Abstract
Pollinators mediate the evolution of secondary floral traits through both natural and sexual selection. Gender-biased nectar, for example, could be maintained by one or both, depending on the interactions between plants and pollinators. Here, I investigate pollinator responses to gender-biased nectar using the dichogamous herb Chrysothemis friedrichsthaliana (Gesneriaceae) which produces more nectar during the male floral phase. Previous research showed that the hummingbird pollinator Phaethornis striigularis visited male-phase flowers more often than female-phase flowers, and multiple visits benefited male more than female fecundity. If sexual selection maintains male-biased rewards, hummingbirds should prefer more-rewarding flowers independent of floral gender. If, however, differential rewards are partially maintained through natural selection, hummingbirds should respond to asymmetry with visits that reduce geitonogamy, i.e. selfing and pollen discounting. In plants with male biases, these visit types include single-flower visits and movements from low to high rewards. To test these predictions, I manipulated nectar asymmetry between pairs of real or artificial flowers on plants and recorded foraging behaviour. I also assessed maternal costs of selfing using hand pollinations. For plants with real flowers, hummingbirds preferred more-rewarding flowers and male-phase morphology, the latter possibly owing to previous experience. At artificial arrays, hummingbirds responded to extreme reward asymmetry with increased single-flower visits; however, they moved from high to low rewards more often than low to high. Finally, selfed flowers did not produce inferior seeds. In summary, sexual selection, more so than geitonogamy avoidance, maintains nectar biases in C. friedrichsthaliana, in one of the clearest examples of sexual selection in plants, to date.
Keywords: hummingbird pollination, nectar, Phaethornis striigularis, Chrysothemis friedrichsthaliana, floral evolution, sexual selection
1. Introduction
Many plant species depend on nectar-seeking animals to outcross, yet their per-flower nectar production rates differ from those preferred by pollinators. Pollinators make more and longer visits to plants and flowers with relatively high nectar production, which often confers higher plant fitness (Real & Rathcke 1991; Mitchell 1993; Schemske & Bradshaw 1999). Nevertheless, differential nectar production rates among flowers on the same plant are commonly observed (e.g. Cruden et al. 1983; Boose 1997; Cresswell 1998; McDade & Weeks 2004). Notable examples include species that produce more nectar during either the male or female floral phase of dichogamous flowers, i.e. bisexual flowers that separate sexual function through time. This pattern, known as gender-biased nectar production, is present in at least 41 studied species representing 18 families (Carlson & Harms 2006).
Gender-biased nectar production and other patterns of variability may confer a selective advantage over high, constant rewards under certain conditions (Pleasants 1983; Bell et al. 1984; Rathcke 1992). Even so, the adaptive benefits of differential rewards, including those associated with floral gender, have rarely been tested or demonstrated (Biernaskie et al. 2002). Carlson & Harms (2006) outlined two non-mutually exclusive mechanistic explanations for gender-biased nectar production: the sexual selection and geitonogamy avoidance hypotheses.
The sexual selection hypothesis postulates that plants temporally adjust nectar production within flowers to match the differential nectar fitness functions of each floral phase. Male fecundity often (but not always) requires more pollinator visits to be saturated than does female fecundity, because ovules and offspring are relatively few and expensive to produce, whereas pollen grains are plentiful and inexpensive (Bateman 1948; Trivers 1972; Arnold 1994). As a result, attractive floral traits influence a plant's ability to compete for access to mates—with a disproportionate benefit to male versus female fecundity (Bell 1985; Willson 1994; but see Ashman & Morgan 2004; Ashman et al. 2004). Given this relationship, male-biased nectar production should be favoured if pollinators preferentially visit more-rewarding flowers within plants. These predictions have been partially matched in a few species with male-biased rewards (e.g. Bell et al. 1984; Aizen & Basilio 1998). Even so, convincing evidence remains scarce, given the lingering controversy over the importance of sexual selection in plant evolution (i.e. Skogsmyr & Lankinen 2002; Ashman & Morgan 2004).
The geitonogamy avoidance hypothesis predicts that differential rewards are favoured because they reduce reproductively costly geitonogamy relative to constant rewards (Pleasants 1983; Rathcke 1992; Carlson & Harms 2006). Geitonogamy, or pollen transfer among flowers on the same plant, is associated with two fitness costs. First, self-fertilization can result in fewer or less vigorous offspring than does outcrossing (i.e. inbreeding depression), thus reducing both female and male plant fitness (Charlesworth & Charlesworth 1987; de Jong et al. 1993). Second, geitonogamy further depresses male fitness due to pollen discounting, or the wastage of pollen that could have fertilized outcrossed ovules (de Jong et al. 1993; Fishman 2000). If plants experience both pollen discounting and intra-male mate competition, sexual and natural selection may even converge to favour nectar asymmetry, to move pollen away from self and towards receptive outcrossed ovules.
Differential rewards are expected to reduce geitonogamy and increase outcrossing based on the marginal value theorem. This theory predicts that foragers will act in a manner that maximizes their rate of energy intake, leaving an inflorescence only when the profitability declines below that which could be gained by moving to another plant (e.g. Stephens & Krebs 1986). Given that nectar distributions vary temporally within and among inflorescences (e.g. Carlson & Harms 2006), pollinators should include the most rewarding types on every visit, but sometimes exclude less-rewarding types. When less-rewarding types are included, optimal foragers should visit more profitable flowers first, followed by less profitable ones (Pyke 1978a). The sum of these behaviours will reduce geitonogamy most when rewards are female biased, and the least when rewards are unbiased. Nevertheless, certain foraging predictions appear to be taxon specific. Although work on bees has proffered strong support for optimal foraging (e.g. Pyke 1978a; Best & Bierzychudek 1982), hummingbirds rarely adhere to simple decision rules for visit order (Pyke 1978b; Healy & Hurly 2001). For example, Devlin & Stephenson (1985) showed that ruby-throated hummingbirds (Archilochus colubris) generally foraged up Lobelia cardinalis racemes, from less (female)- to more (male)-rewarding floral phases. When hummingbirds decide to include less-rewarding flowers in a foraging bout, they may prefer to visit those flowers first, i.e. when inflorescence structure affects sampling ease. If so, the frequency of geitonogamy-causing male- to female-phase moves may be greater for plants with female- rather than male-biased rewards.
Recent work on gender-biased rewards has focused on the protandrous herb Chrysothemis friedrichsthaliana (Gesneriaceae) and its hummingbird pollinator Phaethornis striigularis saturatus (Trochilidae: Phaethornithinae; figure 1). In a 3-year study, Carlson (2007b) demonstrated that first, nectar production rates were higher during the male versus female floral phase. Second, Phaethornis striigularis spent 53% more time per visit and made 86% more visits to male- compared with female-phase flowers. Third, female fecundity was maximized by one outcrossed visit, whereas male fecundity continued to increase up to at least three visits. In summary, a sexual selection explanation was broadly supported by field-biased observations; however, it remains to be proven that P. striigularis floral preferences are nectar driven, and that the bird can distinguish more- and less-rewarding flowers based on visual cues.
