Abstract
Despite the importance of predator recognition in mediating predator–prey interactions, we know little about the specific characteristics that prey use to distinguish predators from non-predators. Recent experiments indicate that some prey who do not innately recognize specific predators as threats have the ability to display antipredator responses upon their first encounter with those predators if they are similar to predators that the prey has recently learned to recognize. The purpose of our present experiment is to test whether this generalization of predator recognition is dependent on the level of risk associated with the known predator. We conditioned fathead minnows to chemically recognize brown trout either as a high or low threat and then tested the minnows for their responses to brown trout, rainbow trout (closely related predator) or yellow perch (distantly related predator). When the brown trout represents a high-risk predator, minnows show an antipredator response to the odour of brown trout and rainbow trout but not to yellow perch. However, when the brown trout represents a low-risk predator, minnows display antipredator responses to brown trout, but not to the rainbow trout or yellow perch. We discuss these results in the context of the Predator Recognition Continuum Hypothesis.
Keywords: learned predator recognition, threat-sensitive learning, generalization, antipredator behaviour, predator odours, fathead minnows Pimephales promelas
1. Introduction
Predation is a strong selective force acting on the behaviour, morphology and life history of prey (Lima & Dill 1990; Chivers & Smith 1998; Lima & Bednekoff 1999). To be successful, prey individuals have to balance their time and energy between necessary but costly predator avoidance and fitness-related activities such as foraging, mating or territorial defence. One way to effectively balance this trade-off is through threat-sensitive predator avoidance, where prey respond to predatory threats with an intensity that matches the level of risk associated with the predator (Helfman 1989; Chivers et al. 2001; Ferrari et al. 2006a). This allows the prey to optimize their predator avoidance/foraging trade-off by minimizing the time spent over-responding during less risky situations.
A prerequisite for prey to show adaptive responses to predation threats is that prey actually recognize the predator as dangerous. Some prey have been shown to display antipredator responses to novel predators upon their first encounter (i.e. innate predator recognition—birds: Veen et al. 2000; fishes: Hawkins et al. 2004). However, many other species require learning to recognize novel predators as threats (birds: Curio et al. 1978; fishes: Mathis et al. 1993; mammals: McLean et al. 1996). Although many studies have investigated the existence of predator recognition in a variety of species, very little is known about the specific characteristics of the predator that the prey learn to recognize. Three recent studies have revolutionized the way in which ecologists view predator recognition (Griffin et al. 2001; Ferrari et al. 2007; Stankowich & Coss 2007). These studies revealed that prey animals have the ability to display an antipredator response to a novel predator if it is closely related to a predator they recently learned to recognize. Ferrari et al. (2007) refer to this phenomenon as generalization of predator recognition. In the first study, Griffin et al. (2001) showed that naive tammar wallabies (Macropus eugenii) do not innately recognize stuffed feral cats (Felis catus) or red foxes (Vulpes vulpes) as dangerous but they do show recognition of cats if they are trained to recognize foxes as a predator. In this case, the wallabies generalize the recognition of the fox to the cat based on characteristics the cat and the fox share in common. However, the generalization by the wallabies was not extended to a stuffed non-predatory juvenile goat (Capra hircus). Similarly, Stankowich & Coss (2007) showed that black-tailed deer (Odocoileus hemionus colombianus) displayed antipredator behaviours not only to model cougars, their predators, but also to model tigers, a novel predator. Interestingly, deer did not respond to model jaguars. Stankowich & Coss (2007) attributed their results to the spotted pattern of the jaguar's coat, which would camouflage the jaguar's body shape. These results indicate that learned predator recognition requires labelling of specific characteristics of predators and that predatory traits shared by closely related species of predators can be used by prey to label them as dangerous, prior to any experience with the novel threats. The extent of generalization of predator recognition is unknown. Presumably, generalization of predator recognition would be beneficial for prey, as it would increase their chances of surviving their first encounter with unknown predators, similar to the benefits that innate predator recognition represents. Responding to novel and potentially non-threatening species would, on the other hand, represent a waste of time and energy, which could have been allocated to other fitness-related activities. This paradox raises the question of whether generalization is a rigid phenomenon or is only expressed in situations which would probably benefit the prey.
