Abstract
Sex ratio theory provides a powerful source of testable predictions about sex allocation strategies. Although studies of invertebrates generally support predictions derived from the sex ratio theory, evidence for adaptive sex ratio biasing in vertebrates remains contentious. This may be due to the fact that most studies of vertebrates have focused on facultative adjustment in relation to maternal condition, rather than processes that might produce uniform sex biases across individuals. Here, we examine the effects of local resource enhancement (LRE) and local resource competition (LRC) on birth sex ratios (BSRs). We also examine the effects of sex differences in the costs of rearing male and female offspring on BSRs. We present data from 102 primate species and show that BSRs are skewed in favour of the dispersing sex in species that do not breed cooperatively, as predicted by the LRC model. In accordance with the LRE model, BSRs are generally skewed in favour of the more beneficial sex in cooperatively breeding primate species. There is no evidence that BSRs reflect the extent of sexual size dimorphism, an indirect measure of the costs of rearing male and female offspring. These analyses suggest that adaptive processes may play an important role in the evolution of BSRs in vertebrates.
Keywords: sex ratio, sex allocation, local resource competition, local resource enhancement, sex ratio adjustment, primates
1. Introduction
Sex ratio theory represents one of the triumphs of evolutionary theory. Studies of sex ratio adjustment in a variety of invertebrate taxa provide strong support for quantitative predictions derived from theoretical models (Seger & Stubblefield 2002; West et al. 2005). By contrast, vertebrate sex ratios provide a poor fit to theoretical models (Cockburn et al. 2002; Uller 2006). This may reflect the fact that most research on vertebrate sex ratios has focused on Trivers & Willard's (1973) model of facultative adjustment in relation to maternal condition (Clutton-Brock & Iason 1986; Brown & Silk 2002; Cockburn et al. 2002) rather than processes that might generate uniform biases across individuals, such as local resource competition (LRC) and local resource enhancement (LRE). LRC occurs when individuals of one sex are philopatric and experience competition over access to local resources, and is expected to favour the evolution of birth sex ratios (BSRs) that are biased in favour of the sex that disperses from the natal area (Hamilton 1967; Clark 1978; Silk 1984). LRE occurs in cooperatively breeding species when there are differences in the effectiveness of male and female helpers. If the benefits derived from helpers do not vary markedly across parents, BSRs will be expected to be biased in favour of the more helpful sex (Gowaty & Lennartz 1985; Emlen et al. 1986; Pen & Weissing 2000). Here, we examine the role of LRC and LRE on BSRs within the primate order.
There is some evidence that LRC generates male-biased BSRs in primate species with male dispersal. The intensity of competition over access to resources was linked to BSRs in seven primate genera with female philopatry (Johnson 1988). Johnson also showed that BSRs were higher (more male-biased) in nine genera with female-biased philopatry than in seven other genera without female-biased philopatry when the extent of home range overlap among females was taken into account. Faust & Thompson (2000) compiled captive birth records for 14 species of primates with known dispersal patterns. In the 13 species with male dispersal, the total number of male births significantly exceeded the total number of female births. These studies suggest that LRC has shaped BSRs in primates, but they have several important limitations. First, both of these studies were based on a relatively small sample of primate taxa. Second, neither study systematically considered the effects of phylogeny, which may distort the results of comparative analyses. Third, only one female-dispersing species was included in these analyses, limiting the power of comparisons between BSRs in female-dispersing and male-dispersing species. Fourth, both studies were based on captive populations; it is possible that sex ratios may vary between wild and captive populations (Glaston 1997). Fifth, these studies did not distinguish between cooperatively and non-cooperatively breeding species, potentially conflating the effects of LRC and LRE.
In cooperatively breeding species of birds, the extent of the bias in BSRs is associated with differences in the benefits derived from male and female helpers (Griffin et al. 2005). Similarly, in alpine marmots (Marmota marmota) and wild dogs (Lycaon pictus), BSRs are biased in favour of males, and males are more effective helpers than females (Allainé 2004; Griffin et al. 2005; McNutt & Silk 2008). In cooperatively breeding primates, males also seem to be more active helpers and have more beneficial effects on the reproductive performance of the breeding female than do females (Garber et al. 1984; Baker et al. 1993; Koenig 1995; Bales et al. 2001). However, it is not yet known whether BSRs are biased in cooperatively breeding primates.
