Abstract
Scents, detected through both the main and vomeronasal olfactory systems, play a crucial role in regulating reproductive behaviour in many mammals. In laboratory mice, female preference for airborne urinary scents from males (detected through the main olfactory system) is learnt through association with scents detected through the vomeronasal system during contact with the scent source. This may reflect a more complex assessment of individual males than that implied by laboratory mouse studies in which individual variation has largely been eliminated. To test this, we assessed female preference between male and female urine using wild house mice with natural individual genetic variation in urinary identity signals. We confirm that females exhibit a general preference for male over female urine when able to contact urine scents. However, they are only attracted to airborne urinary volatiles from individual males whose urine they have previously contacted. Even females with a natural exposure to many individuals of both sexes fail to develop generalized attraction to airborne male scents. This implies that information gained through contact with a specific male's scent is essential to stimulate attraction, providing a new perspective on the cues and olfactory pathways involved in sex recognition and mate assessment in rodents.
Keywords: scent communication, mate choice, sex recognition, individual recognition, sexual selection, Mus musculus domesticus
1. Introduction
Signals produced by one sex and received by the other play a critical role in mediating reproductive interactions. The information conveyed by such signals can range from simply alerting animals to the presence or location of opposite-sex conspecifics, to a more complex advertisement of the signaller's individual identity and potential quality as a mate (Andersson 1994). Scent is a major signalling modality in many animals, providing genetically encoded information on species, sex, individual identity and kinship of the owner, as well as information on the animal's current reproductive, social and health status (Wyatt 2003; Brennan & Kendrick 2006; Johansson & Jones 2007), all of which may influence mate selection. An extensive literature based largely on studies of laboratory mice and hamsters has started to identify the olfactory and neural pathways involved in sex-biased responses to conspecific scent signals (see recent reviews by Restrepo et al. (2004), Brennan & Kendrick (2006), Keller et al. (2008), Maras & Petrulis (2008) and Meredith et al. (2008)). Analysis so far suggests that both the main and accessory olfactory systems play an integral part in the detection and processing of signals that coordinate sex recognition and reproductive behaviour. While the main olfactory epithelium (MOE) detects airborne scents (largely volatile chemical components), potentially at some distance from their source, the accessory olfactory system detects volatile and involatile molecules that are pumped to the vomeronasal organ (VNO) during contact with the scent source (Meredith 1994; Breer et al. 2006). These two systems detect at least partially overlapping sets of social chemosignals, which mediate different sexual and social responses through each system (Restrepo et al. 2004; Spehr et al. 2006b).
A common approach that has been taken to distinguish the roles that these two olfactory systems play in sexual communication is to examine deficits in response when one of the two systems is removed or severely debilitated. This has provided conflicting evidence, particularly concerning the ability of animals to recognize the sex of conspecifics through scents detected only through the MOE. Knockout male mice lacking a trp2 cation channel (required for normal odorant-activated transduction in the VNO) appear unable to recognize the sex of conspecifics, abnormally showing the same mating and courtship behaviour towards both males and females (Leypold et al. 2002; Stowers et al. 2002). Nevertheless, these animals retain some ability to detect urine scents through the VNO (Leypold et al. 2002; Kelliher et al. 2006). Surgical removal to completely eliminate VNO inputs, by contrast, can completely eliminate male mating and courtship behaviours even in response to female scents. However, the extent to which sexual behaviour is impaired if animals still have a functional main olfactory system depends on whether males had sexual contact with females prior to vomeronasal ablation (Meredith 1986; Wysocki & Lepri 1991; Pankevich et al. 2004). Males appear to learn to recognize sex-specific airborne volatiles detected through the MOE by association with VNO inputs, so that subsequently these can stimulate normal sexual responses directed towards females even if the VNO is removed. Similarly, female mice must learn to recognize airborne volatiles from males. Without any prior direct contact with adult male scents, females show no inherent preference for airborne volatiles from males over those from females (Moncho-Bogani et al. 2002, 2005) or from castrated males (Martínez-Ricós et al. 2007, 2008). However, after repeated direct contact with male scents detected through the VNO, females are subsequently attracted to male airborne volatiles alone detected through the MOE (Moncho-Bogani et al. 2002, 2005; Martínez-Ricós et al. 2008). Male mice excrete several androgen-dependent volatile pheromones in their urine (Schwende et al. 1986; Novotny et al. 1999; Lin et al. 2005) and, not surprisingly, differences in the airborne scents of males and females are readily detected in simple scent discrimination tests (Pankevich et al. 2004, 2006; Keller et al. 2006). It is thus unclear why appropriate sex-specific responses are not stimulated inherently through odours detected solely through the MOE, but must instead be learned in association with VNO inputs.
The interpretation of these responses in terms of sex recognition is complicated by the use of laboratory rodents. These animals are derived from extremely small gene pools (mice: Ferris et al. 1982; Beck et al. 2000; hamsters: Alder 1948) and are deliberately inbred and kept under highly standardized conditions to reduce individual variation for biomedical research. Laboratory mice lack normal individual variation in highly polymorphic major urinary proteins (MUPs) that are detected through the vomeronasal system (More 2006; Chamero et al. 2007; Kimoto et al. 2007) and underlie individual recognition (Hurst et al. 2001; Cheetham et al. 2007), inbreeding avoidance (Sherborne et al. 2007) and genetic heterozygosity assessment (Thom et al. 2008) in wild mice. Animals of the same inbred laboratory strain also lack normal variation in MHC type which influences both volatile (Schaefer et al. 2002; Willse et al. 2006) and peptide (Leinders-Zufall et al. 2004; Spehr et al. 2006a) scent profiles, along with other genetic and non-genetic differences that would normally contribute to individual scent profiles (Boyse et al. 1987; Brown 1995). Thus, although preference or discrimination between ‘male’ and ‘female’ scents is usually interpreted as sex recognition when other differences between scent donors have been eliminated (Pankevich et al. 2004, 2006; Keller et al. 2006), it is essential to understand whether this really represents the recognition of the animal's sex per se (and would apply across other genetically variable individuals) or reflects more complex responses that depend on individual-specific as well as sex-specific signals.