In the present study, we investigate whether generalization of predator recognition is dependent on the level of risk associated with the known predator, i.e. if there is a threat-threshold associated with the known predator that would determine whether or not closely related species should be labelled as dangerous. Keeping with the previous example, we could ask whether wallabies would still be scared of cats if the red foxes only represented a mild threat. To answer the question of whether the level of threat of the predator influences the generalization to other predators by the prey, we used fathead minnows as our test subject. Ferrari et al. (2007) showed that fathead minnows conditioned to recognize lake trout (Salvelinus namaycush) odour as a threat, generalize their recognition to novel brook trout (Salvelinus fontinalis) and rainbow trout (Oncorhynchus mykiss), but not to distantly related predatory pike (Esox lucius) or non-predatory white suckers (Catostomus commersoni). Using the same system, we conditioned fathead minnows to recognize the odour of predatory brown trout (Salmo trutta) as a high or low risk. We then tested the minnows for a response to the odour of brown trout (reference predator), closely related rainbow trout (same family) or distantly related yellow perch (Perca flavescens; table 1). We hypothesized that if generalization of predator recognition is a constant phenomenon, then the minnows should display antipredator behaviour when exposed to both brown and rainbow trout, regardless of the level of threat associated with the brown trout (as long as the brown trout represent a threat). Alternatively, if generalization of predator recognition is dependent on the level of risk associated with the reference predator, we hypothesized that minnows should recognize rainbow trout as dangerous only when brown trout are already labelled as highly threatening and minnows should not respond to the odour of rainbow trout when the brown trout represent a low threat. In all cases, minnows are not expected to respond to the odour of yellow perch, as they are distantly related.
Table 1.
Simplified representation of the taxonomic relationship between the fish species used in the experiment.
| division: Teleostei |
| subdivision: Euteleostei |
| superorder: Protacanthopterygii |
| order: Salmoniformes |
| family: Salmonidae |
| genus: Salmo, brown trout |
| genus: Oncorhynchus, rainbow trout |
| superorder: Acanthopterygii |
| order: Perciformes |
| family: Percidae, yellow perch |
2. Material and methods
(a) Test fish
Fathead minnows were captured from Feedlot pond, a pond located on the University of Saskatchewan campus, using Gee's Improved minnow traps in October 2007. Feedlot pond contains minnows and brook stickleback (Culaea inconstans), but lacks any predatory fish species. The minnows were housed in a 6000 l flow-through pool filled with dechlorinated tap water at 11°C and fed ad libitum with commercial fish flakes (Nutrafin basix, Rolf C. Hagen, Inc., Montreal, Quebec, Canada).
Brown trout and rainbow trout were obtained from the Fort Qu'Appelle fish hatchery, Saskatchewan, in July 2006 and April 2007, respectively. The two species were housed separately in 6000 l flow-through pools filled with dechlorinated tap water and fed daily with commercial trout pellets (Martin's, Elmira, Ontario, Canada). Yellow perch were captured from Blackstrap Lake, Saskatchewan, in July 2005 using seine nets. They were housed similarly in a 6000 l flow-through pool filled with dechlorinated tap water and fed live prey (minnows, dace, stickleback or goldfish). All fishes were kept under a 14 L : 10 D cycle.