It also possible that sex differences in the costs of rearing male and female offspring might contribute to variation in BSRs across species. According to Fisher (1930), mothers will balance the overall investment in male and female offspring. This means that if one sex is more costly to rear than the other, BSRs will be biased in favour of the less costly sex. In primates, where adult males are often considerably larger than females, we would expect more dimorphic species to have lower (less male-biased) BSRs. Johnson (1988) did not find a significant effect of sexual dimorphism on BSRs in primates, but his analysis was restricted to a relatively small number of primate genera.
Here we examine the effects of LRC, LRE and the extent of sexual dimorphism on BSRs. These analyses extend the previous studies of the effects of dispersal patterns on BSRs by drawing on a considerably larger dataset and employing phylogenetic methods for analysis. If sex differences in investment affect BSRs in primates, then the extent of sexual dimorphism will be expected to be negatively related to BSRs. If LRC shapes BSRs, then species in which females are the primary dispersers are expected to have lower (less male-biased) BSRs than species in which males are the primary dispersers. The species in which both males and females disperse are expected to have intermediate BSRs, as long as both sexes disperse over equal distances and impose similar competitive costs on mothers and their resident offspring. If LRE shapes BSRs in cooperatively breeding primates and the benefits of helping do not vary substantially across groups, then we would expect these species to have male-biased BSRs because males seem to be the more helpful sex.
2. Material and methods
We compiled information about BSRs from a variety of sources (see electronic supplementary material). We collated published information on the numbers of males and females born in wild and captive populations in primates by (i) surveying all issues of specialized primatology journals published between 1990 and 2005, (ii) carrying out keyword searches of the ISI Web of Knowledge, using the terms ‘sex ratio’, ‘BSR’ and ‘sex allocation’, and (iii) using published reports that were discovered in searches linked to previous analyses of the effects of maternal rank on BSR (Brown & Silk 2002; Silk et al. 2005), and cross checking sources of data mentioned in any these publications.
We obtained unpublished information about BSR by (i) soliciting information from the directors of long-term field research projects, directors of captive primate colonies and stud book keepers, (ii) posting a request for unpublished data on the Primate-Science e-mail list, administered by Primate Info Net (National Primate Research Center, University of Wisconsin–Madison), and (iii) requesting permission to reuse unpublished information solicited for previous analyses. Only datasets with at least 10 births of known sex were included. Where more than one report from a specific field site or captive colony was available, only the study with the largest sample size was included.
For all of the species in our database, we compiled subsidiary information about dispersal patterns, body size dimorphism and group size mainly from published sources. The adult body size dimorphism was calculated as ln(male weight)−ln(female weight). We included group size in the analyses as a potential confounding variable. Dispersal patterns were classified into three categories as follows: species in which females are the primary dispersers and males are philopatric (or in which females disperse further than males); species in which both sexes disperse; and species in which males are the primary dispersers and females are philopatric (or in which males disperse further than females). This classification scheme treats dispersal as a categorical phenomenon, and not a quantitative one. This is problematic to the extent that it ignores the possibility of sex differences in dispersal probabilities or dispersal distances in species in which both sexes disperse. However, quantitative estimates of sex differences in dispersal frequency and dispersal distance are not available for most species.
(a) Analysis
We defined BSR as the proportion of male births (number of male births divided by the number of male births plus the number of female births). Births of unknown sex were reported in 66% of the samples, and on average accounted for 15±0.01% of all births in these cases. We did not include births of unknown sex in our estimates of BSR, which is equivalent to assuming that there were no biases in assignment to the unknown category.
Ascertainment bias may affect the analysis in two ways. First, females may be more likely to be classified as ‘unknown’ than males because their genitalia are less conspicuous or readily classified. Second, infants may be systematically misidentified as males or females. Such biases could distort the results of our analyses. However, biases in ascertainment are likely to have a bigger impact on the estimates of BSRs in wild populations with poor visibility than in captive populations where animals can be inspected at a close range. If data obtained from wild and captive populations generate similar patterns, we can have more confidence in the results.