Here, we use naive or sexually experienced wild female house mice (Mus musculus domesticus) to assess how prior olfactory experience with odours from genetically heterogeneous conspecifics affects adult female preference for male over female odours. Each experiment comprised an odour pre-exposure phase followed by a test of preference between male and female odours. Females were pre-exposed to urine from a male and a female which they could either contact (gaining access to both volatile and involatile scent components) or not (gaining airborne volatiles only). The preference for male over female urine was then assessed when females were allowed full contact with the odour sources (experiment 1) or could access airborne urinary volatiles only (experiments 2 and 3), and when scents were from either the same individuals (familiar) or different individuals (unfamiliar) to those encountered during the pre-exposure phase. Experiment 1 confirmed that naive wild-derived female mice are more attracted to male than to female urine when allowed direct contact with scents, whether or not these have been previously contacted. Experiment 2 confirmed that previous direct contact with urine is a prerequisite for naive females to develop an attraction to airborne urinary volatiles from males versus females. However, it also revealed that learnt attraction to male airborne scents is specific to scent from the same individual that was previously contacted. Experiment 3 further revealed that even females with extensive prior natural social experience with many different individuals of both sexes fail to develop a generalized preference for the airborne scents of males over those from females. Instead, females are only attracted to airborne scents from individual males whose scents they have previously investigated through direct contact. Taken together, the experiments provide a novel functional perspective on the interaction between the main and accessory olfactory systems in sex recognition and mate assessment.
2. Material and methods
(a) Subjects and urine donors
The subjects were captive bred adult female M. m. domesticus (F2–F4) from a colony derived from wild ancestors captured from five different populations in the northwest of England, UK. Different females were used in each experiment. To control for prior olfactory and sexual experience in experiments 1 and 2, we used females from litters that had only experienced contact with adult male odours from their father until he was removed and cages cleaned at 4–14 days post-partum, and exposed to juvenile male odours from sibs until weaning at days 21–24. To ensure that contact with other male odours was prevented, each breeding cage was handled with different gloves and kept inside separated enclosures (1.2×1.2×0.8 m). At weaning, the females were transferred into individually ventilated cages (IVCs; 37×15.6×13 cm 1145T Sealsafe cages, housed in a Tecniplast TouchSLIMLine IVC unit, Tecniplast UK Ltd, London) in single-sex family groups (two or three sisters per cage). Approximately one week before the start of experiments 1 and 2, experimental females (n=16 and 32, respectively) were placed in clean IVCs in sister pairs (females aged 4–6 months). For experiment 3, we used sexually experienced females captured from freely breeding populations housed in large (250 m2) outdoor semi-natural enclosures (see Sherborne et al. (2007) for further details). These females were housed singly in 43×11.5×12 cm cages (M3, North Kent Plastics, UK) at the start of the experiment. Throughout, all animals were housed on a reversed 12 : 12 hours light : dark cycle with lights off at 09.00, and were maintained on Corn Cob Absorb 10/14 substrate with paper wool nest material and ad libitum access to water and food (Lab Diet 5002 Certified Rodent Diet, Purina Mills, St Louis, MO, USA). Cardboard tubes were provided periodically for additional environmental enrichment.
Urine donors came from the same colony but were always from separate breeding lines from the subject and thus were not close relatives. Male donors were housed singly in M3 cages from 8 to 10 weeks old because wild-derived adult males frequently become highly aggressive and intolerant of their cage mates. Female donors were housed in single-sex family groups as for subjects. Urine was collected by holding a stimulus mouse by the scruff of the neck over a clean 1.5 ml Eppendorf tube. Urine was collected up to two weeks prior to testing and stored at −18°C until use.
(b) Experimental procedures
Three days prior to each trial, soiled nest material and substrate from an unfamiliar male (unrelated to those used in the experiment) were introduced into the female's home cage to induce oestrus during the preference test (Marsden & Bronson 1964). We have shown that this procedure works reliably in our mouse colony, with vaginal smears showing that 86 out of 90 females were in oestrus or proestrus 3 days after similar scent exposure in prior tests that used females from the same stock and housing conditions (Cheetham et al. 2007). However, as wild-derived females are very sensitive to direct handling and physical restraint, we did not carry out vaginal smears during the experiments reported here in case this disturbed female behaviour after their first test. Nonetheless, our previous studies indicate that nearly all females were likely to be in oestrus or proestrus during the preference tests. In experiments using naive females (experiments 1 and 2), equivalent scents from an unfamiliar female were also introduced to control for different levels of exposure to odours from the two sexes.
All trials were conducted in a clean 45×28×13 cm cage (MB1, North Kent Plastics, UK). A Perspex lid with evenly distributed 6 mm holes drilled 2 cm apart allowed free passage of airborne volatile substances into the cage below but prevented physical contact with the urine sources where desired. Females were presented with urine from a male at one end of the cage and from a female at the other end, approximately 25 cm apart and equidistant from the cage walls. In trials testing response to airborne urinary volatiles only (experiment 2 and 3, see below), the urine was presented as 10 μl streaks on 5×2.5 cm2 Benchkote strips suspended 2 cm above the Perspex lid using a 5 cm diameter Perspex cylinder. In trials testing response when mice could contact the stimulus urine (experiment 1), 10 μl of urine were streaked directly onto the underside of the Perspex lid in the same orientation. The position of male and female urine was randomized and in both the test and subsequent analysis of behaviour the experimenter was blind to each odour source. All tests were carried out during the dark phase under dim red lighting.
Trials lasted 10 min, during which female behaviour towards the two urine sources was recorded remotely on a DVD in a neighbouring laboratory. We measured two types of behaviour which reflect different types of ‘preference’ between two scents. The time spent sniffing up at the cage lid within the 5 cm diameter circle in which a test urine was presented indicates the female's interest in gaining further information from a scent source. As this is part of the gathering and processing of scent information, the time spent sniffing mostly occurs in the first few encounters with a scent and is likely to depend, in part, on prior familiarity of a scent and on any differences in the processing time required to interpret male and female scents. To assess whether females are attracted to spend more time near to male compared with female scent, we deliberately placed the scents so that mice had to move away from the side walls to be close to the scent source, in a location where they normally spend little time in the absence of attractive scents. The time under the urine source when not investigating the urine (defined as the subject's nose being within the 5 cm diameter circle but not sniffing up at the lid) thus reflects non-investigatory attraction to spend time close to the scent source. The durations of these behaviours were not normally distributed, hence differences in response to male versus female test odours within each test were assessed using Wilcoxon signed-rank tests. Data were log transformed (s+1) to compare the log ratio of response to (male/female) scent between tests using repeated measures ANOVA and t-tests, as in each case the transformed data approximated normality (Kolmogorov–Smirnov tests, NS).