(b) Stimulus collection
(i) Minnow skin extract
For aquatic prey, one widespread mode of learning is through the simultaneous pairing of predator stimuli (i.e. sight or odour) with the alarm cues from injured conspecifics (Chivers & Smith 1994a,b; Woody & Mathis 1998; Wisenden & Millard 2001). In such cases, a single pairing is enough for the prey to learn to recognize the predator (reviewed by Chivers & Smith 1998). Recent work suggested that this mode of learning not only allows prey to learn to recognize the predator as dangerous but also to learn to respond to the predator in a threat-sensitive manner (Ferrari et al. 2005, 2006b; Ferrari & Chivers 2006). Thus, we used a high and a low concentration of conspecific alarm cues to mediate the differential learning of predatory brown trout by the minnows. Minnows conditioned with high concentrations of alarm cues recognize the trout as a high level of threat, while those conditioned with a low concentration of alarm cues recognize the trout as a low level of threat (Ferrari et al. 2005).
To produce alarm cues, we collected the skin extract from four fathead minnows (fork length (FL): mean±s.d.=5.50±0.18 cm). The minnows were killed with a blow to the head (in accordance with the Canadian Council on Animal Care) and skin fillets were removed from both sides of the body and placed in chilled distilled water. The fillets were then homogenized using a Polytron and filtered through glass wool to remove any remaining tissues. We collected a total of 13.9 cm2 of skin in a total of 278 ml of distilled water. This solution was diluted to obtain a final solution containing approximately 1 cm2 of skin per 40 l. This concentration has been shown to elicit overt antipredator responses in fathead minnows (Ferrari et al. 2005, 2006b, 2007). Skin extracts were frozen in 20 ml aliquots at −20°C until required.
(ii) Fish odour
Prey animals often respond to predators based on the presence of conspecific alarm cues in the diet of the predator (Mathis & Smith 1993; Chivers & Mirza 2001). Thus, the perch were deprived of food for 5 days prior to stimulus collection. After this period, two perch (FL: 17.3 and 17.4 cm), two rainbow trout (FL: 16.0 and 19.1 cm) and two brown trout (FL: 17.4 and 17.6 cm) were captured from their holding pool and placed in pairs in three 74 l tanks filled with dechlorinated tap water at 18°C. The fishes were chosen so as to minimize the difference in size between the three species. To control for the effect of diet in our experiment, all fishes were fed two earthworms (obtained from a local bait store) the following day. The earthworms were cut in approximately 1 cm long pieces to facilitate feeding. Two days after feeding, the two fish of each species were rinsed and placed in a 74 l tank filled with 50 l of dechlorinated tap water and left to soak for 24 hours. Each tank was equipped with an airstone but no filter. After this period, the fishes were returned to their original holding pool and fed. The fish-conditioned water was stirred and frozen in 60 ml aliquots until required.
(c) Experimental procedure
(i) Conditioning phase
Twenty-four hours prior to being conditioned, groups of three minnows were placed in 37 l tanks (50×25×30 cm) containing 30 l of dechlorinated tap water and a gravel substrate. The tanks were also equipped with an airstone to which was attached a 2 m long piece of tubing used to inject the stimuli into the tanks. Minnows were fed after being transferred and also 1 hour prior to being conditioned the next day. Prior to injecting the stimuli in the tank, we withdrew and discarded 60 ml of water from the injection tubes (to remove any stagnant water) and an additional 60 ml of water was withdrawn and retained to flush the stimuli into the tank.
The conditioning consisted of injecting sequentially 5 ml of a high or low concentration of alarm cues or dechlorinated tap water, followed by 20 ml of brown trout odour, and finally 60 ml of the retained tank water. For the high concentration of alarm cues, we injected 5 ml of the prepared solution of alarm cues (1 cm2 of skin per 40 l, see §2b(i) above). For the low concentration of alarm cues, we withdrew 1 ml of the prepared solution of alarm cues and 4 ml of dechlorinated tap water in a 5 ml syringe and injected the content of the syringe (equivalent of 1 cm2 of skin per 200 l) into the tank. As such, a non-differential training procedure was used for pairing brown trout odour with either high- or low-concentration alarm cue reinforcement. Previous work by Ferrari et al. (2005) showed that fathead minnows acquire recognition of the odour of a novel predator through similar conditioning using alarm cues at a concentration as low as 1 cm2 of skin per 240 l. On each conditioning day, a third of the tanks received the high concentration of alarm cue treatment, a third received the low concentration of alarm cue treatment and the last third received the water treatment. The three treatments were randomly assigned to the conditioning tanks in the experimental room. At least 1 hour after being conditioned, the groups of three minnows were randomly transferred to identical 37 l tanks (used for testing) containing clean dechlorinated tap water and were fed.