A substantial fraction of the BSR data came from captive, semi-free ranging or provisioned populations (N=145 samples). If captivity systematically affects BSRs, the use of captive data could bias the analysis. Therefore, we also present the results of analyses based on data derived from 72 samples of wild populations representing 42 species. We used the binomial test to determine whether the number of male and female births for each species differed significantly from unity.
To assess the factors that affect BSR, we carried out partial regression analyses using Alan Grafen's Phylogenetic Regression programme implemented in the SAS statistical software package (http://users.ox.ac.uk/∼grafen/phylo/index.html, v. 7.0). The phylogeny used in these analyses was created in the Newick style from the previously published primate supertree (Purvis 1995; Purvis & Webster 1999). As branch lengths were not available for all parts of this tree, we allowed the phylogenetic regression programme to set branch lengths using the default method. In addition, we allowed the scaling parameter, ρ, to be set by the programme using the maximum-likelihood method.
For analyses of LRC, we excluded cooperatively breeding species. We did not conduct phylogenetic analyses of predictions derived from LRE because all of the cooperatively breeding primate species are members of a single subfamily. We used one-way ANOVAs to assess differences in BSRs and size of groups between cooperative and non-cooperative breeders.
3. Results
The final dataset was composed of 217 samples representing 102 species and 45 genera, providing a total of 77 294 births. The estimates of BSRs ranged from 0.36 in woolly spider monkeys (Brachyteles arachnoides) to 0.77 in western tarsiers (Tarsius bancanus; mean±s.e.: 0.53±0.07; see table 1 in the electronic supplementary material). A similar distribution of values was observed in samples derived from wild populations (mean=0.50±0.02, range 0.26–0.76, N=42). BSRs deviate significantly from unity in 35 out of the 102 species we surveyed (35%; table 1 in the electronic supplementary material), which is far more than expected by chance if species are considered as independent data points (binomial, p<0.0001).
The accuracy of BSR estimates is likely to be affected by the size of the sample on which the estimates are based (Palmer 2000). The number of births of known sex for each species ranged from 17 to nearly 12 000, with an average of 753±159. In wild populations, the sample sizes of births of known sex ranged from 12 to 3021, with an average of 283±89. The magnitude of sex ratio skews was negatively related to the sample size (all data: r=−0.2043, n=102, p=0.0394; wild data: r=−0.2940, n=42, p=0.0588), with the largest skews coming from species with the smallest numbers of births. However, in samples with more than 100 births, there is no effect of sample size on the magnitude of sex ratio skews (r=−0.1996, n=68, p=0.1029; wild data: r=−0.2067, n=17, p=0.4261). Thus, samples that exceed 100 provide reliable estimates of BSRs.
4. Sexual size dimorphism
No relationship was found between BSR and sexual size dimorphism (F1,76=0.151, p=0.699). This result held when the analyses were confined to species with samples of 100 or more births (F1,69=0.0795, p=0.779). An analysis of data from wild populations also failed to find a significant relationship between BSR and sexual size dimorphism (F1,34=0.792, p=0.380).
5. Local resource competition
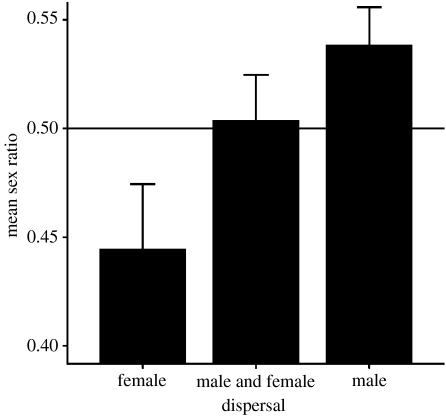
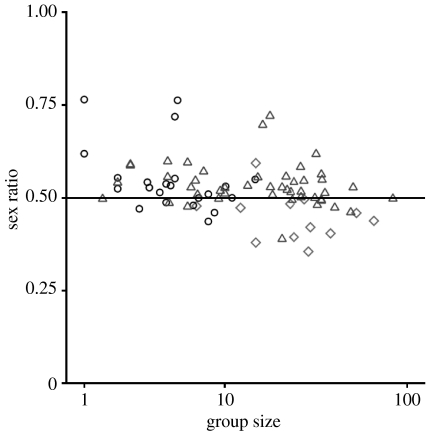
BSR was found to differ significantly across dispersal categories (F2,67=5.421, p=0.007). On average, BSRs are biased towards females in species in which females are the primary dispersers, unbiased in species in which both the sexes disperse and biased in favour of males in species in which males disperse (figure 1). The relationship also held when the analysis was restricted to BSRs from wild populations (F2,34=4.251, p=0.022). The relationship remained when the analyses were restricted to species with samples of 100 or more births (F2,60=4.451, p=0.016). However, group size may contribute to these differences because species in which both sexes disperse live in smaller groups than other species (F2,65=10.486, p<0.001). When group size was controlled in the analyses, the relationship between BSR and dispersal category remained significant (figure 2; F2,67=3.881, p=0.025). This result also held when using sex ratios from wild populations (F2,33=4.408, p=0.020), and remained as a trend when restricted to species with large samples of births (more than 100 births: F2,59=2.994, p=0.058).