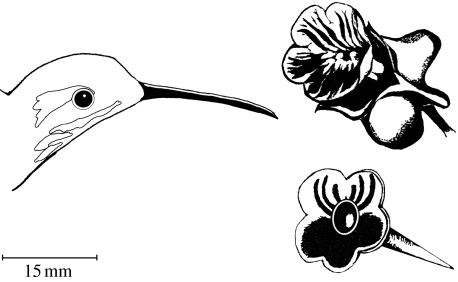
Figure 1.
Phaethornis striigularis with a C. friedrichsthaliana flower and an artificial flower, both of which were used in nectar manipulation experiments. The artificial flower was made of a 10 μl pipettor tip painted orange and bordered to resemble a real flower. Line drawing by J.E.C.
The geitonogamy avoidance hypothesis is also likely to be relevant for C. friedrichsthaliana. Chrysothemis friedrichsthaliana's small floral displays include multiple flowers (typically two) and both genders approximately 33% of the time (Carlson 2007a). Furthermore, P. striigularis hummingbirds move from the male- to female-phase flower within a two-flowered plant on 50% of two-flowered visits. Given that C. friedrichsthaliana experiences geitonogamy frequently, it is possible that selection to reduce geitonogamy acts in concert with sexual selection. If so, two predictions should be met. First, there should be reproductive costs of geitonogamous selfing, such as reduced seed number, weight and germination rate, or significant pollen discounting. Second, pollinators should respond to differential rewards in the following ways: they should feed from only more-rewarding types on some but not all visits, and when both floral types are included, visits should typically proceed from low to high rewards within plants (i.e. female-to-male). Such visit orders are plausible for hummingbirds (i.e. Devlin & Stephenson 1985) and are expected if male-biased nectar evolves predominately through inbreeding avoidance. If the first, but not the second, behavioural prediction is met, selection to reduce geitonogamy may still occur, but is probably subsidiary to sexual selection.
This study uses an experimental approach to test the relative importance of sexual selection and geitonogamy avoidance as adaptive explanations for male-biased nectar in C. friedrichsthaliana. I exposed P. striigularis to pairs of real male- and female-phase flowers with altered nectar production patterns, and measured behavioural responses to nectar and gender-specific morphology. Next, I measured P. striigularis responses to unequal nectar in pairs of artificial flowers without gender-specific cues. Finally, I used hand pollinations to assess the costs of selfing, in terms of seed production and quality. Then experimental results were compared with predictions of the sexual selection and geitonogamy avoidance hypotheses, as outlined above.
2. Material and methods
(a) Species and study site
I studied C. friedrichsthaliana at Centro Tropical of the Fundacíon Neotrópica, in Agua Buena de Rincón, situated within the Golfo Dulce Forest Reserve (8°42′ N, 83°31′ W) on Osa Peninsula, Puntarenas Province, Costa Rica. Plants in this population are pollinated almost exclusively by P. striigularis (Carlson 2007a), a small (2.6 g), non-territorial, traplining hermit hummingbird of lowland forests (Stiles & Skutch 1990; Hinkelmann & Schuchmann 1997; figure 1). In 2004 and 2005, at least 17 different P. striigularis individuals visited plants at Centro Tropical per year (based on acrylic paint marks on heads) and apparently relied heavily on flowers of C. friedrichsthaliana when they were available (J. E. Carlson 2004–2006, personal observation).
The 2-day protandrous flowers of C. friedrichsthaliana decrease nectar production rates between and within each 12 daylight hour sexual phase (Carlson 2007b). Male-phase flowers produce approximately 26% more sugar in the early morning and 65% more sugar in the early afternoon, relative to female-phase flowers (Carlson 2007b). When nectar production is at its peak in either phase, unvisited flowers contain an average of 8–9 μl nectar and 2.5–2.7 mg sugar (Carlson 2007b). Some autogamous self-fertilization occurs as anthers curl back and stigma grows forward as flowers transition between phases. Even so, a single outcrossed visit achieves higher seed production than natural levels of autogamy (Carlson 2007b).
Chrysothemis friedrichsthaliana produces one new flower per day at peak flowering, and new flowers are irregularly distributed within plants (Skutch 1992; Carlson 2007a). I carried out experiments from August to November in 2004, 2005 and 2006, which corresponded to the second half of C. friedrichsthaliana's flowering season and the peak of the rainy season.
(b) Pollinator responses to nectar in real flowers
I used natural patches of C. friedrichsthaliana plants to test the responses of P. striigularis to male-biased, female-biased and unbiased nectar production in real male- and female-phase flowers. Once per week for three consecutive weeks, I applied a different nectar treatment to each of three plants in a patch, followed by a 2 hour observation period. By the end of three weeks, all three plants had received all three treatments in a Latin Square Design (LSD). I replicated the LSD on four patches in 2004 and 2005, and three in 2006. Patches studied within the same year were 50 m or more apart, were visited by different hummingbirds and were observed on different days.
To apply nectar treatments, I arrived to a patch before sunrise (approx. 05.00 hours, local time) and adjusted each floral display to exactly one male-phase flower and one female-phase flower. I drained the standing crop of nectar from all six flowers by piercing the corolla, and immediately added nectar treatments using a pipettor and 25% sucrose solution. In the male-biased treatment, female-phase flowers received no nectar, but male-phase flowers received 10 μl nectar (2.75 mg sugar). In the female-biased treatment, this pattern was reversed between male- and female-phase flowers. In the unbiased treatment, both flowers received 5 μl nectar (1.38 mg sugar). At 40 and 80 min into the experiment, I drained and refilled the flowers with half the original volume to mimic natural reward reduction following a flower's first visit (Carlson 2007b).
Starting at sunrise (05.30 hours, local time), I digitally recorded the patch for two consecutive hours. From slow motion playback, I measured the number of visits per flower and plant, the duration of bill insertion, and the types of visit made to plants. Phaethornis striigularis visited plants in one of three ways (visit types): visited only one flower; visited both flowers in order of male to female phase; or both in order of female to male phase. Throughout the study, single-flower visits were almost always to male-phase flowers (91–93% of visits).
I performed statistical tests on four aspects of P. striigularis visits. For the first and second, I determined whether P. striigularis altered 2 hour visit rates or visit durations in response to nectar treatment, floral gender, patch of plants (i.e. an individual bird) and all fixed-effect interactions. I used two generalized linear mixed models with Poisson distributions, which are appropriate for count data, and made adjustments for heterogeneous variance across years (Littell et al. 2006). Additional random effects included the week and the individual plant, the latter accounting for non-independence among paired flowers. Third, I compared the probability of a pollinator visit to a plant's male- versus female-phase flower during the first foraging bout per day. Fixed effects were nectar treatment and gender, random effects were similar to those above and the distribution was binary for yes/no response data. Fourth, I compared the probabilities of each of the three visit types across the nectar treatments. I used a generalized multinomial distribution, which is appropriate for discrete unordered response variables that fall into three or more categories, such as the three visit types (SAS Institute, Inc. 2005). Nectar treatment was the only fixed effect, and random effects were patch of plants (i.e. bird), plant, week and year. I used linear contrasts to determine which visit types were most frequent in the male-biased, female-biased and unbiased treatments. Unless otherwise specified, all statistical analyses were performed using the Glimmix Procedure in SAS v. 9.1.3 (Cary, NC, USA) and included Kenward–Roger approximations for degrees of freedom (SAS Institute, Inc. 2005).