(ii) Testing phase
The testing phase took place 24 hours after the conditioning phase. Minnows were fed 1 hour prior to testing. During this phase, they were randomly exposed to 20 ml of the odour of brown trout, rainbow trout or perch. The behaviour of the minnows was recorded prior to and following the injection of the stimulus into the tank. The injection procedure was similar to that used for the conditioning, with the difference that only the fish odour was injected.
(iii) Behavioural assay
The behaviours of minnows were quantified for 8 min prior to (to obtain a baseline level of activity for the minnows) and for 8 min following the injection of one of the three fish odours. Fathead minnows are known to exhibit immediate and overt antipredator responses upon detection of risky stimuli. The common antipredator behaviours include dashing, freezing, shelter use, reduced activity and increased shoaling cohesion. In our experiment, we used a well-established protocol (Mathis & Smith 1993; Ferrari et al. 2005, 2007) to quantify the antipredator behaviour of our groups of minnows using shoaling index and number of line crosses. The shoaling index of the three fish (1, no fish within a body length of another; 2, two fish within a body length of each other; 3, all three fish within a body length of each other) was assessed every 15 s. The number of line crosses was calculated (using a click counter) for one of the three minnows (randomly chosen) using the 3×3-grid pattern drawn on the side of the tank. An increase in shoaling and a reduction in movement are well-established antipredator responses in fathead minnows (Brown et al. 1995; Ferrari et al. 2005, 2007; Ferrari & Chivers 2006). The experimenter was blind to the treatments throughout the experiment.
(d) Statistical analysis
The data used for the analysis were obtained from the difference in behavioural measures between the pre- and post-injection periods. The data were normally distributed and homoscedastic (Levene's test: shoaling index, p>0.7; line crosses, p>0.6). Thus, we performed a two-way ANOVA on the change in shoaling index and line crosses to investigate the effect of cue (water, low or high concentration of alarm cues) and the effect of predator (brown trout, rainbow trout or yellow perch) on the responses of minnows. To verify the existence of differential learning of brown trout odour by the minnows, a one-way ANOVA was performed to test the effect of cue on the responses of minnows to brown trout. To further investigate the existence of generalization of predator recognition, subsequent tests (one for each cue) were performed to investigate the responses of minnows to different predators.
Threat-sensitive generalization could result from an additive or synergistic effect of combining threat-sensitive learning with generalization. In the case of an additive effect, there is some point where the combined effect of reduced risk and reduced specificity would fall below the threshold for evoking a significant antipredator response. In the case of a synergistic effect, the difference in the intensity of response between the high- and low-alarm cue conditioning groups exposed to the reference predator should be smaller than the difference in intensity between the high- and low-alarm cue conditioning groups in response to the closely related predator (i.e. the response to the low-risk closely related predator should be lower than expected in the additive scenario). Consequently, to test for a possible interaction between the intensity of threat associated with the known reference predator and the response to the closely related predator, we performed a partial two-way ANOVA, comparing only two levels of threat (high and low) and two predators (brown and rainbow trout).