Figure 1.
The mean+s.e. of BSRs in species in which females are the primary dispersers (n=8 species), both sexes disperse (11 species) and males are the primary dispersers (37 species) are shown. Only species with samples of births which exceed 100 were used to construct this graph.
Figure 2.
BSRs plotted against group size for those species in which females disperse (n=12 species; diamond), males disperse (46 species; triangle) and both sexes disperse (22 species; circle). Cooperatively breeding species were excluded from this graph.
6. Local resource enhancement
Our dataset included 10 cooperatively breeding species. The average sex ratio of these species was 0.54±0.01 (n=9 species with a sample size greater than 100). Cooperatively breeding species had significantly higher sex ratios than other species in which both sexes disperse (0.50±0.01, n=11 species with sample size greater than 100; one-way ANOVA: F1,18=5.02, p=0.038), although they lived in groups of approximately the same size (one-way ANOVA: F1,18=0.06, p=0.809). We are unable to replicate this analysis with data from wild populations because we have data for only two cooperatively breeding species in the wild.
We could not use phylogenetic methods to assess sex ratio biases in cooperatively breeding species because all cooperatively breeding species belong to the same subfamily. However, we can evaluate BSR for one species within the subfamily that does not breed cooperatively, Callimico goeldii. For this species, the sex ratio is 0.51 (n=1890 births), which falls just outside the 95% CI of the mean BSR for cooperatively breeding members of the subfamily.
7. Discussion
BSRs in primate groups conform to predictions derived from LRC and LRE models, but not to predictions based on sex differences in investment costs. BSRs are not linked to the extent of sexual dimorphism within species, suggesting that BSRs do not reflect differences in the costs of rearing male and female offspring or that investment costs need to be measured in a more sophisticated way in primates (Brown 2001), as has already been done for social insects (Boomsma 1989).
As predicted from the LRC model, dispersal patterns were linked to BSRs in primate groups that do not breed cooperatively. Species in which females are the primary dispersers had female-biased BSRs, species in which males are the primary dispersers had male-biased BSRs and species in which both sexes dispersed were unbiased. Although male dispersal predominates within the primate order (Pusey & Packer 1987), female dispersal has evolved in several distantly related taxa, including spider and woolley monkeys (Ateles, Lagothrix and Brachyteles), red colobus (Piliocolobus badius), various langurs and leaf monkeys (Trachypithecus spp.), hamadryas baboons (Papio hamadryas) and both species of chimpanzee (Pan troglodytes, Pan paniscus). In each case, female-dispersing species have lower sex ratios than other closely related species.
Sex ratios are biased towards males in cooperatively breeding primate species. This corresponds to reports which indicate that males are more active and effective helpers than females in these species (Garber et al. 1984; Baker et al. 1993; Koenig 1995; Bales et al. 2001). Thus, females seem to bias BSR in favour of the more beneficial sex, as predicted by some forms of the LRE model. BSRs are higher in the cooperatively breeding members of the subfamily Callitrichinae than in the only member of the subfamily that does not breed cooperatively.