(c) Pollinator responses to nectar in artificial flowers
I used arrays of artificial flowers to test P. striigularis responses to differential rewards in the absence of gender-specific floral cues (figure 1). On the foraging route of a marked hummingbird, I placed an array of 12–16 artificial flowers attached two per plant to live, potted C. friedrichsthaliana plants. I trained the hummingbird to revisit by adding 6 μl of 25% sucrose solution (1.65 mg sugar) hourly to all flowers for several days prior to the experiment.
Starting at 08.00 hours on the first day of the experiment, I added 25% sucrose solution to the two flowers per plant according to their assigned nectar treatments. The treatments were analogous to varying degrees of nectar asymmetry, at the high end of C. friedrichsthaliana's natural range of volumes and sucrose contents (Carlson 2007a). I divided six different treatments between two arrays. A hummingbird was exposed to the first array for 8 days, followed by 6 days with the second array. For the first, nectar treatments included 2 μl to the flower on the left and 0 μl to the flower on the right (i.e. 2-0 μl): 4-0 μl; 4-2 μl; and 6-2 μl. For the second array, which had two fewer plants, treatments were 6-0 μl, 3-3 μl and 4-2 μl. For the remaining plants in each array, I used the same nectar treatments but exchanged volumes between left and right flowers. In analyses, these near-identical treatments were combined (e.g. 0-2 and 2-0 μl). I reapplied treatments hourly until 15.00 hours and assigned new treatments each morning until each plant received each treatment in an LSD.
I collected data on two foraging bouts per day (at approx. 14.00 and 15.00 hours) by recording the plants and flowers which were visited and the types of visits made. I classified hummingbird visits into one of three types: visit to one of the two flowers; visit to both flowers in order of higher to lower rewards; or visit to both flowers in order of lower to higher rewards. Single-flower visits were almost invariably to the more-rewarding flower (more than 93% of visits). Treatment 3-3 had only two visit types: visit to only one flower or visit to both flowers.
Between August and October 2005 and 2006, I moved arrays to the traplines of five different hummingbirds and observed all necessary visits to the first array. Only two individuals continued visiting the second array for the six additional days needed. Given that the two hummingbirds behaved similarly within both arrays (F<0.6, p>0.4 for visit probabilities and types), I analysed the two arrays as one in the final analyses.
I used logistic regressions to test pollinator responses in the absence of floral cues, measuring responses as discrete successes or failures to visit flowers or plants. First, I tested whether the probability of a visit was dependent on a flower's nectar volume (0, 2, 3, 4, 6 μl nectar), the volume of its companion flower or the specific treatment combination of the plant (2-0, 4-0, 6-0, 3-3, 4-2 or 6-2 μl nectar). I included the hummingbird's identity and its interaction with nectar volume as additional fixed effects. A binary distribution was used, which accounted for non-independence with random effects of plant and day (Littell et al. 2006). Second, I tested whether the probability of a plant receiving any visit type was dependent on the nectar treatment or hummingbird, excluding the 3-3 μl treatment. I used the same random effects, but changed the distribution to generalized multinomial (SAS Institute, Inc. 2005). Third, I returned the 3-3 μl treatment to the preceding model and made the following modifications: all visits were recategorized into two types, single- or two-flower visits, and the distribution was changed to binary. Linear contrasts were used to compare the probabilities of receiving each visit type among treatments for the latter two regressions.
(d) Costs of selfing
To determine whether selfed C. friedrichsthaliana flowers produced seeds of lower qualities or quantities than outcrossed flowers, I hand-pollinated flowers and tested germination of the resulting seeds. The evening before treatments were applied, I bagged two male-phase flowers on a large robust plant. The day's visits had removed most pollen from anthers, such that emasculation was unnecessary (Carlson 2007b). Once in the female phase each flower was randomly assigned a hand-pollination treatment: one flower received a visit from each of two virgin male-phase flowers on the same plant (selfed), and the other received a visit from each of two virgin male-phase flowers on a neighbouring plant (outcrossed). A Phaethornis sp. bill was used to transfer pollen from male- to female-phase flowers, and the bill was cleaned with alcohol after each female-phase visit. Flowers were bagged until corolla abscission, and fruit capsules were collected as they dehisced (approx. 35 days later). Seeds were subsequently dried, counted and weighed.
I stored seeds through their natural dormancy period (Skutch 1992) and then tested germination of a random subsample of 100 seeds per capsule. The seeds were placed onto Petri plates on moistened Whatman no. 10 filter paper, and the plates were kept in an incubator for 23 days at 26°C and 55% humidity with a 12 L : 12 D cycle. I checked for germinated seeds and rearranged the plates within the incubator every other day. I collected data from 14 to 19 capsules per treatment in both 2004 and 2005.
I performed three 2-way ANOVAs to test the effects of selfing versus outcrossing on seed number, mass and germination in C. friedrichsthaliana. I logit transformed the variable percentage of seeds germinated and used a binomial distribution to correct for non-normality. The remaining two variables, seed count per flower and seed mass, were normally distributed and therefore analysed using mixed linear models (Mixed Procedure, SAS v. 9.1.3). Since I replicated the experiment once per year for only two years, I included year as an additional fixed effect, as well as its interaction with hand-pollination treatment. In all analyses, plant was a random effect accounting for paired treatments within plants; however, a few plants experienced herbivory and did not contain both treatments by the experiment's end.
3. Results
(a) Pollinator responses to nectar in real flowers
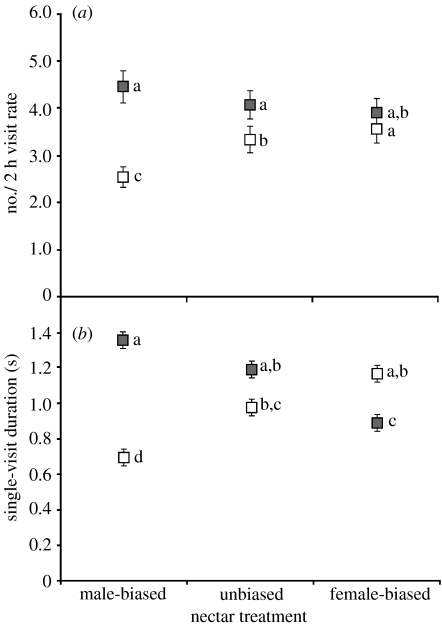
Phaethornis striigularis increased visit rates and durations as rewards increased, but their responses to nectar were the strongest in female-phase flower (table 1). Female-phase flowers with more nectar (i.e. those in the female-biased treatment) received significantly more and longer visits than female-phase flowers with less nectar (figure 2). A similar response to nectar was observed in male-phase flowers, but only for visit durations (table 1; figure 2a). Visit rates to male-phase flowers were consistently high and exceeded those for female-phase flowers, regardless of nectar treatment. A preference for male-phase morphology was even apparent during a hummingbird's first visit of the day, when prior visits to a flower could not influence preferences. Ninety-four per cent of the day's first visits included the male-phase flower, whereas only 81% included the female-phase flower (gender: F1,254=7.99, p=0.001; treatment: F2,254=1.66, p=0.19; interaction: F2,254=0.45, p=0.64).