3. Results
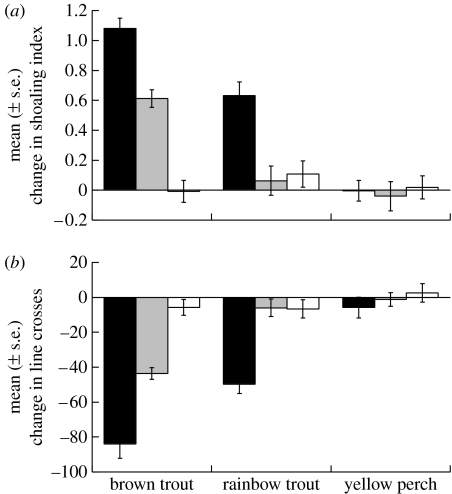
The results of the ANOVA revealed a statistically significant main effect of predator (shoaling index: F2,144=36.9, p<0.001; line crosses: F2,144=49.9, p<0.001), a significant effect of cue (shoaling index: F2,144=33.2, p<0.001; line crosses: F2,144=51.3, p<0.001) and a significant interaction between the two factors (shoaling index: F4,144=13.6, p<0.001; line crosses: F4,144=13.0, p<0.001; figure 1).
Figure 1.
Mean (±s.e.) change in (a) shoaling index or (b) line crosses for minnows responding to the odour of brown trout, rainbow trout or yellow perch, but initially conditioned with brown trout odour paired with a high concentration (black bars) or a low concentration (grey bars) of alarm cues or a water control (white bars).
The one-way ANOVA investigating the effect of cue (high or low concentrations of alarm cues or water) on the responses of minnows to brown trout only revealed threat-sensitive learning by minnows (shoaling index: F3,48=113.1, p<0.001; line crosses: F3,48=91.6, p<0.001; figure 1), i.e. minnows conditioned with a high concentration of alarm cues responded to brown trout odour with a greater response intensity than the ones conditioned with a low concentration of alarm cues (both p<0.001) or with the water control (both p<0.001). Moreover, minnows conditioned with a low concentration of alarm cues showed a higher response intensity to brown trout odour than did the water control ones (both p<0.001).
When investing the effect of predator (brown or rainbow trout or perch) by cue, we found that, as expected, minnows conditioned with water did not differ in their responses to the novel odour of brown trout, rainbow trout or yellow perch (shoaling index: F2,48=0.575, p=0.566; line crosses: F2,48=0.995, p=0.377; figure 1), indicating that minnows did not innately respond to any of the fishes. Consistent with previous results, minnows conditioned with the high concentration of alarm cues paired with brown trout odour showed generalization of predator recognition, i.e. they responded to both brown and rainbow trout odour with an antipredator response (all p<0.001). Moreover, minnows responded with a greater response intensity to the brown trout odour than the rainbow trout odour (both p<0.001). Interestingly, when conditioned to recognize brown trout odour with a low concentration of alarm cues, minnows showed an antipredator response to brown trout odour (both p<0.001), but failed to show a response to the odour of rainbow trout (both p>0.4; figure 1).
The results of the partial two-way ANOVA indicated no significant interaction between predator and cues for either shoaling index (F1,64=0.4, p>0.5) or line crosses (F1,64=0.04, p>0.7).
4. Discussion
Consistent with previous results (Ferrari et al. 2005, 2006b), we showed that the level of risk associated with a new learned threat is dependent on the concentration of alarm cues that prey experienced during the conditioning event. In this case, minnows conditioned with the high concentration of alarm cues labelled brown trout as high-risk predators, while minnows conditioned with the low concentration of alarm cues labelled brown trout as lower risk predators. Moreover, our results clearly suggest that the generalization of predator recognition is not a fixed phenomenon, but depends on the level of risk associated with the reference predator (the brown trout in this case). When brown trout are labelled as high-risk predators, minnows responded to closely related rainbow trout but not to distantly related yellow perch. These results are consistent with the results of Ferrari et al. (2007). Most interestingly, minnows failed to recognize rainbow trout as threatening when brown trout are labelled as low-risk predators. In this study, we combined threat-sensitive learning (see Ferrari et al. 2005) with the concept of generalization of predator recognition (see Ferrari et al. 2007) and refer to the phenomenon as threat-sensitive generalization of predator recognition. Future studies examining this phenomenon should test whether threat-sensitive generalization results from additive or synergistic effects of combining these two phenomena. In the additive scenario, there is some point where the combined effect of reduced risk and reduced specificity falls below the threshold for evoking a significant antipredator response. In the synergistic scenario, the difference in the intensity of response between the high- and low-alarm cue conditioning groups exposed to the reference predator should be smaller than the difference in the intensity between the high- and low-alarm cue conditioning groups in response to the closely related predator. In our experiment, we observed an additive effect. However, it is important to note that we could have missed a synergistic effect due to a zero-truncation problem (i.e. the response of the minnows to the low-risk closely related predator was not different to the control and hence any additional decrease would not have been observable).