The patterns of BSRs reported here may result from the selection on genetic factors that bias BSRs in a species-typical manner or from facultative adjustment by individual females to prevailing social or ecological conditions. There is some evidence that female primates facultatively adjust BSRs in response to the current levels of LRC. Over a 30-year period, BSRs tracked fluctuations in population density in a population of wild red howler monkeys, Alouatta seniculus (Rudran & Fernandez-Duque 2003). BSRs were female-biased when population densities were low and groups were small, but became progressively more male-biased as population densities increased, groups became larger and competition among females over recruitment opportunities became more intense.
It might also be profitable for females to facultatively adjust their BSRs in relation to the size of their groups. Griffin et al. (2005) predicted that groups with fewer numbers of helpers would produce relatively more of the more helpful sex. In wild dogs, Lycaon pictus, sex ratio biases are most pronounced in litters produced by young mothers (Creel et al. 1998; McNutt & Silk 2008). We do not yet know whether maternal age or group size affects BSR in cooperatively breeding primates, but this would be an interesting issue to investigate.
The data presented here suggest that LRC and LRE have more consistent impacts on BSRs in the primate order than does variation in maternal condition (Brown & Silk 2002; Silk et al. 2005). This corresponds to the results of broader comparative analyses, which show that sex ratio manipulation is generally more responsive to forces that shape sex ratios at the population level, than to forces that adjust sex ratios in relation to environmental conditions that have sex-specific effects on offspring (West et al. 2005). LRC may play an important role in shaping BSRs in a wide range of mammalian taxa. Sex biases in philopatry occur in species as diverse as white-nosed coatis (Nasua narica: Gompner et al. 1997), spotted hyenas (Crocuta crocuta: van Horn et al. 2003) and sperm whales (Physeter macrocephalus: Christal & Whitehead 2001). Also, in a number of species that are normally categorized as ‘solitary’, adults typically forage alone, but kin occupy overlapping home ranges (Waser & Jones 1983). For example, female brushtailed possums (Trichosurus vulpecula) settle within their mothers' home ranges and compete for access to den sites, while males disperse further. In areas in which the extent of competition for dens is high, sex ratios are more strongly biased in favour of males (Johnson et al. 2001). Thus, it may be profitable to explore further the effects of competition and cooperation on BSRs in mammals.
Acknowledgments
Elisabeth Willoughby contributed to early data collection efforts. We are grateful to all those who provided us with information for these analyses: D. Aden, J. Altmann, S. Alberts, S. Averill, A. Baker, J. Ballou, L. Barrett, V. Bentley-Condit, C. Borries, R. Deleu, M. Eberle, M. Emery-Thompson, L. Fairbanks, C. Fichtel, H. Fitch-Snyder J. French, S. Friedrichs, D. Gibson, D. Haring, S. P. Henzi, B. Jacobs, M. Jorgensen, P. Kappeler, R. Lesser, I. Leinfelder, J. Littleton, C. McCann, D. Meikle, L. Nash, M. Norconk C. Packer, J. Parga, L. Perkins, S. Perry, S. Pochron, A. E. Pusey, U. Reichard, G. Reinartz, V. Reynolds, A. Robbins, M. Robbins, C. Ross, S. Ross, C. P. van Schaik, J. Setchell, N. Shelmidine S. Siers, M. Warneke, D. Wharton, E. Wickings and P. Wright. We thank Alan Grafen, Craig Packer, Stuart West and an anonymous reviewer for their helpful comments on the manuscript.