(i) Experiment 1: naive female response to full contact with urine
An hour prior to the first trial, each female pair was pre-exposed for 30 min in their home cage to full contact with a 5×2.5 cm2 Benchkote strip streaked with 10 μl male urine and 10 μl female urine from unfamiliar individuals. After 30 min, the odour source was removed and the females transferred into individual test cages where they were allowed to settle for 30 min. The first female in each pair was then tested by placing the male and female test urine on the underside of the cage lid to allow contact as described above and behaviour was recorded for 10 min. The second female was tested in the same way with fresh scents immediately afterwards and then both the females were returned to their home cage.
Each female (n=16) was tested twice: once with pre-exposure and test urine from the same individuals (test urine familiar), and once with pre-exposure and test urine from different individuals (test urine unfamiliar). The two tests were conducted in a balanced order (one half of the females tested with familiar test urine first and the other half with unfamiliar urine first) with trials separated by 4 days to coincide with sequential oestrus periods. Each female was pre-exposed to urine from the same individuals in both the tests, using different donors between pairs of females, while the donors of familiar and unfamiliar test urine were not closely related.
(ii) Experiment 2: naive female response to airborne urinary volatiles
An hour prior to the first trial, each female pair was pre-exposed for 30 min in their home cage to a 5×2.5 cm2 Benchkote strip streaked with 10 μl male urine and 10 μl female urine from unfamiliar individuals. Half of the subjects assigned at random (n=16 females) were pre-exposed to airborne volatiles only, with direct contact prevented by enclosing the Benchkote strip inside a clean hinged mesh sphere (44 mm diameter and 1 mm square mesh size). The other set of females (n=16) could contact the introduced odours, with the Benchkote strip held outside the hinged mesh sphere. After 30 min, the odour source was removed from the home cage and the females transferred into individual test cages where they were allowed to settle for 30 min. The first female in each pair was then tested by placing the male and female test odours above the test cage so that the urine could not be contacted (described above), and the response to airborne scents recorded for 10 min. The second female was tested in the same way with fresh scents immediately afterwards, then both females were returned to their home cage.
As for experiment 1, each female was tested twice: once with pre-exposure and test urine sourced from the same individuals (test urine familiar), and once with pre-exposure and test urine sourced from different individuals (test urine unfamiliar). The two tests were conducted in a balanced order with trials separated by 4 days. Each female was pre-exposed to urine from the same individuals in both the tests, using different donors between pairs of females, while the donors of familiar and unfamiliar test urine were not closely related.
(iii) Experiment 3: experienced female response to airborne urinary volatiles
Experiment 3 did not contain a separate 30 min pre-exposure phase. Instead, females with a substantial experience of scent cues from many different individuals of both sexes prior to capture, and very likely to have sexual experience, were tested with airborne urinary volatiles from either the same male donor that provided soiled bedding left in the home cage for 3 days to bring females into oestrus on the day of testing (test urine familiar), or from a different male (test urine unfamiliar). In this test, females were only pre-exposed to an individual male's scent. This allowed us to check that the subsequent preference for male over female odours was due to attraction to male scent rather than avoidance of a familiar female scent. The order of presentation was balanced so that one half were tested with familiar male odour first and the other half unfamiliar male odour first. In all trials, the female test odour was unfamiliar to the subject. Sample sizes were n=11 and 10 for the familiar and unfamiliar odour tests, respectively, with all but one female tested twice as before.
3. Results
(a) Experiment 1: naive female response to full contact with urine
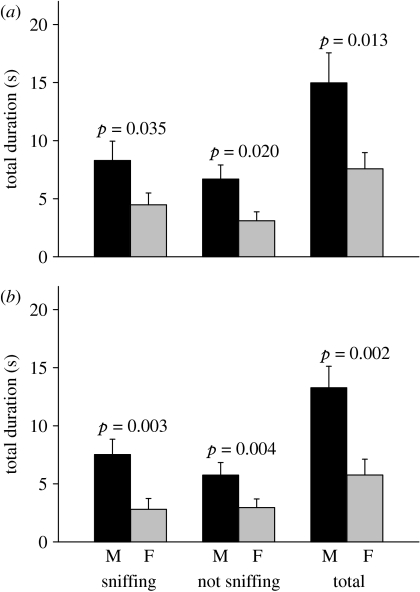
When females could contact urine stimuli during the preference test, they consistently spent more time with a male urine stimulus than with a female stimulus, irrespective of whether the urine was familiar or unfamiliar from their contact pre-exposure prior to the test. Females spent more time both sniffing male scent to gain further information and more time under the scent source not sniffing, with no difference in their bias towards male scents whether the scents were familiar or unfamiliar (figure 1; t-test of log ratio in sniffing: t15=−1.36, p=0.19; under not sniffing: t15=0.24, p=0.81). We conclude from experiment 1 that when able to contact the urine of a male and a female conspecific, wild-derived female house mice show a general preference for male scents regardless of prior familiarity with scents from individual donors.
Figure 1.
Preference for male (M) over female (F) urine when naive females can contact urine stimuli. Females were either (a) familiar with urine from the same individuals from pre-exposure prior to the test or (b) unfamiliar with the stimuli after pre-exposure to urine from different individuals. The response is broken down into time spent sniffing the stimulus, time under the stimulus not sniffing and total time under the stimulus urine (mean+s.e., n=16). The p-values indicate Wilcoxon signed-rank tests. The log ratio of total time under the male/female stimulus did not differ between familiar and unfamiliar tests (matched-pair t-test, t15=−0.89, p=0.39).
(b) Experiment 2: naive female response to airborne urinary volatiles
Naive female laboratory mice do not show an innate attraction to airborne urinary volatiles from males but learn this attraction during direct contact with involatile components in male scent, resulting in subsequent attraction to airborne volatiles alone (Moncho-Bogani et al. 2002). If this process of associative learning results in a simple recognition of male airborne volatiles (Maras & Petrulis 2008; Martinez-Garcia et al. 2008), contact with male urine will lead to general recognition of and attraction to airborne volatiles from other intact males. However, if this associative learning represents a more sophisticated recognition of specific individual males, we predicted that females will only be attracted to airborne volatiles from the individual male whose scent they have previously contacted, because these have become associated with the involatile genetically determined individual identity signal of that particular male (Hurst & Beynon 2004). Using naive females, we thus varied both the nature of their urine pre-exposure (direct contact or airborne volatiles only) and their familiarity with scents from individual donors (same or different urine donors in pre-exposure and preference test).