Recently, Ferrari et al. (2007) presented the Predator Recognition Continuum Hypothesis, highlighting some of the situations that would lead prey to display either innate or learned recognition of predators. Intuitively, innate predator recognition represents a great advantage to prey, as it probably dramatically increases the prey chances of survival upon their first encounter with a novel predator. Prey showing learned predator recognition need the first encounter with a novel predator to label it as dangerous. The fact that many prey do not show innate predator recognition indicates that either predator and prey did not co-occur for a long enough period of time to allow the genetic fixation of the recognition, and/or that innate predator recognition is costly. In the Predator Recognition Continuum Hypothesis, Ferrari et al. (2007) hypothesized that recognition of predators is dependent on (but not limited to) the temporal and spatial predictability of predation and the diversity of the predators. Indeed, prey would probably benefit from innate predator recognition in environments where the predictability of attack from a predator is high and the diversity of predators is low (i.e. a few but constant predators). Alternatively, prey exposed to a great variety of predator species that are unpredictable in their probability of attack (e.g. due to seasonal diet switches) should benefit more from learned predator recognition, which allows a case-by-case learning of potential threats. In addition, prey exposed to the greatest variability of predation contexts should display the greatest plasticity in their responses to predators, of which generalization of predator recognition is included. This would allow the prey to increase their chances of survival from the first ‘learning trial’ by using their knowledge on close relatives of the novel predator. Our present results refine this aspect of generalization. Prey animals seem to generalize their recognition to close relatives of known predators only for highly threatening species and not for those that represent a low threat. Put back in the context of optimizing trade-offs, differential generalization should allow the prey to be able to match the intensity of their antipredator response to the threat posed by the predator.
Our results indicate that if a predator represents a high-level threat, then prey should exhibit antipredator responses to close relatives of that predator, as closely related species usually share similar foraging habits. When predators are only mildly threatening however, prey seem to restrict their antipredator responses to the specific species of the predator that they learned. While initially counterintuitive, these results may indicate that the more dangerous the predator is, the less specific is its recognition. It may be interesting to consider the phenomenon of generalization in the context of recognition templates. While both groups of minnows have the opportunity to acquire the same amount of information regarding the predator characteristics, it may be that the degree of matching of the predator characteristics to the template varies for the two groups of minnows. When a predator represents a mild threat, minnows might respond to any predator, whose characteristics match exactly the template used for recognition, i.e. species-specific recognition. As the level of threat associated with the learned predator increases, the window of matching necessary to elicit a fright response might become wider and wider, allowing prey to generalize their recognition to all species that fit more or less the characteristics possessed by the reference predator.
In this experiment, we investigated generalization from a chemical perspective. We conditioned minnows to recognize the brown trout as a threat using a constant concentration of trout odour paired with different concentrations of alarm cues. When the minnows recognize the brown trout as a high-level threat, they generalize this threat to rainbow trout. The reduction in intensity of response of these minnows to rainbow trout indicates that the rainbow trout odour does not match the brown trout odour exactly (i.e. there are fewer chemicals in common or the concentrations of specific chemicals are different). When the brown trout is recognized as a low-level threat the mismatch between the rainbow trout odour and brown trout odour is the same. However, given that the level of threat of the brown trout is lower, the reduction in the intensity of antipredator response as a result of the mismatch is enough to eliminate the response to rainbow trout. From a proximate perspective, this could be interpreted as an effect of diluting the concentration of the specific chemicals or suite of chemicals that elicit the response. Future researchers should use this framework to address how the specific visual characteristics of predators are likewise diluted to eliminate the recognition in a generalization context. This would allow us to address the specific characteristics that prey use to recognize predators.