Supplementary Material
Supplementary tables
References
- Allainé D. Sex ratio variation in the cooperatively breeding alpine marmot Marmota marmota. Behav. Ecol. 2004;15:997–1002. doi:10.1093/beheco/arh105 [Google Scholar]
- Baker A.J, Dietz J.M, Kleiman D.G. Behavioural evidence for monopolization of paternity in multi-male groups of golden lion tamarins. Anim. Behav. 1993;46:1091–1103. doi:10.1006/anbe.1993.1299 [Google Scholar]
- Bales K, O'Herron M, Baker A.J, Dietz J.M. Sources of variability in numbers of live births in wild golden lion tamarins (Leontopithecus rosalia) Am. J. Primatol. 2001;54:211–221. doi: 10.1002/ajp.1031. doi:10.1002/ajp.1031 [DOI] [PubMed] [Google Scholar]
- Boomsma J.J. Sex-investment ratios in ants: has female bias been systematically overestimated? Am. Nat. 1989;133:517–532. doi:10.1086/284933 [Google Scholar]
- Brown G.R. Sex-biased investment in nonhuman primates: can Trivers & Willard's theory be tested? Anim. Behav. 2001;61:683–694. doi:10.1006/anbe.2000.1659 [Google Scholar]
- Brown G.R, Silk J.B. Reconsidering the null hypothesis: is maternal rank associated with birth sex ratios in primate groups? Proc. Natl Acad. Sci. USA. 2002;99:11 252–11 255. doi: 10.1073/pnas.162360599. doi:10.1073/pnas.162360599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christal J, Whitehead H. Social affiliations within sperm whale (Physeter macrocephalus) groups. Ethology. 2001;107:323–340. doi:10.1046/j.1439-0310.2001.00666.x [Google Scholar]
- Clark A.B. Sex ratio and local resource competition in a prosimian primate. Science. 1978;201:163–165. doi: 10.1126/science.201.4351.163. doi:10.1126/science.201.4351.163 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Iason G.R. Sex ratio variation in mammals. Q. Rev. Biol. 1986;61:339–374. doi: 10.1086/415033. doi:10.1086/415033 [DOI] [PubMed] [Google Scholar]
- Cockburn A, Legge S, Double M.C. Sex ratios in birds and mammals: can the hypotheses be disentangled? In: Hardy I.C.W, editor. Sex ratios: concepts and research methods. Cambridge University Press; Cambridge, UK: 2002. pp. 266–286. [Google Scholar]
- Creel S, Creel N, Monfort S.L. Birth order, estrogens and sex-ratio adaptation in African wild dogs (Lycaon pictus) Anim. Reprod. Sci. 1998;53:315–320. doi: 10.1016/s0378-4320(98)00121-3. doi:10.1016/S0378-4320(98)00121-3 [DOI] [PubMed] [Google Scholar]
- Emlen S.T, Emlen J.M, Levin S.A. Sex-ratio selection in species with helpers-at-the-nest. Am. Nat. 1986;127:1–8. doi: 10.1086/303148. doi:10.1086/284463 [DOI] [PubMed] [Google Scholar]
- Faust L.J, Thompson S.D. Birth sex ratio in captive mammals: patterns, biases, and implications for management and conservation. Zoo Biol. 2000;19:11–25. doi:10.1002/(SICI)1098-2361(2000)19:1<11::AID-ZOO2>3.0.CO;2-V [Google Scholar]
- Fisher R.A. Oxford University Press; Oxford, UK: 1930. The genetical theory of natural selection. [Google Scholar]
- Garber P.A, Moya L, Malaga C. A preliminary field study of the moustached tamarin monkey (Saguinus mystax) in northeastern Peru: questions concerned with the evolution of a communal breeding system. Folia Primatol. 1984;42:17–32. [Google Scholar]
- Glaston A.R. Sex ratio research in zoos and its implications for captive management. Appl. Anim. Behav. Sci. 1997;51:209–216. doi:10.1016/S0168-1591(96)01105-7 [Google Scholar]
- Gompner M.E, Gittleman J.L, Wayne R.K. Genetic relatedness, coalitions and social behaviour of white-nosed coatis, Nasua narica. Anim. Behav. 1997;53:781–797. doi:10.1006/anbe.1996.0344 [Google Scholar]
- Gowaty P.A, Lennartz M.R. Sex ratios of nestling and fledgling red-cockaded woodpeckers (Picoides borealis) favor males. Am. Nat. 1985;126:347–353. doi:10.1086/284421 [Google Scholar]
- Griffin A.S, Sheldon B.C, West S.A. Cooperative breeders adjust offspring sex ratios to produce helpful helpers. Am. Nat. 2005;166:628–632. doi: 10.1086/491662. doi:10.1086/491662 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D. Extraordinary sex ratios. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. doi:10.1126/science.156.3774.477 [DOI] [PubMed] [Google Scholar]
