Abstract
For prey animals to negotiate successfully the fundamental trade-off between predation and starvation, a realistic assessment of predation risk is vital. Prey responses to conspicuous indicators of risk (such as looming predators or fleeing conspecifics) are well documented, but there should also be strong selection for the detection of more subtle cues. A predator's head orientation and eye-gaze direction are good candidates for subtle but useful indicators of risk, since many predators orient their head and eyes towards their prey as they attack. We describe the first explicit demonstration of a bird responding to a live predator's eye-gaze direction. We present wild-caught European starlings (Sturnus vulgaris) with human ‘predators’ whose frontal appearance and gaze direction are manipulated independently, and show that starlings are sensitive to the predator's orientation, the presence of eyes and the direction of eye-gaze. Starlings respond in a functionally significant manner: when the predator's gaze was averted, starlings resumed feeding earlier, at a higher rate and consumed more food overall. By correctly assessing lower risk and returning to feeding activity earlier (as in this study), the animal gains a competitive advantage over conspecifics that do not respond to the subtle predator cue in this way.
Keywords: predation risk, starvation predation trade-off, eye-gaze direction, predator orientation, anti-predator vigilance, European starlings Sturnus vulgaris
1. Introduction
All prey animals are under pressure to be vigilant against predation. While vigilance and feeding are not always mutually exclusive activities (Cresswell et al. 2003; Cowlishaw et al. 2004), feeding often reduces vigilance (Lima & Bednekoff 1999; Devereux et al. 2006). Because the costs of failing to identify predation risk can be high, animals are expected to overestimate the risk of predation (Kavaliers & Choleris 2001) and to maintain a baseline expectation of risk at all times (Lima & Dill 1990). However, long-term costs of anti-predator responses (review by Lima 1998) provide an incentive to delay or reduce feeding only when really necessary, adjusting behaviour in line with the current level of risk. The risk may depend on the current situation of the prey (e.g. the effect of being in a group: theory reviewed by Bednekoff & Lima (1998)) or on the predator's characteristics (the threat-sensitivity hypothesis: Helfman 1989). While responses to conspicuous indicators (such as looming predators or fleeing conspecifics) are well documented, responses to subtle predator cues have been studied in less depth. We investigate responses to aspects of predator orientation, including eye-gaze direction, which is a very subtle and somewhat overlooked cue of predatory risk. Until now there has been no evidence of prey animals responding to the direction of a predator's eye-gaze, which may be a common signal in mammalian predators, despite the functional advantage it could provide (particularly for competitive socially feeding prey species, where even small competitive advantages can be very important).
Some of the more conspicuous cues of predatory risk that prey respond to include the proximity of a potential predator (Ydenberg & Dill 1986), whether the predator is looming towards the prey (Schiff et al. 1962; Wang & Frost 1992; Carlile et al. 2006) and its speed or direction of movement (Stankowich & Blumstein 2005). Responses to even these relatively simple cues can be refined as a result of experience (Burger & Gochfeld 1981, 1990). A more subtle indicator of risk is the predator's orientation, since predators typically face their prey when stalking or attacking. Some evidence of responses to a human's orientation has been suggested in field studies (Ristau 1991; Watve et al. 2002). Because many predators rely on binocular vision and focus their eyes towards their prey as they attack, eye-gaze direction would be an even more sensitive cue of predation risk. Eye-gaze cues are likely to be of limited use when predators are distant or in visually cluttered environments, but could provide the necessary sensitivity to allow prey to function efficiently when their potential predators are nearby.
Sensitivity to eyes and eye-like stimuli is a widespread phenomenon that is expressed in many contexts (reviewed by Emery 2000). Birds respond to human gaze cues in a non-predation context (Bugnyar et al. 2004), and to artificial eye-like stimuli in a predation context (reviewed by Curio 1993), often from a very early age (e.g. Gallup et al. 1971; Jones 1980). The movement of artificial eye-like stimuli also has an effect: chicks show greater fear responses to artificial eyes that appear to track the subject rather than avoiding it (Scaife 1976).