The ability of prey to avoid predators is a fundamental issue in biology. The specific ecological and evolutionary pressures that lead to learning versus fixed recognition have received surprisingly little attention (but see Blumstein 2002, 2006; Ferrari et al. 2007). Our results expand on the theoretical framework of the Predator Recognition Continuum Hypothesis demonstrating that the ability of prey to generalize their recognition of predators is dependent on the relative threat posed by the predator.
Acknowledgments
All work reported herein was in accordance with the Guidelines to the Care and Use of Experimental Animals published by the Canadian Council on Animal Care and was conducted under the University of Saskatchewan Committee of Animal Care and Supply protocol no. 20070083.
The Natural Sciences and Engineering Research Council of Canada and the University of Saskatchewan provided financial support to D.P.C. and F.M.
References
- Blumstein D.T. Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J. Biogeogr. 2002;29:685–692. doi:10.1046/j.1365-2699.2002.00717.x [Google Scholar]
- Blumstein D.T. The multipredator hypothesis and the evolutionary persistence of antipredator behaviour. Ethology. 2006;112:209–217. doi:10.1111/j.1439-0310.2006.01209.x [Google Scholar]
- Brown G.E, Chivers D.P, Smith R.J.F. Localized defecation of pike: a response to labelling by cyprinid alarm pheromone? Behav. Ecol. Sociobiol. 1995;36:105–110. doi:10.1007/BF00170715 [Google Scholar]
- Chivers, D. P. & Mirza, R. S. 2001 Predator diet cues and the assessment of predation risk by aquatic vertebrates: a review and prospectus. In Chemical signals in vertebrates, vol. 9 (eds D. A. Marchlewska-Koj, J. J. Lepri & D. Müller-Schwarze), pp. 277–284. New York, NY: Plenum Press.
- Chivers D.P, Smith R.J.F. Fathead minnows, Pimephales promelas, acquire predator recognition when alarm substance is associated with the sight of unfamiliar fish. Anim. Behav. 1994a;48:597–605. doi:10.1006/anbe.1994.1279 [Google Scholar]
- Chivers D.P, Smith R.J.F. The role of experience and chemical alarm signalling in predator recognition by fathead minnows, Pimephales promelas. J. Fish Biol. 1994b;44:273–285. doi:10.1111/j.1095-8649.1994.tb01205.x [Google Scholar]
- Chivers D.P, Smith R.J.F. Chemical alarm signaling in aquatic predator/prey interactions: a review and prospectus. Ecoscience. 1998;5:338–352. [Google Scholar]
- Chivers D.P, Mirza R.S, Bryer P.J, Kiesecker J.M. Threat-sensitive predator avoidance by slimy sculpins: understanding the importance of visual versus chemical information. Can. J. Zool. 2001;79:867–873. doi:10.1139/cjz-79-5-867 [Google Scholar]
- Curio E, Ernst U, Vieth W. Cultural transmission of enemy recognition: one function of mobbing. Science. 1978;202:899–901. doi: 10.1126/science.202.4370.899. doi:10.1126/science.202.4370.899 [DOI] [PubMed] [Google Scholar]
- Ferrari M.C.O, Chivers D.P. The role of learning in the development of threat-sensitive predator avoidance: how do fathead minnows incorporate conflicting information? Anim. Behav. 2006;71:19–26. doi:10.1016/j.anbehav.2005.02.016 [Google Scholar]
- Ferrari M.C.O, Trowell J.J, Brown G.E, Chivers D.P. The role of leaning in the development of threat-sensitive predator avoidance in fathead minnows. Anim. Behav. 2005;70:777–784. doi:10.1016/j.anbehav.2005.01.009 [Google Scholar]