- Johnson C.N. Dispersal and the sex ratio at birth in primates. Nature. 1988;332:726–728. doi: 10.1038/332726a0. doi:10.1038/332726a0 [DOI] [PubMed] [Google Scholar]
- Johnson C.N, Clinchy M, Taylor A.C, Krebs C.J, Jarman P.J, Payne A, Ritchie E.G. Adjustment of offspring sex ratios in relation to the availability of resources for philopatric offspring in the common brushtail possum. Proc. R. Soc. B. 2001;268:2001–2005. doi: 10.1098/rspb.2001.1723. doi:10.1098/rspb.2001.1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig A. Group size, composition, and reproductive success in wild common marmosets (Callithrix jacchus) Am. J. Primatol. 1995;35:311–317. doi: 10.1002/ajp.1350350407. doi:10.1002/ajp.1350350407 [DOI] [PubMed] [Google Scholar]
- McNutt J.W, Silk J.B. Pup production, sex ratios, and survivorship in African wild dogs, Lycaon pictus. Behav. Ecol. Sociobiol. 2008;62:1061–1067. doi:10.1007/s00265-007-0533-9 [Google Scholar]
- Palmer A.R. Quasireplication and the contract of error: lessons from sex ratios, heritabilities and fluctuating asymmetry. Annu. Rev. Ecol. Syst. 2000;31:441–480. doi:10.1146/annurev.ecolsys.31.1.441 [Google Scholar]
- Pen I, Weissing F.J. Sex-ratio optimization with helpers at the nest. Proc. R. Soc. B. 2000;267:539–543. doi: 10.1098/rspb.2000.1034. doi:10.1098/rspb.2000.1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A. A composite stimate of primate phylogeny. Phil. Trans. R. Soc. B. 1995;348:405–421. doi: 10.1098/rstb.1995.0078. doi:10.1098/rstb.1995.0078 [DOI] [PubMed] [Google Scholar]
- Purvis A, Webster A.J. Phylogenetically independent comparisons and primate phylogeny. In: Lee P.C, editor. Comparative primate socioecology. Cambridge University Press; Cambridge, UK: 1999. pp. 44–70. [Google Scholar]
- Pusey A.E, Packer C. Dispersal and philopatry. In: Smuts B.B, Cheney D.L, Seyfarth R.M, Wrangham R.W, Struhsaker T.T, editors. Primate societies. University of Chicago Press; Chicago, IL: 1987. pp. 250–266. [Google Scholar]
- Rudran R, Fernandez-Duque E. Demographic changes over thirty years in a red howler population in Venezuela. Int. J. Primatol. 2003;24:925–947. doi:10.1023/A:1026241625910 [Google Scholar]
- Seger J, Stubblefield J.W. Models of sex ratio evolution. In: Hardy I.C.W, editor. Sex ratios: concepts and research methods. Cambridge University Press; Cambridge, UK: 2002. pp. 2–25. [Google Scholar]
- Silk J.B. Local resource competition and the evolution of male-biased sex ratios. J. Theor. Biol. 1984;108:203–213. doi: 10.1016/s0022-5193(84)80066-1. [DOI] [PubMed] [Google Scholar]
- Silk J.B, Willoughby E, Brown G.R. Maternal rank and local resource competition do not predict birth sex ratios in wild baboons. Proc. R. Soc. B. 2005;272:859–864. doi: 10.1098/rspb.2004.2994. doi:10.1098/rspb.2004.2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R.L, Willard D. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. doi:10.1126/science.179.4068.90 [DOI] [PubMed] [Google Scholar]
- Uller T. Sex-specific sibling interactions and offspring fitness in vertebrates: patterns and implications for maternal sex ratios. Biol. Rev. 2006;81:207–217. doi: 10.1017/S1464793105006962. doi:10.1017/S1464793105006962 [DOI] [PubMed] [Google Scholar]
- van Horn R.C, McElhinny T.L, Holekamp K.E. Age estimation and dispersal in the spotted hyena (Crocuta crocuta) J. Mammal. 2003;84:1019–1030. doi:10.1644/BBa-023 [Google Scholar]
- Waser P.M, Jones W.T. Natal philopatry among solitary mammals. Q. Rev. Biol. 1983;58:355–390. doi:10.1086/413385 [Google Scholar]
- West S.A, Shuker D.M, Sheldon B.C. Sex-ratio adjustment when relatives interact: a test of constraints on adaptation. Evolution. 2005;59:1211–1228. doi:10.1111/j.0014-3820.2005.tb01772.x [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables