126/science.1111322. doi:10.1126/science.1111322 [DOI] [PubMed] [Google Scholar]
- Rudstam L.G, Hansson S, Johansson S, Larsson U. Dynamics of planktivory in a coastal area of the northern Baltic Sea. Mar. Ecol. Prog. Ser. 1992;80:159–173. doi:10.3354/meps080159 [Google Scholar]
- Shiomoto A, Tadokoro K, Nagasawa K, Ishida Y. Trophic relations in the subarctic North Pacific ecosystem: possible feeding effect from pink salmon. Mar. Ecol. Prog. Ser. 1997;150:75–85. doi:10.3354/meps150075 [Google Scholar]
- Shurin J.B, Borer E.T, Seabloom E.W, Anderson K, Blanchette C.A, Broitman B, Cooper S.D, Halpern B.S. A cross-ecosystem comparison of the strength of trophic cascades. Ecol. Lett. 2002;5:785–791. doi:10.1046/j.1461-0248.2002.00381.x [Google Scholar]
- Voss R, Köster F.W, Dickmann M. Comparing the feeding habits of co-occurring sprat (Sprattus sprattus) and cod (Gadus morhua) larvae in the Bornholm Basin, Baltic Sea. Fish. Res. 2003;63:97–111. [Google Scholar]
- Ware D.M, Thomson R.E. Bottom-up ecosystem trophic dynamics determine fish production in the Northeast Pacific. Science. 2005;308:1280–1284. doi: 10.1126/science.1109049. doi:10.1126/science.1109049 [DOI] [PubMed] [Google Scholar]
- Wasmund N, Nausch G, Matthäus W. Phytoplankton spring blooms in the southern Baltic Sea—spatio-temporal development and long-term trends. J. Plankton Res. 1998;20:1099–1117. doi:10.1093/plankt/20.6.1099 [Google Scholar]
- Wieland K, Jarre-Teichmann A, Horbowa K. Changes in the timing of spawning of Baltic cod: possible causes and implications for recruitment. ICES J. Mar. Sci. 2000;57:452–464. doi:10.1006/jmsc.1999.0522 [Google Scholar]
- Winder M, Schindler D.E. Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology. 2004;85:2100–2106. doi:10.1890/04-0151 [Google Scholar]
- Worm B, Myers R.A. Meta-analysis of cod–shrimp interactions reveals top-down control in oceanic food webs. Ecology. 2003;84:162–173. doi:10.1890/0012-9658(2003)084[0162:MAOCSI]2.0.CO;2 [Google Scholar]
- Worm B, Lotze H.K, Sommer U. Coastal food web structure, carbon storage, and nitrogen retention regulated by consumer pressure and nutrient loading. Limnol. Oceanogr. 2000;45:339–349. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three tables, three figures and references