Table 1.
Results of ANOVAs of 2 hours visit rate and single-visit duration of P. striigularis at nectar-manipulated, two-flowered C. friedrichsthaliana plants. (Nectar treatments were male-biased, female-biased and unbiased production within plants. A patch was three adjacent plants on a hummingbird's foraging route, during a given flowering season. Plant, week and year were random effects, the last effect grouping the three patches (approx. three hummingbirds) in 2006 and the four patches in each 2004 and 2005. d.f., numerator degrees of freedom, denominator degrees of freedom (figure 2).)
| source of variation | visit rate (no./2 hour) | single-visit duration (s) | ||||
|---|---|---|---|---|---|---|
| d.f. | F | p | d.f. | F | p | |
| among-plant fixed effects | ||||||
| treatment | 2,58.9 | 1.1 | 0.35 | 2,76.8 | 2.6 | 0.079 |
| patch | 10,40.7 | 5.3 | <0.0001 | 10,24.4 | 38.8 | <0.0001 |
| patch×treatment | 20,44.0 | 0.6 | 0.92 | 20,25.5 | 1.1 | 0.45 |
| within-plant fixed effects | ||||||
| gender | 1,85.8 | 51.3 | <0.0001 | 1,110.4 | 20.5 | <0.0001 |
| treatment×gender | 2,85.7 | 11.5 | <0.0001 | 2,110.4 | 37.3 | <0.0001 |
| patch×gender | 10,45.5 | 2.1 | 0.041 | 10,54.1 | 1.7 | 0.12 |
| treatment×gender×patch | 20,46.0 | 0.8 | 0.73 | 20,54.9 | 1.1 | 0.37 |
Figure 2.
(a) Visit rate and (b) average single-visit duration of P. striigularis at nectar-manipulated C. friedrichsthaliana plants on the Osa Peninsula, Puntarenas Province, Costa Rica. Between August 2004 and October 2006, 33 different plants were observed three times each, once per nectar treatment. Filled squares, male phase; open squares, female phase. Least-square means with different lowercase letters are significantly different (Tukey–Kramer adjusted, α=0.05) and error bars represent ±1 s.e (see table 1 for complete statistical results).
Hummingbird foraging behaviour was variable among the 11 plant patches. Individual birds and/or the spatial location and arrangement of each patch had a significant effect on visit rates and durations (table 1). Even so, preferences for nectar treatments and floral genders were generally consistent across patches (i.e. two- and three-way interactions with a patch non-significant, except for patch×gender interaction for visit rate; table 1).
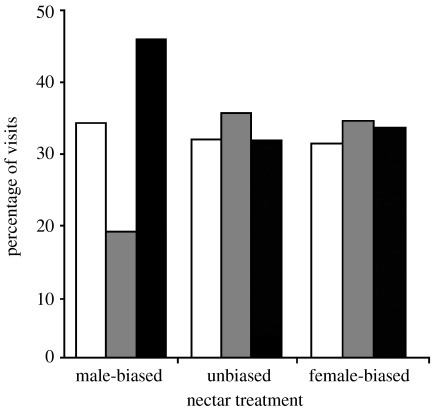
Phaethornis striigularis hummingbirds were nearly twice as likely to make single- versus two-flower visits when rewards were male biased, as opposed to those female biased or unbiased (F5,20=3.11, p=0.031; figure 3). Contrary to geitonogamy avoidance predictions, they were also half as likely to make a female-then-male visit in the male-biased versus other treatments (linear contrasts for female-then-male visit: t159>3.61, p<0.0005). Visit types that could result in geitonogamy (male-then-female-phase visit) were made at a constant rate across treatments, at approximately 32–34% of visits (linear contrasts for male-then-female visit: t120<1.4, p>0.16; figure 3).
Figure 3.
Types of visits made by P. striigularis to two-flowered C. friedrichsthaliana plants with male-biased, female-biased and unbiased nectar production. Percentages are based on total numbers of visits to 33 individual plants, each observed three times, as shown in figure 2 (white bars, moved male to female; grey bars, moved female to male; black bars, one-flower visit). Six hundred and fifty-three visits were made by at least 11 different hummingbirds over the three flowering seasons of study (see §3 for statistical tests).
(b) Pollinator responses to nectar in artificial flowers
The probability of an artificial flower receiving a P. striigularis visit was strongly dependent on the flower's nectar volume (F3,841=77.22, p<0.0001). It was, however, affected neither by the volume of its companion flower (F3,736=0.23, p=0.88) nor by their interaction (F3,872=0.87, p=0.46). Flowers with 2, 3, 4 or 6 μl all had high (93% or more) and statistically equivalent probabilities of being visited, whereas flowers with 0 μl all had similarly low (25% or less) probabilities of being visited. The overall nectar treatment failed to predict pollinator responses; birds only discriminated between flowers containing 0 and greater than 0 μl.
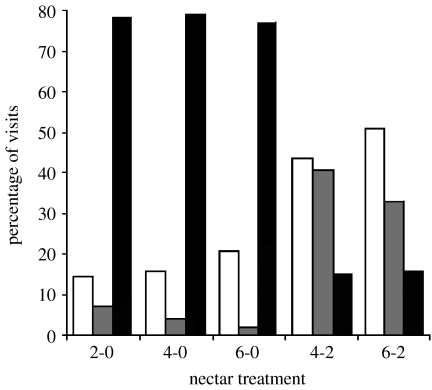
Hummingbirds were more likely to make single- versus two-flower visits to plants with ‘extreme’ nectar differences, i.e. those with an unrewarding artificial flower (overall test: F8,698=29.97, p<0.0001; figure 4). When hummingbirds visited both flowers on these plants, however, they were almost twice as likely to move from high to low as low to high rewards. Plants with both flowers rewarding (treatments 4-2 and 6-2) received different types of visits than those with an unrewarding flower (treatments 2-0, 4-0 and 6-0; linear contrast: t1,698>9.2, p<0.0001), yet the visit types made within each group were statistically indistinguishable (all contrasts: t558<1.1, p>0.31). Plants in treatment 3-3 had a 19% probability of receiving a single-flower visit, making them similar to other plants with two rewarding flowers (contrast with 4-2: t755=0.87, p=0.39; contrast with 6-0: t755=5.06, p<0.0001).
Figure 4.
Types of visits made by P. striigularis to artificial flowers on C. friedrichsthaliana plants, in response to six different nectar production patterns. Raw percentages for artificial flowers are based on 32 foraging bouts by each of the five different hummingbirds for treatments 2-0, 4-0, 4-2 and 6-2 (white bars, moved high to low; grey bars, moved low to high; black bars, one-flower visit). For treatments 6-0, 4-2 and 3-3, they are based on 24 bouts per treatment by two of these hummingbirds. Plants in treatment 3-3 (not shown) received the same proportions of single- and two-flower visits as in 4-2 and 6-2 (see §3 for statistical tests).