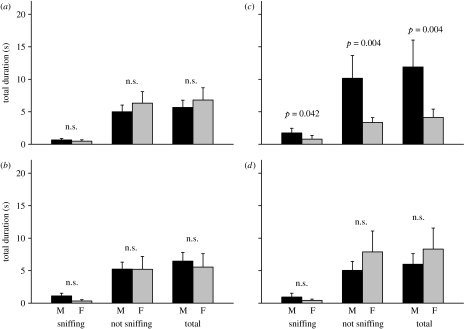
After pre-exposure only to airborne urinary volatiles, the females showed no preference for male over female airborne urinary volatiles, regardless of whether the volatile scents from individual donors were familiar (figure 2a) or unfamiliar (figure 2b). Thus, naive wild-derived female mice do not show an innate preference for airborne urinary volatiles from males over females.
Figure 2.
Preference for airborne male (M) over female (F) urinary odours according to prior exposure to urine stimuli. Prior to the preference test, naive females had pre-exposure to either airborne urinary odours (a,b) or contact with urine stimuli (c,d), where pre-exposure and test stimuli were either from the same individuals ((a,c) familiar) or from different individuals ((b,d) unfamiliar). The p-values indicate Wilcoxon signed-rank tests. Females preferred male over female airborne odours only in test (c) when they had contact with urine from the same individuals prior to the test (interaction term for log ratio in total time: F1,30=4.16, p=0.050). The preference in test (c) differed strongly from that following contact with urine from different individual donors ((c) versus (d), matched-pair t-test of log ratio in total time, t15=3.20, p=0.006), and more weakly from that following pre-exposure to airborne scent from the same individual donors ((c) versus (a), t-test: t30=1.78, p=0.085).
After pre-exposure to full contact with urine, female preference for male over female airborne scent was limited to volatiles from the same individual male donor whose urine they had previously contacted. When pre-exposure and test urine came from the same individual donors, females spent significantly more time investigating airborne urinary volatiles from the familiar male than from the familiar female. They showed an even stronger preference for spending more time under the male scent when not sniffing (figure 2c). This preference for familiar airborne male scent after contact with the stimuli was no different from that shown in experiment 1 when females could contact the stimuli during both pre-exposure and preference test (log ratio of total time under male/female stimulus in experiment 1 versus experiment 2: t30=−0.026, p=0.98). By contrast, when pre-exposure and test urine came from different individual donors, females failed to investigate the unfamiliar airborne scents from male urine more than those from female urine, and they were not attracted to spend more time close to male scent when not sniffing (figure 2d). This failure to be attracted to the airborne odour of an unfamiliar male differed significantly from their attraction to unfamiliar male scent that they could contact in experiment 1 (log ratio of total time under male/female stimulus in experiment 1 versus experiment 2: t30=3.48, p=0.002). Thus, prior contact with male urine did not lead to a general attraction to male airborne volatiles through a learnt association with attractive involatile components in male urine. Instead, contact with scents led to specific attraction to airborne urinary volatiles from the individual donor male (compared with an equally familiar individual female donor's scent).
(c) Experiment 3: experienced female response to airborne urinary volatiles
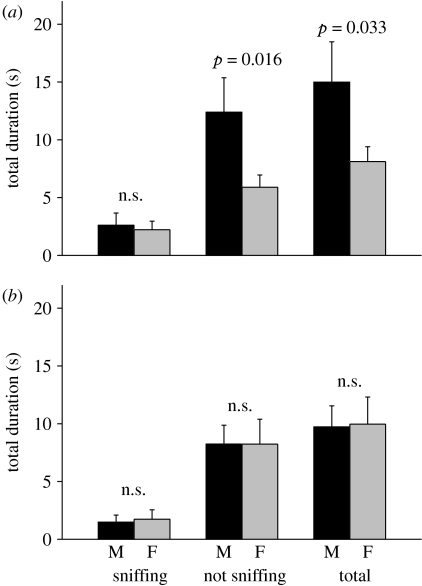
To test whether normal prior exposure to odours from many different males and females leads to a more generalized attraction to male airborne urinary volatiles, we tested socially and sexually experienced female subjects that had been reared in large semi-natural populations. When tested with airborne volatiles from an unfamiliar male and female, females showed no preference for the male scent, either with respect to sniffing at the scents (z=−0.28, p=0.78) or time under the scents when not sniffing (z=−0.15, p=0.88; figure 3b). Thus, even very experienced females failed to show a generalized attraction to male airborne urinary volatiles. However, when the same females had contact with scent from the individual male prior to the test so that this individual's scent was familiar, females were attracted to the male's airborne urinary volatiles and spent more time under the male's scent not sniffing than under the female's scent (z=−2.40, p=0.016; figure 3a; log ratio of time under male/female not sniffing in familiar versus unfamiliar test, t9=2.67, p=0.026). Females did not spend more time sniffing volatiles from the familiar male scent compared with the female scent to gain further information (z=−0.05, p=0.96), probably because females had received prolonged pre-exposure to the familiar male's scent in their home cage while the female's scent was unfamiliar in this experiment. This design thus additionally confirms that preference for familiar airborne male scent is a response to prior exposure to male scent rather than any avoidance of familiar female scent, as females still preferred to spend more time close to the familiar male scent than to the unfamiliar female scent. The failure of naive female mice to show a generalized attraction to male airborne urinary volatiles thus also applies to highly experienced animals that had encountered scents from a wide range of individual males under naturalistic social conditions. Instead, attraction to male airborne urinary volatiles is specific to the individual male scents that females have previously contacted.
Figure 3.
Preference of socially experienced females for airborne male (M) over female (F) urinary odours. Experienced females were either (a) familiar with soiled bedding from the same individual male prior to the test or (b) unfamiliar after pre-exposure to bedding from a different individual male. The p-values indicate Wilcoxon signed-rank tests. The log ratio of total time under the male/female stimulus differed between familiar and unfamiliar tests (matched-pair t-test, t9=3.08, p=0.013).
4. Discussion
Our findings using wild-derived mice agree in part with the discovery that female laboratory mice are not inherently attracted to airborne volatiles emitted from the urine of adult male conspecifics (Moncho-Bogani et al. 2002, 2005; Martínez-Ricós et al. 2007, 2008). Attraction to male airborne scent is a learnt response following direct contact with male urine scents that are inherently attractive. Our experiments confirm that females that are able to contact scents show a consistent preference for individual male over individual female scents, regardless of whether they were previously familiar with the individual donor scents, and that this preference is shown by females with no sexual experience. Not only do they show a more prolonged chemoinvestigation of male urine scents (which might simply relate to the processing time required to gain information from male versus female scents) but most importantly they choose to spend longer in close vicinity to male scents. After contact with a male's urine, females also become attracted to airborne volatiles emanating from that male's scent, now showing the same preference for spending time close to the source of these airborne volatiles that they show in response to contact. However, our tests using genetically heterogeneous wild mice reveal that this attraction is restricted to airborne urinary scents from the same individual male whose scent they have previously contacted. This is in contrast to the implication from experiments with laboratory mice. Laboratory mouse studies suggest that young female mice learn to recognize male airborne volatiles through association with inherently attractive male scents detected through the vomeronasal system when they first contact scents from adult males during development, thereafter being attracted to airborne male scents as a general response (Moncho-Bogani et al. 2002, 2005; Maras & Petrulis 2008). Instead, females throughout their life learn to recognize the airborne odour signatures of specific individual males during contact investigation of their urine. Only once females have investigated an individual male's urine scent through direct contact are they then attracted to airborne scents from the same individual male source.
Previous reports of a learnt generalized female attraction to airborne volatiles from males used scents pooled from several different laboratory male donors and are likely to be an artefact of unnatural similarity between individual laboratory mice. Although the CD-1 mouse strain used by Moncho-Bogani et al. (2002, 2005) is generally referred to as ‘outbred’, all of the classical strains of laboratory mice have been derived from such an extremely small gene pool (probably from a single female based on mitochondrial DNA; Ferris et al. 1982) that even outbred strain individuals are likely to have very little genetic diversity compared with normal wild animals. Correspondingly, we have found that mice within and between strains that derive from a shared genetic lineage, including those within the CD-1 strain, all share the same involatile MUP identity signature (S. A. Cheetham, R. J. Beynon, A. L. Smith & J. L. Hurst 2006, unpublished data). Although mice are highly sensitive to even small genetic or non-genetic differences between laboratory animals in scent discrimination tests (reviewed by Thom & Hurst 2004), the recognition of individual urine donors depends on whether each expresses a different MUP pattern, regardless of the many other genetic or non-genetic differences between wild individuals (Hurst et al. 2001; Cheetham et al. 2007). This fixed individual genetic identity signature allows individuals to be identified even though their volatile scent profiles are susceptible to variation according to current physiological status, diet, bacterial flora and health status (Brown 1995; Hurst & Beynon 2004). Thus, while females detect differences in airborne scents from different individual laboratory males even when these are genetically identical (e.g. Penn & Potts 1998; Nakamura et al. 2007), all of these scents will be associated with the same shared involatile MUP individual identity signature during the contact investigation of the scents. A crucial difference in our study is that stimulus donors were outbred wild mice that were not closely related to subjects or each other and thus had clearly distinct individual genetic identity scents.
Our findings provide a novel perspective on how females select potential mates using scent signals, and the roles that the main and accessory olfactory systems are likely to play in such sexual assessment. It is extremely unlikely that animals are prevented from recognizing a general difference between male- and female-specific airborne odours through the main olfactory system by simple neurophysiological constraints, forcing mice to learn each individual-specific volatile profile from the scents that they contact. The marked difference in airborne odours emanating from male and female mouse urine is readily apparent even to an untrained human nose, with adult male mice excreting a number of male-specific volatile pheromones in their urine, including 2-sec-butyl-4,5-dihydrothiazole (Schwende et al. 1986), E,E-α-farnesene and E-β-farnesene (Harvey et al. 1989) and methylthiomethanethiol (Lin et al. 2005). Female mice are sensitive to these volatile pheromones (Jemiolo et al. 1985, 1991; Lin et al. 2005) and therefore should easily be able to learn this difference, even if recognition is not inherently encoded through specific receptors and neural pathways in the main olfactory system. Instead, the clear implication from our study is that female attraction to males involves more than simple sex recognition, requiring females to gain information about an individual male through close contact scent investigation before that individual male's scent becomes attractive.
Females are generally choosy in selecting their mates, needing not only to recognize whether a conspecific is male but also to assess his suitability in terms of both quality and genetic compatibility as a potential mate (Andersson 1994; Andersson & Simmons 2006). The ability to recognize and assess different individual males is thus an important component of sexual selection and reproductive behaviour. As all male scent donors used in our experiments were unrelated to females, in good health and individually housed ‘territory owners’, all were expected to be attractive (Pusey & Wolf 1996; Hurst & Beynon 2004; Kavaliers et al. 2005). However, under natural circumstances, not all males are equally desirable and a general attraction to any male airborne odours is unlikely to be a useful discriminating response. While females may be able to gain some information about male social status from androgen-dependent airborne urinary volatiles (Harvey et al. 1989; Jemiolo et al. 1991), other essential information is held by involatile components that can be detected only through a close contact investigation presumed to involve the vomeronasal system. Competitive scent marking allows males to advertise their territory ownership and competitive ability and is an important sexual signal in many mammals (Hurst & Beynon 2004). Female mice identify the individual owners of scent marks and countermarks through the fixed individual MUP signature in scent marks, requiring a direct contact investigation of these involatile proteins (Nevison et al. 2003; Cheetham et al. 2007) that stimulate receptors in the VNO (Chamero et al. 2007). By only being attracted to a male's airborne scent once that scent has been associated with a known individual male identity signature (through contact investigation), females may ensure that they have already gained information about that individual male from his scent marks. Correspondingly, females prefer males whose scent marks they have previously contacted relative to males whose scent is unfamiliar (Coopersmith & Banks 1983; Tang-Martinez et al. 1993; Johnston et al. 1997; Roberts & Gosling 2004; Cheetham et al. 2007). This contact may provide additional information through the vomeronasal system that is essential to assess the suitability of mates. The MUP pattern in mouse urine provides not only an individual genetic identity signature but also a genetic marker of kinship, with wild mice showing strong avoidance of inbreeding with relatives that share the same MUP pattern as themselves (Sherborne et al. 2007). As yet, we know little about the specific scent components that female mice might use to assess male quality, but identifying the scent information gained through contact investigation that leads to subsequent attraction to individual males (including attraction to the individual's airborne scents) will help us to understand further how females assess the suitability of mates through scent signals. Another key challenge for the future will be to understand how information gained about an individual male through contact investigation involving the accessory olfactory system becomes associated with individual airborne scent profiles detected through the main olfactory system, and how long such associations are remembered.