- Ferrari M.C.O, Messier F, Chivers D.P. The nose knows: minnows determine predator proximity and density through detection of predator odours. Anim. Behav. 2006a;72:927–932. doi:10.1016/j.anbehav.2006.03.001 [Google Scholar]
- Ferrari M.C.O, Kapitania-Kwok T, Chivers D.P. The role of learning in the development of threat-sensitive predator avoidance: the use of predator cue concentration by fathead minnows. Behav. Ecol. Sociobiol. 2006b;60:522–527. doi:10.1007/s00265-006-0195-z [Google Scholar]
- Ferrari M.C.O, Gonzalo A, Messier F, Chivers D.P. Generalization of learned predator recognition: an experimental test and framework for future studies. Proc. R. Soc. B. 2007;274:1853–1859. doi: 10.1098/rspb.2007.0297. doi:10.1098/rspb.2007.0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin A.S, Evans C.S, Blumstein D.T. Learning specificity in acquired predator recognition. Anim. Behav. 2001;62:577–589. doi:10.1006/anbe.2001.1781 [Google Scholar]
- Hawkins L.A, Magurran A.E, Armstrong J.D. Innate predator recognition in newly-hatched Atlantic salmon. Behaviour. 2004;141:1249–1262. doi:10.1163/1568539042729694 [Google Scholar]
- Helfman G.S. Threat-sensitive predator avoidance in damselfish–trumpetfish interactions. Behav. Ecol. Sociobiol. 1989;24:47–58. doi:10.1007/BF00300117 [Google Scholar]
- Lima S.L, Bednekoff P.A. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 1999;153:649–659. doi: 10.1086/303202. doi:10.1086/303202 [DOI] [PubMed] [Google Scholar]
- Lima S.L, Dill L.M. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 1990;68:619–640. [Google Scholar]
- Mathis A, Smith R.J.F. Fathead minnows, Pimephales promelas, learn to recognize northern pike, Esox lucius, as predators on the basis of chemical stimuli from minnows in the pike's diet. Anim. Behav. 1993;46:645–656. doi:10.1006/anbe.1993.1241 [Google Scholar]
- Mathis A, Chivers D.P, Smith R.J.F. Population differences in responses of fathead minnows (Pimephales promelas) to visual and chemical stimuli from predators. Ethology. 1993;93:31–40. [Google Scholar]
- McLean I.G, Lundie-Jenkins G, Jarman P.J. Teaching an endangered mammal to recognise predators. Biol. Cons. 1996;56:51–62. doi:10.1016/0006-3207(95)00038-0 [Google Scholar]
- Stankowich T, Coss R.G. The re-emergence of felid camouflage with the decay of predator recognition in deer under relaxed selection. Proc. R. Soc. B. 2007;274:175–182. doi: 10.1098/rspb.2006.3716. doi:10.1098/rspb.2006.3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen T, Richardson D.S, Blaakmeer K, Komdeur J. Experimental evidence for innate predator recognition in the Seychelles warbler. Proc. R. Soc. B. 2000;267:2253–2258. doi: 10.1098/rspb.2000.1276. doi:10.1098/rspb.2000.1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisenden B.D, Millard M.C. Aquatic flatworms use chemical cues from injured conspecifics to assess predation risk and to associate risk with novel cues. Anim. Behav. 2001;62:761–766. doi:10.1006/anbe.2001.1797 [Google Scholar]
- Woody D.R, Mathis A. Acquired recognition of chemical stimuli from an unfamiliar predator: associative learning by adult newts, Notophthalmus viridescens. Copeia. 1998;1998:1027–1031. doi:10.2307/1447352 [Google Scholar]