All five hummingbirds in the artificial flower experiment used similar strategies when faced with differential nectar rewards. Visit probabilities and visit types were similar across hummingbirds, independent of nectar treatment (per-flower visit probability: F4,162=0.05, p=0.99; per-plant visit types: F<1.813, p>0.36). Hummingbirds also responded similarly to specific nectar volumes within flowers; they all tended to avoid flowers with no nectar and tended to visit flowers with 2, 3, 4 or 6 μl at equal frequencies (hummingbird×treatment for per-flower visit probability: F14,1200=1.21, p=0.33).
(c) Costs of selfing
Seed production and quality in C. friedrichsthaliana did not change in response to pollination with selfed versus outcrossed pollen (table 2; figure 1 in the electronic supplementary material). Number, mass and germinability of seeds were equivalent between hand-pollination treatments, across 2004 and 2005 (table 2). Even so, there was a non-significant trend for outcrossed seeds to be slightly more germinable than selfed seeds. There were large differences between experiments performed in 2004 and 2005 for all response variables (table 2; figure 1 in the electronic supplementary material). Averaged across all treatments, flowers produced 1154±43 seeds (l.s. means±s.e.) in 2004 and 980±48 seeds in 2005. Germination rate was 65±5% in 2004 but only 19±4% in 2005.
Table 2.
Results of two-way ANOVAs on seed production and seed quality of C. friedrichsthaliana in response to hand-pollination treatments. (Treatments were applied with a Phaethornis sp. bill and consisted of pollination by two virgin male-phase flowers from the same plant (selfed) or two virgin male-phase flowers on a different plant (outcrossed). Experiments were replicated in the flowering seasons of 2004 and 2005. Germination trials were performed approx. 5 months after seed collection, and each lasted 23 days. Plant was a random effect in all models. Numerator degrees of freedom is 1 for all effects and variables; d.d.f., denominator degrees of freedom.)
| source of variation | seed count (no./flower) | mean weight per seed (mg) | percent germination | ||||||
|---|---|---|---|---|---|---|---|---|---|
| d.d.f. | F | p | d.d.f. | F | p | d.d.f. | F | p | |
| treatment | 58 | 0.01 | 0.93 | 40 | 0.06 | 0.80 | 32 | 3.27 | 0.08 |
| year | 34 | 7.28 | 0.01 | 36 | 8.10 | 0.007 | 37 | 32.54 | <0.0001 |
| year×treatment | 58 | 0.55 | 0.46 | 20 | 2.38 | 0.13 | 32 | 0.03 | 0.86 |
4. Discussion
(a) Sexual selection hypotheses
Phaethornis striigularis hummingbirds responded to nectar manipulations in both real and artificial flowers, making possible hummingbird-mediated sexual selection in C. friedrichsthaliana. Many other hummingbird species also increase visit rates to real or artificial flowers with higher rewards, when visual cues are either present (Zimmerman 1983; Melendez-Ackerman et al. 1997) or absent (Miller et al. 1985; Hurly 1996; Irwin 2000). Preferences for more-rewarding flowers are also observed in other pollinator taxa, such as bumblebees and honeybees (e.g. Gonzalez et al. 1995; Aizen & Basilio 1998; Johnson & Nilsson 1999). This study is one of the few to use real flowers to show that nectar directly contributes to hummingbird preferences. In doing so, it builds on earlier work (Carlson 2007b) to produce one of the most rigorous demonstrations of sexual selection in plants thus far.
Although P. striigularis discriminated among most flowers based on nectar, thereby matching sexual selection predictions, they also used gender-specific floral cues. Birds visited male-phase flowers more frequently regardless of nectar content, and male-phase preferences were evident even during the first foraging bout of the day. This suggests that hummingbirds purposefully targeted male-phase flowers while foraging, possibly by using morphological differences between phases, such as the relative positions of anthers and stigma. Future studies that disassociate sexual parts from floral phases should reveal how and even why P. striigularis hummingbirds discriminate among flowers. At present, these questions are largely unanswered for hermit hummingbirds, and they represent a fruitful next step for studies of hummingbird-mediated selection in the tropics.
In C. friedrichsthaliana, P. striigularis probably prefers male-phase flowers independent of rewards for one of the following reasons. Hummingbirds may find male-phase flowers easier to encounter or feed from (i.e. Temeles et al. 2002), or they may anticipate more nectar in subsequent male-phase visits, based on their experiences in the population at large. Of the two explanations, the latter seems more likely, given that non-reproductive floral parts are largely unchanging between the phases (J. E. Carlson, personal observation). Responses to nectar availability have been studied recently in another traplining species, female purple-throated caribs (Eulampis jugularis; Temeles et al. 2006). When nectar rewards were experimentally reduced in high-quality Heliconia patches on a single bird's foraging route, the birds increased rather than decreased their visit frequency to those patches. From these behaviours, the authors inferred that birds were defending a valued food source by draining flowers before competitors (Gill 1988; Temeles et al. 2006). Similarly, P. striigularis hummingbirds probably remain faithful to unrewarding male-phase flowers because they associate male-phase morphology with faster refill rates and generally higher rewards. As a result, birds are undeterred when nectar competition disassociates overall quality from current nectar volume.
Hummingbirds prefer visual cues over nectar volumes in other plant taxa. Melendez-Ackerman & Campbell (1998) found that real Ipomopsis flowers painted red were preferentially visited over real flowers painted pink or white, regardless of nectar content and underlying morphology. Hummingbird experiences at larger temporal and spatial scales appeared to best explain these responses, since neighbouring populations of pink- and white-flowered congeners naturally produced less nectar than red-flowered I. aggregata (Melendez-Ackerman & Campbell 1998). Earlier studies that altered rewards at a larger scale and observed responses of the same species did find a reversal in preference based on nectar (Melendez-Ackerman et al. 1997). This suggests that if hummingbirds encounter plants with atypical patterns often enough, they can learn to rely on new visual or spatial cues (e.g. Healy & Hurly 2001).
At artificial arrays, P. striigularis preferred rewarding over unrewarding artificial flowers within plants, but did not discriminate among rewarding artificial flowers with unequal volumes. As a result, only the so-called extreme nectar differences (0 versus ≥2 μl) elicited the responses predicted by the sexual selection hypothesis. Birds might have failed to discriminate because 2 μl was above the departure threshold value for the plant or array (i.e. Pyke 1978b). Neighbouring unmanipulated patches were probably of lesser value (Carlson 2007b) and distances within arrays were relatively small, which could increase the profitability of even relatively poor food items in the patch (Stephens & Krebs 1986). A contrasting view is that even under natural conditions, P. striigularis discriminates strongly only if some flowers are entirely or nearly unrewarding. Unrewarding C. friedrichsthaliana flowers do occur naturally; female-phase flowers are often empty in the afternoon, when male-biased preferences are in fact the strongest (Carlson 2007b).