Experiments with live predators have claimed to show that birds are sensitive to the ‘gaze’ of a predator (Gallup et al. 1972), but often do not clearly distinguish between eye-gaze direction and head orientation. One notable exception is a study comparing the responses of captive house sparrows (Passer domesticus) with a human predator whose head and eyes could either face towards or away from the prey independently and in various combinations (Hampton 1994). This study showed that house sparrows are sensitive to the head orientation of a nearby human predator, but found no evidence that they respond to the direction of eye-gaze. It may be important for prey to distinguish a predator's eye-gaze direction from head orientation, since these cues do not always correlate in predators. As a consequence of the limited field of view, and particularly the limited area of high-quality vision in birds and mammals, eye movements may be used to focus the eye's area of best vision onto the target (Carpenter 1977). For example, a stalking cat might minimize conspicuous movements of its body or head, while moving its eyes laterally to retain focus on its target prey. In this situation, a nearby non-target bird may be in direct line of the predator's body, but off its directed line of sight. The benefit from recognizing this lower risk would be avoiding an unnecessary anti-predator response. Even very small adjustments in the timing of escape responses can be biologically meaningful (Quinn & Cresswell 2005a), and the nature of the response, as well as the return to normal activity after predation, could have important fitness consequences (Lima 1998).
We test whether European starlings (Sturnus vulgaris) are able to respond to increasingly subtle cues of risk from a nearby live predator, including the ability to use eye-gaze cues when the body and head orientation are controlled. The starling has already been shown to be responsive to more conspicuous indicators of risk in other experimental studies of anti-predator behaviour (e.g. Lima & Bednekoff 1999; Devereux et al. 2006). In the wild, starlings have exploited the anthropogenic environment for nesting, roosting and feeding (Feare 1984; Cramp & Perrins 1994), but have been labelled and targeted as a pest species and consequently experience a predation risk from humans (e.g. lethal control measures: Woolnough et al. 2006). There is also anecdotal evidence suggesting that starlings adjust their anti-predator responses appropriately in response to humans. In captivity, starlings can be successfully habituated to the presence of humans who provide valued resources, and hence their prolific use in behavioural research (Hawkins et al. 2001), but still maintain typical anti-predator behaviours when humans present a clear risk such as when catching the birds for experimental or husbandry purposes. In urban habitats, it is common for starlings to continue feeding in the vicinity of approaching humans, not fleeing until the humans get very close (within 2 m) and begin to present an imminent threat (J. Carter & A. R. Goldsmith 2007, personal observation). The starlings' apparent ability to adjust their responses to the real-time risk posed by a potential human predator may well have played a part in their ability to exploit the human environment rapidly and extensively.
Using captive flocks of starlings and human predators, we manipulated the cues given by a nearby live predator and measured responses to increasingly subtle differences in the predator's orientation and gaze cues. Because birds may show individual behavioural syndromes regarding responses to predation risk (Quinn & Cresswell 2005b) and respond differently according to sex or dominance (Hegner 1985; Kavaliers & Choleris 2001), paired designs were used to assess the response of each bird to the cues that represented ‘higher’ and ‘lower’ risk in each experiment. We considered an increase in feeding behaviour as an indication that the birds perceived a lower level of risk (because feeding reduces vigilance; Devereux et al. 2006). We first tested whether starlings respond to the body and face orientation of a nearby predator. Second, we tested whether starlings are affected by the presence of eyes on a nearby predator that is facing them (there is already evidence that starlings can respond to the presence of artificial eye-like stimuli; Inglis et al. 1983). Finally, we tested whether starlings respond to the eye-gaze direction of a live predator that is nearby and facing the starlings directly (i.e. controlling for the head and body orientation).
2. Material and methods
(a) Subjects
Twenty wild European starlings were decoy-trapped as juveniles on farmland in southwest England (under English Nature licence) and subsequently tested as adults. A total of 10 males and 10 females were randomly assigned into non-breeding male–female pairs, and were housed and tested in outdoor aviaries at the University of Bristol. All pairs were in visual and acoustic contact with the others, in a wire-mesh aviary system consisting of two rows of five units (each 1.8 m long×0.9 m wide×1.8 m tall) either side of a central corridor. The starlings had access to water and water baths at all times, access to high-protein chick crumb food when the experiment was not in progress and were accustomed to feeding from a dish of mealworms with a human present (mealworms, larvae of the Tenebrio molitor beetle, are a valued food reward for starlings). On completion of the study, all subjects were inspected by the University Veterinary Officer before being released back into the wild at sites in the Bristol area where stable wild starling flocks were present.
(b) Design and procedure
Ten pairs of starlings took part in three repeated-measures experiments, each experiment comprising two trials (higher and lower risks) presented to each pair. At the beginning of each experimental session, the crumb food was removed. Trials began 2–90 min after food removal, depending on trial order. For each pair, the two trials in a given experiment took place 24 hours apart, and the food deprivation period was the same for both trials. The food source provided for the experiments was a clear plastic dish (10 cm diameter) filled with mealworms. In each experimental trial, the potential predator was a human situated directly in front of the birds' aviary unit, with the food source introduced for 300 s to the floor of the unit 1 m in front of the predator. A second person, with their face obscured, recorded the starlings' responses without providing any confounding facial cues (see figure 1 for further details).
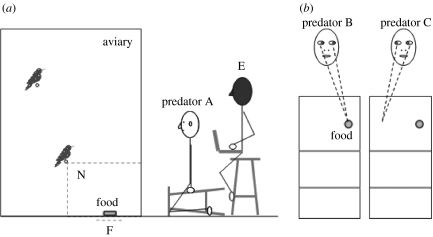
Figure 1.
(a) Side and (b) plan views of experimental situation. In experiment 1, the predator faced directly towards the aviary unit (predator A) or directly away. In experiment 2, the predator faced directly towards the aviary (predator A) with eyes showing or eyes covered. In experiment 3, the predator faced directly towards the aviary with eye-gaze directed either laterally towards the food source (predator B) or laterally away from the food source (predator C). The experimenter (E), sitting behind the predator, recorded the starlings' responses to the human predator without providing face cues (head is obscured by a fine-mesh stocking hood). A starling was considered near to the food in zone N (forward and below the lowest perch) and within bird's reach of the food source in region F.
In experiment 1, the ‘higher risk’ cue was a predator facing directly towards the aviary unit with its eye-gaze directed towards a centrally placed food source, and the ‘lower risk’ cue was a predator facing directly away from the aviary unit. In experiment 2, the higher risk cue was a predator facing directly towards the aviary unit with its eyes unobstructed (and with one of three alternative face areas covered by a horizontal white cloth band, 5 cm wide, across the forehead, nose or mouth) and its eye-gaze directed towards a centrally placed food source, and the lower risk cue was a predator facing directly towards the aviary unit with the band covering its eyes. In experiment 3, the higher risk cue was a predator facing directly towards the aviary unit with its eye-gaze directed towards a laterally placed food source, and the lower risk cue was a predator facing directly towards the aviary unit with its eye-gaze directed away from the laterally placed food source (figure 1).
To minimize between-subject carry-over effects, the predator had opaque barriers either side of its face so that only the pair being tested could see the predator's facial features. In each experiment, half of the pairs received the higher risk presentation before the lower risk presentation, and half the converse. Experiment 3 was balanced for the lateral position of the food source (left or right). Two predators (N.J.L. and H.L.C.) were used in alternate trials within each experiment to reduce pseudoreplication (Hurlbert 1984).
(c) Analysis
Data were recorded using EthoLog v. 2.25 behavioural transcription software (Ottoni 2000) and analysed using Minitab v. 14 (with normality of differences or residuals, and homogeneity of variances confirmed as necessary for parametric tests). Experiment 1 measured the behaviour of pairs: latency for the first bird to go near the food source (into near zone); latency until feeding began; and the total number of mealworms eaten by both birds. In experiments 2 and 3, we measured the behaviour of individuals within each pair separately: latency for each bird to approach the food source (feet within 50 mm of the food source: bird within reach of the food); latency until each bird began feeding; and the number of mealworms eaten by each bird. Effect sizes are presented as ‘mean difference±1 s.e’.
Where no approach or feeding occurred within a 5 min trial (only ever in the higher risk condition), a value of 300 s latency was substituted; the effect of predatory cues on the latency to approach or feed is then an underestimate. Latency data were usually log transformed (to correct positive skew and unequal variances). Absolute food intake rate was calculated by dividing the number of mealworms consumed by the feeding time (time remaining in the trial after feeding had begun).
Paired t-tests were used to analyse the behaviour of the pairs in experiment 1. Balanced repeated-measures ANOVAs were used for analysing the behaviour of individual birds in experiments 2 and 3: for each measure in each experiment, a single ANOVA modelled ‘predation’ (higher and lower risks) and ‘sex’ as fixed main factors, ‘pair’ as a random factor and all two-way interactions of these factors. Post hoc analyses were performed to investigate any significant predation×sex interactions using paired t-tests for males and females separately.
3. Results
(a) Experiment 1: head and body orientation
A pair of starlings took significantly longer to go near a food source (difference 9.0±3.3 s; t9=2.78, p=0.021), took longer to start feeding (68.1±34.0 s; log transformed t9=4.04, p=0.003), ate at a slower rate (0.03±0.01 mealworms per sec; t9=−2.34, p=0.044) and ate fewer mealworms in total (10.8±3.7 mealworms; t9=−2.96, p=0.016), if a predator was facing towards them than if it was facing away from them.