Finally, our findings may help to explain the different but integrated roles that the main and accessory olfactory systems play in coordinating female assessment of and attraction to males. While detection of airborne scents (through the MOE) alerts animals to the presence of a scent source and stimulates approach and close contact investigation, particularly when airborne stimuli are novel, scents detected through the VNO during close contact investigation appear to provide essential information concerning the individual genetic identity of the scent owner (including species, sex, individual and kinship) and allow the assessment of their individual attractiveness as potential mates. Thus, the accessory olfactory system plays a key role in stimulating sexual responses including attraction (present study; Pankevich et al. 2004; Keller et al. 2006; Martínez-Ricós et al. 2008) and female sexual receptivity (Keller et al. 2006). However, processing of scents through the accessory olfactory system is very slow (Luo et al. 2003) and requires physical contact with each scent. By learning an association between the individual-specific information gained through the VNO and the airborne volatile profile detected simultaneously through the main olfactory system, animals are able to use airborne odours alone to recognize individual scent owners that have already been assessed. It is difficult to see the adaptive value of this dual system if it underpins only simple sex recognition. On the contrary, a system that enables choosy females to recognize and differentially respond to specific individuals—as our results imply—has immediate relevance in the context of mate choice.
Acknowledgments
We thank Dr Rick Humphries, John Waters, Felicity Fair, Linda Burgess and Sue Jopson for their technical help, members of the Mammalian Behaviour and Evolution Group for their stimulating discussions and Professor Rob Beynon for his help in improving the manuscript. The study was funded by a research grant to J.L.H. from the Biotechnology and Biological Science Research Council (BBC503897). This research adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research, the legal requirements of the country in which the work was carried out and all institutional guidelines.
References
- Alder S. Origin of the golden hamster (Cricetus auratus) as a laboratory animal. Nature. 1948;162:256–257. doi: 10.1038/162256b0. doi:10.1038/162256b0 [DOI] [PubMed] [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Andersson M, Simmons L.W. Sexual selection and mate choice. Trends Ecol. Evol. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. doi:10.1016/j.tree.2006.03.015 [DOI] [PubMed] [Google Scholar]
- Beck J.A, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig J.T, Festing M.F, Fisher E.M. Genealogies of mouse inbred strains. Nat. Genet. 2000;24:23–25. doi: 10.1038/71641. doi:10.1038/71641 [DOI] [PubMed] [Google Scholar]
- Boyse E.A, Beauchamp G.K, Yamazaki K. The genetics of body scent. Trends Genet. 1987;3:97–102. doi:10.1016/0168-9525(87)90192-2 [Google Scholar]
- Breer H, Fleischer J, Strotmann J. The sense of smell: multiple olfactory subsystems. Cell. Mol. Life Sci. 2006;63:1465–1475. doi: 10.1007/s00018-006-6108-5. doi:10.1007/s00018-006-6108-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P.A, Kendrick K.M. Mammalian social odours: attraction and individual recognition. Phil. Trans. R. Soc. B. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. doi:10.1098/rstb.2006.1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R.E. What is the role of the immune system in determining individually distinct body odours? Int. J. Immunopharmacol. 1995;17:655–661. doi: 10.1016/0192-0561(95)00052-4. doi:10.1016/0192-0561(95)00052-4 [DOI] [PubMed] [Google Scholar]
- Chamero P, Marton T.F, Logan D.W, Flanagan K, Cruz J.R, Saghatelian A, Cravatt B.F, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. doi:10.1038/nature05997 [DOI] [PubMed] [Google Scholar]
- Cheetham S.A, Thom M.D, Jury F, Ollier W.E.R, Beynon R.J, Hurst J.L. The genetic basis of individual recognition signals in the mouse. Curr. Biol. 2007;17:1771–1777. doi: 10.1016/j.cub.2007.10.007. doi:10.1016/j.cub.2007.10.007 [DOI] [PubMed] [Google Scholar]
- Coopersmith C.B, Banks E.M. Effects of olfactory cues on sexual behavior in the Brown Lemming, Lemmus trimucronatus. J. Comp. Psychol. 1983;97:120–126. doi:10.1037/0735-7036.97.2.120 [PubMed] [Google Scholar]
- Ferris S.D, Sage R.D, Wilson A.C. Evidence from mtDNA sequences that common laboratory strains of inbred mice are descended from a single female. Nature. 1982;295:163–165. doi: 10.1038/295163a0. doi:10.1038/295163a0 [DOI] [PubMed] [Google Scholar]
- Harvey S, Jemiolo B, Novotny M. Pattern of volatile compounds in dominant and subordinate male-mouse urine. J. Chem. Ecol. 1989;15:2061–2072. doi: 10.1007/BF01207438. doi:10.1007/BF01207438 [DOI] [PubMed] [Google Scholar]
- Hurst J.L, Beynon R.J. Scent wars: the chemobiology of competitive signalling in mice. Bioessays. 2004;26:1288–1298. doi: 10.1002/bies.20147. doi:10.1002/bies.20147 [DOI] [PubMed] [Google Scholar]
- Hurst J.L, Payne C.E, Nevison C.M, Marie A.D, Humphries R.E, Robertson D.H, Cavaggioni A, Beynon R.J. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. doi:10.1038/414631a [DOI] [PubMed] [Google Scholar]
- Jemiolo B, Alberts J, Sochinski-Wiggins S, Harvey S, Novotny M. Behavioural and endocrine responses of female mice to synthetic analogs of volatile compounds in male urine. Anim. Behav. 1985;33:1114–1118. doi:10.1016/S0003-3472(85)80170-6 [Google Scholar]
- Jemiolo B, Xie T.M, Novotny M. Socio-sexual olfactory preference in female mice: attractiveness of synthetic chemosignals. Physiol. Behav. 1991;50:1119–1122. doi: 10.1016/0031-9384(91)90570-e. doi:10.1016/0031-9384(91)90570-E [DOI] [PubMed] [Google Scholar]
- Johansson B.G, Jones T.M. The role of chemical communication in mate choice. Biol. Rev. 2007;82:265–289. doi: 10.1111/j.1469-185X.2007.00009.x. doi:10.1111/j.1469-185X.2007.00009.x [DOI] [PubMed] [Google Scholar]
- Johnston R.E, Sorokin E.S, Ferkin M.H. Female voles discriminate males' over-marks and prefer top-scent males. Anim. Behav. 1997;54:679–690. doi: 10.1006/anbe.1997.0471. doi:10.1006/anbe.1997.0471 [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Pfaff D.W. Genes, odours and the recognition of parasitized individuals by rodents. Trends Parasitol. 2005;21:423–429. doi: 10.1016/j.pt.2005.07.008. doi:10.1016/j.pt.2005.07.008 [DOI] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum M.J, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur. J. Neurosci. 2006;23:521–530. doi: 10.1111/j.1460-9568.2005.04589.x. doi:10.1111/j.1460-9568.2005.04589.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, M., Baum, M. J. & Bakker, J. 2008 Olfactory control of sex-recognition and sexual behaviour in mice. In Chemical signals in vertebrates, vol. 11 (eds J. L. Hurst, R. J. Beynon, S. C. Roberts & T. Wyatt), pp. 241–250. New York, NY: Springer.
- Kelliher K.R, Spehr M, Li X.H, Zufall F, Leinders-Zufall T. Pheromonal recognition memory induced by TRPC2-independent vomeronasal sensing. Eur. J. Neurosci. 2006;23:3385–3390. doi: 10.1111/j.1460-9568.2006.04866.x. doi:10.1111/j.1460-9568.2006.04866.x [DOI] [PubMed] [Google Scholar]
- Kimoto H, Sato K, Nodari F, Haga S, Holy T.E, Touhara K. Sex- and strain-specific expression and vomeronasal activity of mouse ESP family peptides. Curr. Biol. 2007;17:1879–1884. doi: 10.1016/j.cub.2007.09.042. doi:10.1016/j.cub.2007.09.042 [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Brennan P, Widmayer P, Chandramani P, Maul-Pavicic A, Jager M, Li X.H, Breer H, Zufall F, Boehm T. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. doi: 10.1126/science.1102818. doi:10.1126/science.1102818 [DOI] [PubMed] [Google Scholar]
- Leypold B.G, Yu C.R, Leinders-Zufall T, Kim M.M, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc. Natl Acad. Sci. USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. doi:10.1073/pnas.082127599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D.Y, Zhang S.Z, Block E, Katz L.C. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. doi:10.1038/nature03414 [DOI] [PubMed] [Google Scholar]
- Luo M, Fee M.S, Katz L.C. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–1201. doi: 10.1126/science.1082133. doi:10.1126/science.1082133 [DOI] [PubMed] [Google Scholar]
- Maras, P. M. & Petrulis, A. 2008 The role of early olfactory experience in the development of adult odor preferences in rodents. In Chemical signals in vertebrates, vol. 11 (eds J. L. Hurst, R. J. Beynon, S. C. Roberts & T. Wyatt), pp. 251–260. New York, NY: Springer.
- Marsden H.M, Bronson F.H. Estrous synchrony in mice: alteration by exposure to male urine. Science. 1964;144:1469. doi: 10.1126/science.144.3625.1469. doi:10.1126/science.144.3625.1469 [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia, F., Agustin-Pavon, C., Martinez-Hernandez, J., Martinez-Ricos, J., Moncho-Bogani, J., Novejarque, A. & Lanuza, E. 2008 Have sexual pheromones their own reward system in the brain of female mice? In Chemical signals in vertebrates, vol. 11 (eds J. L. Hurst, R. J. Beynon, S. C. Roberts & T. Wyatt), pp. 261–270. New York, NY: Springer.
- Martínez-Ricós J, Agustín-Pavón C, Lanuza E, Martínez-García F. Intraspecific communication through chemical signals in female mice: reinforcing properties of involatile male sexual pheromones. Chem. Senses. 2007;32:139–148. doi: 10.1093/chemse/bjl039. doi:10.1093/chemse/bjl039 [DOI] [PubMed] [Google Scholar]
- Martínez-Ricós, J., Agustín-Pavón, C., Lanuza, E. & Martínez-García, F. 2008 Role of the vomeronasal system in intersexual attraction in female mice. Neuroscience (doi:10.1016/j.neuroscience.2008.02.002) [DOI] [PubMed]
- Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol. Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. doi:10.1016/0031-9384(86)90362-8 [DOI] [PubMed] [Google Scholar]
- Meredith M. Chronic recording of vomeronasal pump activation in awake behaving hamsters. Physiol. Behav. 1994;56:345–354. doi: 10.1016/0031-9384(94)90205-4. doi:10.1016/0031-9384(94)90205-4 [DOI] [PubMed] [Google Scholar]
- Meredith, M., Samuelsen, C., Blake, C. & Westberry, J. 2008 Selective response of medial amygdala subregions to reproductive and defensive chemosignals from conspecific and heterospecific species. In Chemical signals in vertebrates, vol. 11 (eds J. L. Hurst, R. J. Beynon, S. C. Roberts & T. Wyatt), pp. 367–378. New York, NY: Springer.