If the observed responses also occur on larger temporal and spatial scales, pollinator-mediated sexual selection may still favour male-biased nectar production in C. friedrichsthaliana, albeit through an indirect mechanism. Since P. striigularis responded to nectar in female- but not male-phase flowers, a slight increase in nectar production during the female phase could potentially attract more visits. Even so, female fecundity is not strongly pollen limited (Carlson 2007b), which suggests that that unbiased or female-biased plants would suffer higher energetic costs of nectar production (e.g. Southwick 1984; Pyke 1991; but see Ordano & Ornelas 2005) with no additional fitness benefits. In this way, male-biased nectar production would currently be maintained by negative selection on female-phase production, rather than by positive selection on male-phase production.
(b) Geitonogamy avoidance hypothesis: pollinator responses to nectar
Phaethornis striigularis behaviours at nectar-manipulated plants only partially suggest that selection for reduced geitonogamy acts in concert with sexual selection. In agreement with geitonogamy avoidance (and sexual selection) predictions, hummingbirds typically probed only the male-phase flower when rewards were male biased. Similarly, they tended to probe only one of two artificial flowers on plants with extreme nectar asymmetry. An early departure, like that observed here, is a common response to reward asymmetry in dichogamous and non-dichogamous species alike, and has been used to explain the evolution of reward variability independent of gender biases (e.g. Rathcke 1992; Biernaskie et al. 2002). Nevertheless, this explanation is insufficient when rewards vary with gender, since visit orders also affect geitonogamy in these species.
Phaethornis striigularis visit orders did not correspond with geitonogamy avoidance predictions. Male-biased nectar should have encouraged visit orders from low to high rewards (i.e. female to male phase) if natural selection equalled or overwhelmed sexual selection on nectar in C. friedrichsthaliana. Nevertheless, hummingbirds typically travelled from high to low, or male to female, when they visited both flowers on male-biased plants. Similarly, their two-flower visits to plants with extreme nectar differences most often proceeded from high to low rewards. This suggests that throughout the trait's evolution, sexual selection has been stronger than natural selection to reduce geitonogamy, because if otherwise, female rather than male-biased nectar production should have been favoured (assuming no constraints on direction of bias).
Although hummingbird visit orders differed from geitonogamy avoidance predictions, they did match a basic prediction of foraging theory, i.e. flowers should be visited in order of decreasing rewards (Pyke 1978a; Best & Bierzychudek 1982). Such simple foraging rules have been thought not to apply to hummingbirds, particularly because they were derived from studies of bees (i.e. Healy & Hurly 2001). Most supporting evidence comes from bees foraging within many-flowered displays with nectar gradients (i.e. Pyke 1978a; Waddington & Heinrich 1979). Surprisingly, P. striigularis hummingbirds foraged from high to low rewards as well, even though real and artificial displays were small and unstructured. Rufous hummingbirds (Selasphorus rufus) have also been observed moving from rewarding to unrewarding artificial flowers in small clusters (Hurly 1996). Although hummingbirds probably forage from high to low rewards for different reasons than bees, this study shows that certain movement rules can apply across taxa.
(c) Geitonogamy avoidance hypothesis: costs of selfing
Current and previous studies confirm that C. friedrichsthaliana exhibits few immediate costs of self-fertilization. The following observations support this conclusion: maximal autogamy produces nearly as many seeds as a single outcrossed visit (Carlson 2007b), and two geitonogamous visits are equivalent to two outcrossed visits in terms of seed quality and quantity (current study). Even if statistical power were increased by larger sample sizes, non-significant trends suggest that outcrossing could only improve germination by 11%. These findings therefore provide very limited support for the geitonogamy avoidance hypothesis in this species. It is possible that the greatest costs of selfing in C. friedrichsthaliana emerge as seedlings approach adulthood (e.g. Wolfe 1993). If, however, selfed and outcrossed offspring remain equally fit as they mature and reproduce, selection would actually favour selfing, rather than reduce it (e.g. Schoen et al. 1996; Fishman & Wyatt 1999).
The current study's plants were larger than those used previously (Carlson 2007b), which helps explain discrepancies in seed production between this and earlier studies. A positive relationship between plant size and seed production has been observed in many species (e.g. Klinkhamer & de Jong 1987; Dudash 1991; Stöcklin & Favre 1994), but remains to be tested directly in C. friedrichsthaliana. If larger plants are more fecund, they may also be more capable of overriding costs of selfing than small plants (Wolfe 1993). Such maternal effects are pervasive in plants, and they should be particularly strong during the early life stages of offspring (Roach & Wulff 1987; Wolfe 1993). Given this possibility, i.e. the low costs of selfing I have observed in large vigorous plants may not be representative of the population as a whole.
Despite weak evidence that there exist costs of selfing in C. friedrichsthaliana, an important cost of geitonogamy—pollen discounting—remains unmeasured. Pollen discounting imposes reproductive costs even when selfing does not, and it potentially intensifies mate limitation on male fitness (i.e. sexual selection) by reducing pollen availability for outcrossing. If male-biased nectar in C. friedrichsthaliana is even partially maintained by selection to reduce geitonogamy, it is most likely through pollen discounting. Only a few studies have experimentally assessed pollen discounting (e.g. Fishman 2000), and further research is needed to clarify its importance in geitonogamy avoidance and sexual selection hypotheses.
In summary, experimental results suggest that male-biased nectar in C. friedrichsthaliana functions predominately to promote male-phase visits, but also reduces geitonogamy to a limited extent. By linking nectar, pollinator responses and plant fitness in C. friedrichsthaliana, I provide a strong mechanistic basis for the adaptive benefits of male-biased nectar production.
Acknowledgments
The author is grateful to K. E. Harms for unfailing support, guidance and editorial advice; M. A. Carlson and G. Reed for laboratory assistance; J. T. Cronin, W. J. Platt, R. D. Stevens, P. R. Gagnon, R. A. Valverde, E. J. Temeles and K. E. Holsinger for thoughtful editorial suggestions and comments; B. A. Moser, J. A. Myers, H. A. Passmore, J. R. Eberhard and C. E. T. Paine for advice on statistical and experimental designs (the latter two also provided excellent editorial suggestions); and W. G. Eberhard, M. J. West-Eberhard and the 2004–2006 staff of Centro Tropical for field support. J. T. Cronin generously allowed the use of his incubator. Funding was provided by LSU Biograds, the Garden Club of America, Sigma Xi, The Explorers Club and a Louisiana Board of Regents Graduate Fellowship.
Supplementary Material
Seed production and quality in Chrysothemis friedrichsthaliana following hand-pollination with selfed and outcrossed pollen in 2004 and 2005. Costs of inbreeding are measured in terms of: (a) number of seeds produced per capsule, (b) percentage of seeds that germinated, and (c) the mean seed weight per seed. Germination trials were performed on 100 seeds each, and lasted 23 days. Least square means with different lowercase letters are significantly different (Tukey-Kramer adjusted, α=0.05), and error bars represent ±1 s.e.