(b) Experiment 2: presence of eyes
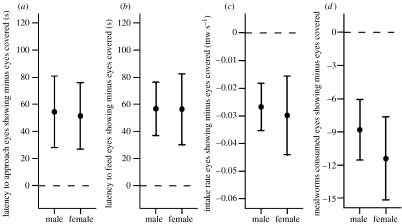
Starlings took significantly longer to approach a food source (log transformed; predation×sex interaction: F1,9=1.82, p=0.210; main effect sex: F1,9=1.05, p=0.333; main effect predation: F1,9=13.83, p=0.005) and took longer to start feeding (log transformed; predation×sex interaction: F1,9=1.38, p=0.271; main effect sex: F1,9=1.86, p=0.206; main effect predation: F1,9=13.75, p=0.005), if a predator's eyes were showing than if they were covered. Starlings also ate at a slower rate (predation×sex interaction: F1,9=0.03, p=0.875; main effect sex: F1,9=5.51, p=0.043, females ate faster than males overall; main effect predation: F1,9=17.04, p=0.003), and ate fewer mealworms in total (predation×sex interaction: F1,9=0.26, p=0.619; main effect sex: F1,9=4.64, p=0.060; main effect predation: F1,9=22.90, p=0.001), if a predator's eyes were showing than if they were covered (see figure 2 for effect sizes).
Figure 2.
Starlings were slower to (a) approach and (b) feed from a food source if a nearby (human) predator's eyes were visible rather than covered: mean difference in latency (eyes showing minus eyes covered) was positive for approach and feeding in both sexes. Starlings also (c) fed at a slower rate and (d) ate fewer mealworms (mw) in total from a food source if a nearby (human) predator's eyes were visible rather than covered: mean difference in latency (eyes showing minus eyes covered) was negative in both sexes. Values represent mean difference ±1 s.e.
(c) Experiment 3: eye-gaze direction
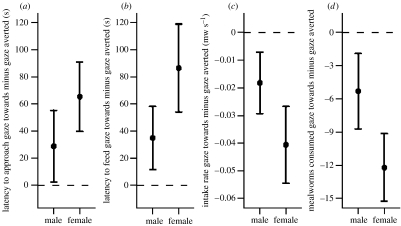
Starlings took significantly longer to approach a food source (log transformed; predation×sex interaction: F1,9=1.28, p=0.287; main effect sex: F1,9<0.01, p=0.983; main effect predation: F1,9=23.12, p=0.001) and took longer to start feeding (log transformed; predation×sex interaction: F1,9=0.67, p=0.435; main effect sex: F1,9=0.01, p=0.910; main effect predation: F1,9=19.44, p=0.002) if a predator's eye-gaze was directed towards the food source than if it was directed away from the food source. Starlings also ate at a slower rate (predation×sex interaction: F1,9=7.05, p=0.026, greater predation effect for females than males: post hoc tests males t9=−1.63, p=0.137, females t9=−2.92, p=0.017), and ate fewer mealworms in total (predation×sex interaction: F1,9=9.94, p=0.012, greater predation effect for females than males: post hoc tests males t9=−1.56, p=0.154, females t9=−3.97, p=0.003), if a predator's eye-gaze was directed towards the food source than if it was directed away from the food source (see figure 3 for effect sizes).
Figure 3.
Starlings were slower to (a) approach and (b) feed from a food source if a nearby (human) predator's eye-gaze was directed towards the food source rather than away from the food source: mean difference in latency (gaze towards minus gaze averted) was positive for approach and feeding in both sexes. Starlings also (c) fed at a slower rate and (d) ate fewer mealworms (mw) in total from a food source if a nearby (human) predator's eye-gaze was directed towards the food source rather than away from the food source: mean difference (gaze towards minus gaze averted) was negative in both sexes. Values represent mean difference ±1 s.e.
4. Discussion
Starlings increase their feeding efficiency by responding to increasingly subtle risk cues of a nearby live predator, including the direction of the predator's eye-gaze. Starlings show less delay in approaching a food source and feeding, and a higher rate of mealworm intake when the predator cue suggests lower risk: when the predator is facing away, with eyes covered or with eye-gaze directed to the side, compared with the higher risk cue of a predator directly facing or looking at the food source. The functional outcome of the starlings' responses to these predation cues is a greater mealworm intake in the lower risk conditions.
The females' rate of mealworm intake (and consequently total intake) was affected more than the males' intake by the predator's eye-gaze direction, though both males and females did show considerable effects in the same direction: increased intake when the eye-gaze was averted to the side. The sample sizes for the post hoc tests were quite small, so the lack of statistical significance for the male subsample may be the result of low statistical power. The result may however represent a genuine sex difference (females being more sensitive than males to the predator's eye-gaze direction), or alternatively a dominance relationship between two individuals housed together as a pair: male–female pairs were used in our design, so sex and dominance may be confounded.
Previous empirical fieldwork measuring anti-predator responses to a predator's orientation and gaze direction has often confounded several cues, or else not made the distinction explicit: for example, body orientation confounded with direction of approach (Burger & Gochfeld 1981; Ristau 1991) or head orientation (head gaze) with eye-gaze direction (Gallup et al. 1972; Watve et al. 2002). Controlled laboratory studies with artificial eye-like stimuli do not necessarily transfer to situations with a live predator (when proximity, body and face direction already indicate high risk). Our study, similar to that of Hampton (1994), used wild-caught birds in a controlled laboratory setting with a live predator. It precisely controlled the cues of a nearby live human predator to de-confound the birds' responses to the body, face and eye cues of the predator. We go beyond the studies of eye-like stimuli in demonstrating that birds respond to the eyes of a nearby live predator, and find evidence (unlike Hampton's 1994 sparrow results) that starlings respond to the eye-gaze direction of a predator when the body and head orientation are controlled. To our knowledge, this is the first explicit demonstration of a bird responding appropriately to the direction of a predator's eye-gaze as a cue of relative predation risk.
The starlings' responses to the predator cues have immediate consequences that would be advantageous to starlings in the wild. Wild starlings are highly social (Feare 1984; Cramp & Perrins 1994) and will quickly join conspecifics at a productive foraging patch (Templeton & Giraldeau 1995; Koops & Giraldeau 1996). This leads to foraging situations that are highly competitive. An individual starling that correctly assesses a relatively low predation risk, and responds by returning more quickly to a foraging patch (as in our study) compared with a starling that has not responded to the predator's cues, will gain valuable feeding time before others join the patch. An individual that assesses a relatively low risk, and responds with an increased intake rate once it has returned to the patch (as in our study), will gain a larger share of the depleting resource per unit time than a competitor that has not increased its feeding rate in response to the predator cues. Even in the testing situation, where the focal birds were only in competition with a single conspecific and not under particular pressure to feed (there was no deprivation or stochasticity of food supply), there was a significant functional consequence of these responses: an increased overall intake of mealworms in the 5 min window of opportunity.
The possible mechanisms behind the starlings' ability to discriminate such subtle predator cues merit consideration. At first sight, we might suppose that the responses to the orientation of the predator, to whether the eyes are covered and to the direction of the predator's eye-gaze suggest that starlings are able to recognize when a predator is looking at them and infer the likelihood that they will consequently be chased. Indeed, the idea that ‘from a gaze cue the animal understands where and what a human can see’ is the sort of interpretation that has been offered in some studies of animal responses to human gaze cues (including Smitha et al. 1999; Watve et al. 2002; Kaminski et al. 2004; Bräuer et al. 2005). While we do not deny the interesting possibility that starlings might have a cognitive appreciation of the knowledge state of their predator through recognizing the predator's visual perspective, this type of explanation may not be necessary. The starlings' responses in these experiments could be explained as a result of innate tendencies and conditional learning during their experience prior to the study, as described below.
There are two plausible mechanisms by which starlings could come to respond to eye-gaze cues. First, circular eyespot patterns are likely to be an inherently conspicuous stimulus to the vertebrate eye (Stevens et al. 2007). An eye-gaze stimulus that is directed towards a starling will be more circular and should therefore be more conspicuous than an averted eye-gaze stimulus, and therefore should attract a starling's attention more. Indeed, the morphology of the human eye is particularly well suited for providing gaze cues (compared with other primates: Kobayashi & Kohshima 2001). Second, a human's eye-gaze direction is associated with the likelihood of subsequent predation in our captive environment: the procedure for catching individual birds (which happens on an intermittent basis during husbandry and experimental routines) involves a human looking directly at an individual starling before catching it; a starling is not captured after a human has been looking elsewhere. The starlings could therefore have acquired an eye-gaze discrimination through conditional learning. The starlings might also have exploited the experience of other individuals, further increasing the number of learning opportunities; we already know that starlings are capable of social learning in our captive environment (e.g. Campbell et al. 1999; Fawcett et al. 2002).
The opportunity for conditional learning of gaze cues will also occur in the starlings' wild urban and agricultural environments. Starlings frequently approach humans for food and roosting (e.g. farm buildings, parks, picnic sites), and when the starlings get very close they will typically be chased off (they are considered a pest by farmers in particular); a bird would only be chased when a human has noted it and therefore has looked towards it, thereby providing a conditional eye-gaze cue predicting an increased risk of predation.
Given that it generally pays to overestimate predation risk (Bouskila & Blumstein 1992), it seems likely that starlings and perhaps other animals start off with an innate ‘rule of thumb’ to exhibit anti-predator responses to all nearby live predators, and to fine-tune that rule later, reducing avoidance responses according to cues that have been reliably associated with lower risk. Fine-tuning of anti-predator responses through experience is supported by field evidence in herring gulls (Larus argentatus): reduced anti-predator responses to cues indicating lower risk when animals have increased experience of humans, while full-blown responses to high-risk situations are preserved in both naive and experienced animals (Burger & Gochfeld 1981).
Whether or not the responses involve some sort of mental attribution or theory of mind, and whether or not they are innate or acquired (these are separate issues, as elucidated by Heyes 1998), the result is that starlings are able to discriminate the very subtle eye-gaze cues of a nearby live predator and adjust their anti-predator responses to fluctuations in predation risk in an adaptively beneficial manner.
Acknowledgments
This research adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research, the legal requirements of the country in which the work was carried out and all institutional guidelines. Bristol University Animal Services Ethical Committee approved the procedures under UIN: UB/03/044.
The experiments were designed by A.R.G., J.C., N.J.L. and H.L.C. Trials were conducted by N.J.L. and H.L.C., and data analysed by J.C. J.C. had primary, and A.R.G. secondary, role in manuscript preparation. J.C. was supported by an NERC and University of Bristol studentship. We thank Rob Massie, Sadie Iles-Ryan and Di Flower for animal maintenance. We thank Andrew N. Radford, Innes C. Cuthill and two reviewers for their useful comments on the manuscript.
References
- Bednekoff P.A, Lima S.L. Randomness, chaos and confusion in the study of antipredator vigilance. Trends Ecol. Evol. 1998;13:284–287. doi: 10.1016/s0169-5347(98)01327-5. doi:10.1016/S0169-5347(98)01327-5 [DOI] [PubMed] [Google Scholar]
- Bouskila A, Blumstein D.T. Rules of thumb for predation hazard assessment: predictions from a dynamic model. Am. Nat. 1992;139:161–176. doi:10.1086/285318 [Google Scholar]
- Bräuer J, Call J, Tomasello M. All great ape species follow gaze to distant locations and around barriers. J. Comp. Psychol. 2005;119:145–154. doi: 10.1037/0735-7036.119.2.145. doi:10.1037/0735-7036.119.2.145 [DOI] [PubMed] [Google Scholar]
- Bugnyar T, Stöwe M, Heinrich B. Ravens, Corvus corax, follow gaze direction of humans around obstacles. Proc. R. Soc. B. 2004;271:1331–1336. doi: 10.1098/rspb.2004.2738. doi:10.1098/rspb.2004.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Discrimination of the threat of direct versus tangential approach to the nest by incubating herring and great black-backed gulls. J. Comp. Physiol. Psychol. 1981;95:676–684. doi:10.1037/h0077811 [Google Scholar]
- Burger J, Gochfeld M. Risk discrimination of direct versus tangential approach by basking black iguanas (Ctenosaura similis): variation as a function of human exposure. J. Comp. Psychol. 1990;104:388–394. doi:10.1037/0735-7036.104.4.388 [Google Scholar]
- Campbell F.M, Heyes C.M, Goldsmith A.R. Stimulus learning and response learning by observation in the European starling, in a two-object/two-action test. Anim. Behav. 1999;58:151–158. doi: 10.1006/anbe.1999.1121. doi:10.1006/anbe.1999.1121 [DOI] [PubMed] [Google Scholar]
- Carlile P.A, Peters R.A, Evans C.S. Detection of a looming stimulus by the Jacky dragon: selective sensitivity to characteristics of an aerial predator. Anim. Behav. 2006;72:553–562. doi:10.1016/j.anbehav.2005.10.027 [Google Scholar]
- Carpenter R.H.S. Pion; London, UK: 1977. Movements of the eyes. [Google Scholar]
- Cowlishaw G, Lawes M.J, Lightbody M, Martin A, Pettifor R, Rowcliffe J.M. A simple rule for the costs of vigilance: empirical evidence from a social forager. Proc. R. Soc. B. 2004;271:27–33. doi: 10.1098/rspb.2003.2522. doi:10.1098/rspb.2003.2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramp, S. & Perrins, C. M. (eds) 1994 Sturnus vulgaris starling. In Handbook of the birds of Europe, the Middle East and North Africa: the birds of the Western Palearctic, vol. 8 crows to finches. Oxford, UK: Oxford University Press.
- Cresswell W, Quinn J.L, Whittingham M.J, Butler S. Good foragers can also be good at detecting predators. Proc. R. Soc. B. 2003;270:1069–1076. doi: 10.1098/rspb.2003.2353. doi:10.1098/rspb.2003.2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curio E. Proximate and developmental aspects of antipredator behaviour. Adv. Study Behav. 1993;22:135–238. [Google Scholar]
- Devereux C.L, Whittingham M.J, Fernández-Juricic E, Vickery J.A, Krebs J.R. Predator detection and avoidance by starlings under differing scenarios of predation risk. Behav. Ecol. 2006;17:303–309. doi:10.1093/beheco/arj032 [Google Scholar]
- Emery N.J. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. doi:10.1016/S0149-7634(00)00025-7 [DOI] [PubMed] [Google Scholar]
- Fawcett T.W, Skinner A.M.J, Goldsmith A.R. A test of imitative learning in starlings using a two-action method with an enhanced ghost control. Anim. Behav. 2002;64:547–556. doi:10.1006/anbe.2002.3092 [Google Scholar]
- Feare C. Oxford University Press; Oxford, UK: 1984. The starling. [Google Scholar]
- Gallup G.G, Nash R.F, Ellison A.L. Tonic immobility as a reaction to predation: artificial eyes as a fear stimulus for chickens. Psychon. Sci. 1971;23:79–80. [Google Scholar]
- Gallup G.G, Cummings W.H, Nash R.F. Experimenter as an independent variable in studies of animal hypnosis in chickens (Gallus gallus) Anim. Behav. 1972;20:166–169. doi: 10.1016/s0003-3472(72)80187-8. doi:10.1016/S0003-3472(72)80187-8 [DOI] [PubMed] [Google Scholar]
- Hampton R.R. Sensitivity to information specifying the line of gaze of humans in sparrows (Passer domesticus) Behaviour. 1994;130:41–51. doi:10.1163/156853994X00136 [Google Scholar]
- Hawkins P, et al. Laboratory birds: refinements in husbandry and procedures. Lab. Anim. 2001;35:S1–S163. doi:10.1258/0023677011911967 [PubMed] [Google Scholar]
- Hegner R.E. Dominance and anti-predator behavior in blue tits (Parus caeruleus) Anim. Behav. 1985;33:762–768. doi:10.1016/S0003-3472(85)80008-7 [Google Scholar]
- Helfman G.S. Threat-sensitive predator avoidance in damselfish–trumpetfish interactions. Behav. Ecol. Sociobiol. 1989;24:47–58. doi:10.1007/BF00300117 [Google Scholar]
- Heyes C.M. Theory of mind in nonhuman primates. Behav. Brain Sci. 1998;21:101–148. doi: 10.1017/s0140525x98000703. doi:10.1017/S0140525X98000703 [DOI] [PubMed] [Google Scholar]
- Hurlbert S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984;54:187–211. doi:10.2307/1942661 [Google Scholar]
- Inglis I.R, Huson L.W, Marshall M.B, Neville P.A. The feeding behaviour of starlings (Sturnus vulgaris) in the presence of eyes. Z. Tierpsychol. J. Comp. Ethol. 1983;62:181–208. [Google Scholar]
- Jones R.B. Reactions of male domestic chicks to two-dimensional eye-like shapes. Anim. Behav. 1980;28:212–218. doi:10.1016/S0003-3472(80)80025-X [Google Scholar]
- Kaminski J, Call J, Tomasello M. Body orientation and face orientation: two factors controlling apes' begging behavior from humans. Anim. Cogn. 2004;7:216–223. doi: 10.1007/s10071-004-0214-2. doi:10.1007/s10071-004-0214-2 [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E. Antipredator responses and defensive behavior: ecological and ethological approaches for the neurosciences. Neurosci. Biobehav. Rev. 2001;25:577–586. doi: 10.1016/s0149-7634(01)00042-2. doi:10.1016/S0149-7634(01)00042-2 [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J. Hum. Evol. 2001;40:419–435. doi: 10.1006/jhev.2001.0468. doi:10.1006/jhev.2001.0468 [DOI] [PubMed] [Google Scholar]
- Koops M.A, Giraldeau L.A. Producer–scrounger foraging games in starlings: a test of rate-maximizing and risk-sensitive models. Anim. Behav. 1996;51:773–783. doi:10.1006/anbe.1996.0082 [Google Scholar]
- Lima S.L. Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv. Study Behav. 1998;27:215–290. [Google Scholar]
- Lima S.L, Bednekoff P.A. Back to the basics of antipredatory vigilance: can nonvigilant animals detect attack? Anim. Behav. 1999;58:537–543. doi: 10.1006/anbe.1999.1182. doi:10.1006/anbe.1999.1182 [DOI] [PubMed] [Google Scholar]
- Lima S.L, Dill L.M. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 1990;68:619–640. [Google Scholar]
- Ottoni E.B. EthoLog 2.2: a tool for the transcription and timing of behavior observation sessions. Behav. Res. Methods Instrum. Comput. 2000;32:446–449. doi: 10.3758/bf03200814. [DOI] [PubMed] [Google Scholar]
- Quinn J.L, Cresswell W. Escape response delays in wintering redshank, Tringa totanus, flocks: perceptual limits and economic decisions. Anim. Behav. 2005a;69:1285–1292. doi:10.1016/j.anbehav.2004.10.007 [Google Scholar]
- Quinn J.L, Cresswell W. Personality, anti-predation behaviour and behavioural plasticity in the chaffinch Fringilla coelebs. Behaviour. 2005b;142:1377–1402. doi:10.1163/156853905774539391 [Google Scholar]
- Ristau C.A. Aspects of the cognitive ethology of an injury-feigning bird, the piping plover. In: Ristau C.A, editor. Cognitive ethology: the minds of other animals. Lawrence Erlbaum; Hillsdale, NJ: 1991. pp. 91–126. [Google Scholar]
- Scaife M. Response to eye-like shapes by birds 2: importance of staring, pairedness and shape. Anim. Behav. 1976;24:200–206. doi:10.1016/S0003-3472(76)80116-9 [Google Scholar]
- Schiff W, Caviness J.A, Gibson J.J. Persistent fear responses in rhesus monkeys to the optical stimulus of looming. Science. 1962;136:982–983. doi: 10.1126/science.136.3520.982. doi:10.1126/science.136.3520.982 [DOI] [PubMed] [Google Scholar]
- Smitha B, Thakar J, Watve M. Do bee eaters have theory of mind? Curr. Sci. 1999;76:574–577. [Google Scholar]
- Stankowich T, Blumstein D.T. Fear in animals: a meta-analysis and review of risk assessment. Proc. R. Soc. B. 2005;272:2627–2634. doi: 10.1098/rspb.2005.3251. doi:10.1098/rspb.2005.3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Hopkins E, Hinde W, Adcock A, Connolly Y, Troscianko T, Cuthill I.C. Field experiments on the effectiveness of ‘eyespots’ as predator deterrents. Anim. Behav. 2007;74:1215–1227. doi:10.1016/j.anbehav.2007.01.031 [Google Scholar]
- Templeton J.J, Giraldeau L.A. Public information cues affect the scrounging decisions of starlings. Anim. Behav. 1995;49:1617–1626. doi:10.1016/0003-3472(95)90084-5 [Google Scholar]
- Wang Y, Frost B.J. Time to collision is signalled by neurons in the nucleus rotundus of pigeons. Nature. 1992;356:236–238. doi: 10.1038/356236a0. doi:10.1038/356236a0 [DOI] [PubMed] [Google Scholar]
- Watve M, Thakar J, Kale A, Puntambekar S, Shaikh I, Vaze K, Jog M, Paranjape S. Bee-eaters (Merops orientalis) respond to what a predator can see. Anim. Cogn. 2002;5:253–259. doi: 10.1007/s10071-002-0155-6. doi:10.1007/s10071-002-0155-6 [DOI] [PubMed] [Google Scholar]
- Woolnough A.P, Lowe T.J, Rose K. Can the Judas technique be applied to pest birds? Wildl. Res. 2006;33:449–455. doi:10.1071/WR06009 [Google Scholar]
- Ydenberg R.C, Dill L.M. The economics of fleeing from predators. Adv. Study Behav. 1986;16:229–249. [Google Scholar]