- Moncho-Bogani J, Lanuza E, Hernandez A, Novejarque A, Martinez-Garcia F. Attractive properties of sexual pheromones in mice: innate or learned? Physiol. Behav. 2002;77:167–176. doi: 10.1016/s0031-9384(02)00842-9. doi:10.1016/S0031-9384(02)00842-9 [DOI] [PubMed] [Google Scholar]
- Moncho-Bogani J, Martinez-Garcia F, Novejarque A, Lanuza E. Attraction to sexual pheromones and associated odorants in female mice involves activation of the reward system and basolateral amygdala. Eur. J. Neurosci. 2005;21:2186–2198. doi: 10.1111/j.1460-9568.2005.04036.x. doi:10.1111/j.1460-9568.2005.04036.x [DOI] [PubMed] [Google Scholar]
- More L. Mouse major urinary proteins trigger ovulation via the vomeronasal organ. Chem. Senses. 2006;31:393–401. doi: 10.1093/chemse/bjj043. doi:10.1093/chemse/bjj043 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kikusui T, Takeuchi Y, Mori Y. The critical role of familiar urine odor in diminishing territorial aggression toward a castrated intruder in mice. Physiol. Behav. 2007;90:512–517. doi: 10.1016/j.physbeh.2006.10.014. doi:10.1016/j.physbeh.2006.10.014 [DOI] [PubMed] [Google Scholar]
- Nevison C.M, Armstrong S, Beynon R.J, Humphries R.E, Hurst J.L. The ownership signature in mouse scent marks is involatile. Proc. R. Soc. B. 2003;270:1957–1963. doi: 10.1098/rspb.2003.2452. doi:10.1098/rspb.2003.2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny M.V, Jemiolo B, Wiesler D, Ma W, Harvey S, Xu F, Xie T.M, Carmack M. A unique urinary constituent, 6-hydroxy-6-methyl-3-heptanone, is a pheromone that accelerates puberty in female mice. Chem. Biol. 1999;6:377–383. doi: 10.1016/S1074-5521(99)80049-0. doi:10.1016/S1074-5521(99)80049-0 [DOI] [PubMed] [Google Scholar]
- Pankevich D.E, Baum M.J, Cherry J.A. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J. Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. doi:10.1523/JNEUROSCI.2376-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich D.E, Cherry J.A, Baum M.J. Effect of vomeronasal organ removal from male mice on their preference for and neural Fos responses to female urinary odors. Behav. Neurosci. 2006;120:925–936. doi: 10.1037/0735-7044.120.4.925. doi:10.1037/0735-7044.120.4.925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn D, Potts W.K. Untrained mice discriminate MHC-determined odors. Physiol. Behav. 1998;64:235–243. doi: 10.1016/s0031-9384(98)00052-3. doi:10.1016/S0031-9384(98)00052-3 [DOI] [PubMed] [Google Scholar]
- Pusey A, Wolf M. Inbreeding avoidance in animals. Trends Ecol. Evol. 1996;11:201–206. doi: 10.1016/0169-5347(96)10028-8. doi:10.1016/0169-5347(96)10028-8 [DOI] [PubMed] [Google Scholar]
- Restrepo D, Arellano J, Oliva A.M, Schaefer M.L, Lin W.H. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm. Behav. 2004;46:247–256. doi: 10.1016/j.yhbeh.2004.02.009. doi:10.1016/j.yhbeh.2004.02.009 [DOI] [PubMed] [Google Scholar]
- Roberts S.C, Gosling L.M. Manipulation of olfactory signaling and mate choice for conservation breeding: a case study of harvest mice. Conserv. Biol. 2004;18:548–556. doi:10.1111/j.1523-1739.2004.00514.x [Google Scholar]
- Schaefer M.L, Yamazaki K, Osada K, Restrepo D, Beauchamp G.K. Olfactory fingerprints for major histocompatibility complex-determined body odors II: relationship among odor maps, genetics, odor composition, and behavior. J. Neurosci. 2002;22:9513–9521. doi: 10.1523/JNEUROSCI.22-21-09513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwende F.J, Wiesler D, Jorgenson J.W, Carmack M, Novotny M. Urinary volatile constituents of the house mouse, Mus musculus, and their endocrine dependency. J. Chem. Ecol. 1986;12:277–296. doi: 10.1007/BF01045611. doi:10.1007/BF01045611 [DOI] [PubMed] [Google Scholar]
- Sherborne A.L, Thom M.D, Paterson S, Jury F, Ollier W.E.R, Stockley P, Beynon R.J, Hurst J.L. The genetic basis of inbreeding avoidance in house mice. Curr. Biol. 2007;17:2061–2066. doi: 10.1016/j.cub.2007.10.041. doi:10.1016/j.cub.2007.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehr M, Kelliher K.R, Li X.H, Boehm T, Leinders-Zufall T, Zufall F. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J. Neurosci. 2006a;26:1961–1970. doi: 10.1523/JNEUROSCI.4939-05.2006. doi:10.1523/JNEUROSCI.4939-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehr M, Spehr J, Ukhanov K, Kelliher K.R, Leinders-Zufall T, Zufall F. Parallel processing of social signals by the mammalian main and accessory olfactory systems. Cell. Mol. Life Sci. 2006b;63:1476–1484. doi: 10.1007/s00018-006-6109-4. doi:10.1007/s00018-006-6109-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy T.E, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male–male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. doi:10.1126/science.1069259 [DOI] [PubMed] [Google Scholar]
- Tang-Martinez Z, Mueller L.L, Taylor G.T. Individual odors and mating success in the Golden hamster, Mesocricetus auratus. Anim. Behav. 1993;45:1141–1151. doi:10.1006/anbe.1993.1138 [Google Scholar]
- Thom M.D, Hurst J.L. Individual recognition by scent. Ann. Zool. Fenn. 2004;41:765–787. [Google Scholar]
- Thom, M. D., Stockley, P., Jury, F., Ollier, W. E. R., Beynon, R. J. & Hurst, J. L. 2008 The direct assessment of genetic heterozygosity through scent in the mouse. Curr. Biol.18, 619–623. (doi:10.1016/j.cub.2008.03.056) [DOI] [PubMed]
- Willse A, Kwak J, Yamazaki K, Preti G, Wahl J.H, Beauchamp G.K. Individual odortypes: interaction of MHC and background genes. Immunogenetics. 2006;58:967–982. doi: 10.1007/s00251-006-0162-x. doi:10.1007/s00251-006-0162-x [DOI] [PubMed] [Google Scholar]
- Wyatt T. Cambridge University Press; Cambridge, UK: 2003. Pheromones and animal behaviour. [Google Scholar]
- Wysocki C.J, Lepri J.J. Consequences of removing the vomeronasal organ. J. Steroid Biochem. Mol. Biol. 1991;39:661–669. doi: 10.1016/0960-0760(91)90265-7. doi:10.1016/0960-0760(91)90265-7 [DOI] [PubMed] [Google Scholar]