References
- Aizen M.A, Basilio A. Sex differential nectar secretion in protandrous Alstroemeria aurea (Alstroemeriaceae): is production altered by pollen removal and receipt? Am. J. Bot. 1998;85:245–252. doi:10.2307/2446312 [PubMed] [Google Scholar]
- Arnold S.J. Bateman's principles and the measurement of sexual selection in plants and animals. Am. Nat. 1994;144:S126–S149. doi:10.1086/285656 [Google Scholar]
- Ashman T.L, Morgan M.T. Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context. Proc. R. Soc. B. 2004;271:553–559. doi: 10.1098/rspb.2003.2642. doi:10.1098/rspb.2003.2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman T.L, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. doi:10.1890/03-8024 [Google Scholar]
- Bateman A.J. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. doi:10.1038/hdy.1948.21 [DOI] [PubMed] [Google Scholar]
- Bell G. On the function of flowers. Proc. R. Soc. B. 1985;224:223–265. doi:10.1098/rspb.1985.0031 [Google Scholar]
- Bell G, Lefebvre L, Giraldeau L.A, Weary D. Partial preference of insects for the male flowers of an annual herb. Oecologia. 1984;64:287–294. doi: 10.1007/BF00379123. doi:10.1007/BF00379123 [DOI] [PubMed] [Google Scholar]
- Best L.S, Bierzychudek P. Pollinator foraging on foxglove (Digitalis purpurea)—a test of a new model. Evolution. 1982;36:70–79. doi: 10.1111/j.1558-5646.1982.tb05011.x. doi:10.2307/2407968 [DOI] [PubMed] [Google Scholar]
- Biernaskie J.M, Cartar R.V, Hurly T.A. Risk-averse inflorescence departure in hummingbirds and bumble bees: could plants benefit from variable nectar volumes? Oikos. 2002;98:98–104. doi:10.1034/j.1600-0706.2002.980110.x [Google Scholar]
- Boose D.L. Sources of variation in floral nectar production rate in Epilobium canum (Onagraceae): implications for natural selection. Oecologia. 1997;110:493–500. doi: 10.1007/s004420050185. doi:10.1007/s004420050185 [DOI] [PubMed] [Google Scholar]
- Carlson, J. E. 2007a Floral traits, pollinator behavior and plant reproduction: tests of natural and sexual selection in the hummingbird-pollinated herb Chrysothemis friedrichsthaliana PhD dissertation, Louisiana State University, Baton Rouge, LA, USA.
- Carlson J.E. Male-biased nectar production in a protandrous herb matches predictions of sexual selection theory in plants. Am. J. Bot. 2007b;94:674–682. doi: 10.3732/ajb.94.4.674. doi:10.3732/ajb.94.4.674 [DOI] [PubMed] [Google Scholar]
- Carlson J.E, Harms K.E. The evolution of gender-biased nectar production in hermaphroditic plants. Bot. Rev. 2006;72:179–205. doi:10.1663/0006-8101(2006)72[179:TEOGNP]2.0.CO;2 [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 1987;18:237–268. doi:10.1146/annurev.es.18.110187.001321 [Google Scholar]
- Cresswell J.E. Stabilizing selection and the structural variability of flowers within species. Ann. Bot. 1998;81:463–473. doi:10.1006/anbo.1998.0594 [Google Scholar]
- Cruden R.W, Hermann S.M, Peterson S. Patterns of nectar production and plant–pollinator coevolution. In: Bentley B, Elias T, editors. The biology of nectaries. Columbia University Press; New York, NY: 1983. pp. 80–125. [Google Scholar]
- de Jong T.J, Waser N.M, Klinkhamer P.G.L. Geitonogamy: the neglected side of selfing. Trends Ecol. Evol. 1993;8:321–325. doi: 10.1016/0169-5347(93)90239-L. doi:10.1016/0169-5347(93)90239-L [DOI] [PubMed] [Google Scholar]
- Devlin B, Stephenson A.G. Sex differential floral longevity, nectar secretion, and pollinator foraging in a protandrous species. Am. J. Bot. 1985;72:303–310. doi:10.2307/2443557 [Google Scholar]
- Dudash M.R. Plant size effects on female and male function in hermaphroditic Sabatia angularis (Gentianaceae) Ecology. 1991;72:1004–1012. doi:10.2307/1940600 [Google Scholar]
- Fishman L. Pollen discounting and the evolution of selfing in Arenaria uniflora (Caryophyllaceae) Evolution. 2000;54:1558–1565. doi: 10.1111/j.0014-3820.2000.tb00701.x. doi:10.1111/j.0014-3820.2000.tb00701.x [DOI] [PubMed] [Google Scholar]
- Fishman L, Wyatt R. Pollinator-mediated competition, reproductive character displacement, and the evolution of selfing in Arenaria uniflora (Caryophyllaceae) Evolution. 1999;53:1723–1733. doi: 10.1111/j.1558-5646.1999.tb04557.x. doi:10.2307/2640435 [DOI] [PubMed] [Google Scholar]
- Gill F.B. Trapline foraging by hermit hummingbirds: competition for an undefended, renewable resource. Ecology. 1988;69:1933–1942. doi:10.2307/1941170 [Google Scholar]
- Gonzalez A, Rowe C.L, Weeks P.J, Whittle D, Gilbert F.S, Barnard C.J. Flower choice by honey bees (Apis mellifera L.)—sex-phase of flowers and preferences among nectar and pollen foragers. Oecologia. 1995;101:258–264. doi: 10.1007/BF00317292. doi:10.1007/BF00317292 [DOI] [PubMed] [Google Scholar]
- Healy S.D, Hurly T.A. Foraging and spatial learning in hummingbirds. In: Chittka L, Thomson J.D, editors. Cognitive ecology of pollination. Cambridge University Press; Cambridge, UK: 2001. pp. 127–147. [Google Scholar]
- Hinkelmann C, Schuchmann K.L. Phylogeny of the hermit hummingbirds (Trochilidae: Phaethornithinae) Stud. Neotrop. Fauna Environ. 1997;32:142–163. [Google Scholar]
- Hurly A.T. Spatial memory in rufous hummingbirds: memory for rewarded and non-rewarded sites. Anim. Behav. 1996;51:177–183. doi:10.1006/anbe.1996.0015 [Google Scholar]
- Irwin R.E. Hummingbird avoidance of nectar-robbed plants: spatial location or visual cues. Oikos. 2000;91:499–506. doi:10.1034/j.1600-0706.2000.910311.x [Google Scholar]
- Johnson S.D, Nilsson L.A. Pollen carryover, geitonogamy, and the evolution of deceptive pollination systems in orchids. Ecology. 1999;80:2607–2619. doi:10.1890/0012-9658(1999)080[2607:PCGATE]2.0.CO;2 [Google Scholar]
- Klinkhamer P.G.L, de Jong T.J. Plant size and seed production in the monocarpic perennial Cynoglossum officinale L. New Phytol. 1987;106:773–783. doi: 10.1111/j.1469-8137.1987.tb00178.x. doi:10.1111/j.1469-8137.1987.tb00178.x [DOI] [PubMed] [Google Scholar]
- Littell R, Milliken G, Stroup W, Wolfinger R, Schabenberger O. SAS Publishing; Cary, NC: 2006. SAS for mixed models. [Google Scholar]
- McDade L.A, Weeks J.A. Nectar in hummingbird-pollinated neotropical plants I: patterns of production and variability in 12 species. Biotropica. 2004;36:196–215. doi:10.1111/j.1744-7429.2004.tb00312.x [Google Scholar]
- Melendez-Ackerman E, Campbell D.R. Adaptive significance of flower color and inter-trait correlations in an Ipomopsis hybrid zone. Evolution. 1998;52:1293–1303. doi: 10.1111/j.1558-5646.1998.tb02011.x. doi:10.2307/2411299 [DOI] [PubMed] [Google Scholar]
- Melendez-Ackerman E, Campbell D.R, Waser N.M. Hummingbird behavior and mechanisms of selection on flower color in Ipomopsis. Ecology. 1997;78:2532–2541. doi:10.1890/0012-9658(1997)078[2532:HBAMOS]2.0.CO.2 [Google Scholar]
- Miller R.S, Tamm S, Sutherland G.D, Gass C.L. Cues for orientation in hummingbird foraging—color and position. Can. J. Zool. 1985;63:18–21. [Google Scholar]
- Mitchell R.J. Adaptive significance of Ipomopsis aggregata nectar production—observation and experiment in the field. Evolution. 1993;47:25–35. doi: 10.1111/j.1558-5646.1993.tb01196.x. doi:10.2307/2410115 [DOI] [PubMed] [Google Scholar]
- Ordano M, Ornelas J.F. The cost of nectar replenishment in two epiphytic bromeliads. J. Trop. Ecol. 2005;21:541–547. doi:10.1017/S026646740500266X [Google Scholar]
- Pleasants J.M. Nectar production patterns in Ipomopsis aggregata (Polemoniaceae) Am. J. Bot. 1983;70:1468–1475. doi:10.2307/2443345 [Google Scholar]
- Pyke G.H. Optimal foraging in bumblebees and coevolution with their plants. Oecologia. 1978a;36:281–293. doi: 10.1007/BF00348054. doi:10.1007/BF00348054 [DOI] [PubMed] [Google Scholar]
- Pyke G.H. Optimal foraging in hummingbirds: testing the marginal value theorem. Am. Zool. 1978b;18:739–752. doi:10.1093/icb/18.4.739 [Google Scholar]
- Pyke G.H. What does it cost a plant to produce floral nectar? Nature. 1991;350:58–59. doi:10.1038/350058a0 [Google Scholar]
- Rathcke B.J. Nectar distributions, pollinator behavior and plant reproductive success. In: Hunter M.D, Ohgushi T, Price P.W, editors. Effects of resource distribution on animal–plant interactions. Academic Press, Inc; New York, NY: 1992. pp. 113–138. [Google Scholar]
- Real L.A, Rathcke B.J. Individual variation in nectar production and its effects on fitness of Kalmia latifolia. Ecology. 1991;72:149–155. doi:10.2307/1938910 [Google Scholar]
- Roach D.A, Wulff R.D. Maternal effects in plants. Annu. Rev. Ecol. Syst. 1987;18:209–235. doi:10.1146/annurev.es.18.110187.001233 [Google Scholar]
- SAS Institute, Inc. SAS Institute, Inc; Cary, NC: 2005. The GLIMMIX procedure, Nov. 2005. [Google Scholar]
- Schemske D.W, Bradshaw H.D., Jr Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus) Proc. Natl Acad. Sci. USA. 1999;96:11 910–11 915. doi: 10.1073/pnas.96.21.11910. doi:10.1073/pnas.96.21.11910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen D.J, Morgan M.T, Bataillon T. How does self-pollination evolve? Inferences from floral ecology and molecular genetic variation. Phil. Trans. R. Soc. B. 1996;351:1281–1290. doi:10.1098/rstb.1996.0111 [Google Scholar]
- Skogsmyr I, Lankinen A. Sexual selection: an evolutionary force in plants. Biol. Rev. 2002;77:537–562. doi: 10.1017/s1464793102005973. doi:10.1017/S1464793102005973 [DOI] [PubMed] [Google Scholar]
- Skutch A.F. Tussacia friedrichsthaliana, a terrestrial herb with aquatic flowers. Brenesia. 1992;37:151–156. [Google Scholar]
- Southwick E.E. Photosynthate allocation to floral nectar—a neglected energy investment. Ecology. 1984;65:1775–1779. doi:10.2307/1937773 [Google Scholar]
- Stephens D.W, Krebs J.R. Princeton University Press; Princeton, NJ: 1986. Foraging theory. [Google Scholar]
- Stiles F.G, Skutch A.F. Cornell University Press; Ithaca, NY: 1990. A guide to the birds of Costa Rica. [Google Scholar]
- Stöcklin J, Favre P. Effects of plant size and morphological constraints on variation in reproductive components in two related species of Epilobium. J. Ecol. 1994;82:735–746. doi:10.2307/2261439 [Google Scholar]
- Temeles E.J, Linhart Y.B, Masonjones M, Masonjones H.D. The role of flower width in hummingbird bill length–flower length relationships. Biotropica. 2002;34:68–80. doi:10.1111/j.1744-7429.2004.tb00312.x [Google Scholar]
- Temeles E.J, Shaw K.C, Kudla A.U, Sander S.E. Traplining by purple-throated carib hummingbirds: behavioral responses to competition and nectar availability. Behav. Ecol. Sociobiol. 2006;61:163–172. doi:10.1007/s00265-006-0247-4 [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B.G, editor. Sexual selection and the decent of man. Aldine; Chicago, IL: 1972. pp. 1871–1971. [Google Scholar]
- Waddington K.D, Heinrich B. The foraging movements of bumblebees on vertical ‘inflorescences’: an experimental analysis. J. Comp. Physiol. 1979;134:113–117. doi:10.1007/BF00610469 [Google Scholar]
- Willson M.F. Sexual selection in plants—perspective and overview. Am. Nat. 1994;144:S13–S39. doi:10.1086/285651 [Google Scholar]
- Wolfe L.M. Inbreeding depression in Hydrophyllum appendiculatum: role of maternal effects, crowding, and parental mating history. Evolution. 1993;47:374–386. doi: 10.1111/j.1558-5646.1993.tb02100.x. doi:10.2307/2410058 [DOI] [PubMed] [Google Scholar]
- Zimmerman M. Plant reproduction and optimal foraging—experimental nectar manipulations in Delphinium nelsonii. Oikos. 1983;41:57–63. doi:10.2307/3544346 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Seed production and quality in Chrysothemis friedrichsthaliana following hand-pollination with selfed and outcrossed pollen in 2004 and 2005. Costs of inbreeding are measured in terms of: (a) number of seeds produced per capsule, (b) percentage of seeds that germinated, and (c) the mean seed weight per seed. Germination trials were performed on 100 seeds each, and lasted 23 days. Least square means with different lowercase letters are significantly different (Tukey-Kramer adjusted, α=0.05), and error bars represent ±1 s.e.