Abstract
Ocean acidification is the lowering of pH in the oceans as a result of increasing uptake of atmospheric carbon dioxide. Carbon dioxide is entering the oceans at a greater rate than ever before, reducing the ocean's natural buffering capacity and lowering pH. Previous work on the biological consequences of ocean acidification has suggested that calcification and metabolic processes are compromised in acidified seawater. By contrast, here we show, using the ophiuroid brittlestar Amphiura filiformis as a model calcifying organism, that some organisms can increase the rates of many of their biological processes (in this case, metabolism and the ability to calcify to compensate for increased seawater acidity). However, this upregulation of metabolism and calcification, potentially ameliorating some of the effects of increased acidity comes at a substantial cost (muscle wastage) and is therefore unlikely to be sustainable in the long term.
Keywords: ocean acidification, echinoderm, regeneration, muscle, hypercapnia, Amphiura filiformis
1. Introduction
Since the start of the industrial revolution, atmospheric levels of carbon dioxide (CO2) have been rising at a far greater rate than previously experienced in the Earth's history. This is primarily a result of burning fossil fuels. The oceans are a natural carbon sink and have so far absorbed approximately half of all anthropogenically produced CO2 (Siegenthaler & Sarmiento 1993). When CO2 enters the ocean it reacts with seawater and alters the chemical properties of the sea itself (Zeebe & Wolf-Gladrow 2001). Among other things this process produces hydrogen ions thus increasing the acidity reflected in a lowering of the value of pH. Seawater pH currently ranges between 7.8 and 8.2 and is already on average 0.1 pH unit lower than it was prior to the industrial revolution (Caldeira & Wickett 2003). Predictions, based on realistic scenarios for future CO2 emissions, suggest that ocean pH will decrease by a further 0.3–0.4 by 2100 (Caldeira & Wickett 2003), a phenomenon termed ocean acidification. While the magnitude of impact will vary with depth (Caldeira & Wickett 2003), latitude (Orr et al. 2005) and habitat, the effects of ocean acidification on seawater chemistry will affect all marine organisms. An organism can be affected by ocean acidification in two ways; firstly through reduced pH and secondly through increased CO2 (hypercapnia). Here we use the term ocean acidification to include both.
Different species and groups of marine animals vary in their ability to cope with, and compensate for, hypercapnia and lowered pH (e.g. Pörtner et al. 2004, 2005) with implications for marine trophic interactions. Species with calcium carbonate skeletons, such as molluscs, crustaceans and echinoderms, are particularly susceptible to ocean acidification. As pH decreases, so too does carbonate availability that has led some authors to conclude that ocean acidification will result in reduced rates of calcification (e.g. Gattuso et al. 1999) and shell dissolution (e.g. Feely et al. 2004) for all calcified organisms, as well as metabolic depression resulting in reduced growth (e.g. Michaelidis et al. 2005). Echinoderm skeletons are composed of magnesium calcite that is particularly susceptible to dissolution as ocean pH decreases (Shirayama & Thornton 2005). Work on the effect of acidification on echinoderms is currently restricted to investigations of survival, growth and extracellular acid–base balance in a limited number of groups, mainly echinoids (e.g. Shirayama & Thornton 2005; Miles et al. 2007). One of the key characteristics of many echinoderm species is their ability to regenerate, which involves alterations in calcification rates (Bowmer & Keegan 1983; Bannister et al. 2005). However, we know nothing of the effect of CO2-induced acidification on such regeneration. Consequently, we have investigated the effect of CO2-induced acidification on the ability of a calcifying organism (the ophiuroid brittlestar Amphiura filiformis) to regenerate calcium carbonate structures (arms). In addition, we have examined the potential energetic costs associated with regeneration in terms of metabolism and reproduction. Amphiura filiformis is a key species in many seafloor communities. Using sediment-filled cores supplied with filtered seawater, brittlestars were exposed for 40 days to varying degrees of acidification; nominally a control of pH 8.0, the worst case scenario for the end of the century of pH 7.7, a 2300 scenario of pH 7.3 and finally pH 6.8.
2. Material and Methods
(a) Experimental set-up
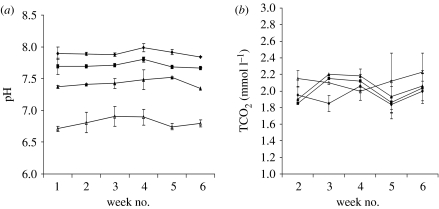
The experiment was carried out in a mesocosm facility (Widdicombe & Needham 2007). Amphiura filiformis collected from Plymouth Sound, UK, were maintained in sediment cores (five individuals per core) supplied with filtered seawater of the allocated pH (pH modified using CO2). Each pH treatment (8.0, 7.7, 7.3 and 6.8) had four cores (20 individuals per pH). Half the individuals in each treatment had one arm removed and the remaining had two arms removed. Total carbon dioxide content (TCO2), pHNIST, salinity and temperature of the seawater were measured for each treatment three times a week (figure 4).
Figure 4.
Water conditions during experiment on (a) pH(NIST) (diamonds, 8.0; squares, pH 7.7; filled triangles, pH 7.3; and open triangles, pH 6.8) and (b) TCO2 (mmol l−1). All values are means ±95% CI.
(b) Oxygen uptake
Closed-bottle respirometry technique adapted from Pomory & Lawrence (1999). Dissolved O2 measured using an automated titration system with photometric endpoint.
(c) Arm regeneration
Regenerated arm discernable from original arm by lighter colour. Measured to 0.05 mm with vernier calipers.
(d) Measurement of calcium content
Arms were digested in nitric acid and the total calcium content was determined using atomic absorption spectrophotometer (Varian SpectrAA 50) as in Spicer & Eriksson (2003).
(e) Arm structure and measurement of egg size
Central discs were embedded in methacrylate (Lewis & Bowen 1985), sectioned with a glass knife and stained (Lee's methylene blue/basic fuchin). Egg feret diameter was measured by image analysis software (Image-Pro Plus v. 4.5 Media Cybernetics) using a digital image (×40 mag. Reichert Polyvar microscope).
(f) Statistical analyses
All statistical analyses were run using Minitab v. 14. The two-way analysis of variance (ANOVA) was used to test for effects of pH treatment or number of arms regenerating on O2 uptake, calcium content, arm regeneration rate, egg size and arm structure. A Kolmogorov–Smirnov test was used to test for normality.
3. Effect of ocean acidification on calcification in A. filiformis
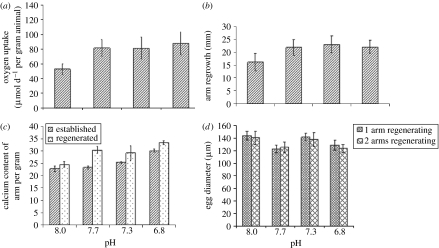
One of the most surprising results is that there was no decrease in the total amount of calcium carbonate in individuals exposed to acidified water. Indeed, individuals from lowered pH treatments had a greater percentage of calcium in their regenerated arms than individuals from control treatments, indicating a greater amount of calcium carbonate (two-way ANOVA using log transformed data, table 1c). Established arms had a significantly lower percentage of calcium carbonate content than regenerated arms (figure 1c); however, number of arms removed had no effect. The interaction between pH and arm type (established or regenerated) was significant due to the different amount of calcium found in each of the arm types; the regenerated arms having significantly greater calcium levels than established ones (figure 1c). This was due to the more developed skeletal structure seen in the established arms. Throughout the exposure, and therefore during the period of regeneration, the brittlestars were maintained in sediment cores (also collected from Plymouth Sound) in order to simulate natural conditions for the species. The inorganic carbon levels (%) for this sediment are 2.207 (±0.176; S. Widdicombe 2005, 2007, unpublished data). However, it is unlikely that the sediment is being used as a carbon source for the calcification process; a previous study found no change in the carbon (TIC) content of the same type of sediment (fine muddy) containing species including A. filiformis after a 20-week exposure to pHs of 7.3, 6.5 and 5.6 (Widdicombe et al. submitted).
Table 1.
Results from two-way ANOVA comparing the impact of different levels seawater pH (8.0, 7.7, 7.3 and 6.8) and number of arms regenerating (1 or 2) on (a) oxygen uptake (μmol d−1 g−1 animal); n=80, data were of normal distribution after square root transformation (n=80). (b) Length of arm regeneration (mm); n=40 and normally distributed. (c) Calcium content of arm (%). Comparing pH treatments (8.0, 7.7, 7.3 and 6.8) and number of arms regenerating (1 or 2) and established arms (denoted as arm regeneration); n=60 and data were normally distributed after log transformation. The significance level was 0.05. (d) Mean egg feret (mm); n=40 and normally distributed.
| source | d.f. | SS | MS | F | p |
|---|---|---|---|---|---|
| a | |||||
| pH | 3 | 54.299 | 18.1 | 7.03 | 0.00 |
| arms regenerating | 1 | 3.954 | 3.9545 | 1.54 | n.s. |
| interaction | 3 | 15.505 | 5.1683 | 2.01 | n.s. |
| error | 72 | 185.45 | 2.5757 | ||
| total | 73 | 259.21 | |||
| b | |||||
| pH | 3 | 200.9 | 67 | 2.99 | 0.05 |
| arms regenerating | 1 | 10 | 10 | 0.45 | n.s. |
| interaction | 3 | 139.4 | 46.5 | 2.07 | n.s. |
| error | 32 | 717.2 | 22.4 | ||
| total | 39 | 1067.5 | |||
| c | |||||
| pH | 3 | 0.7695 | 0.2565 | 16.58 | 0.00 |
| arms regenerating | 2 | 0.2719 | 0.1359 | 8.79 | 0.001 |
| interaction | 6 | 0.274 | 0.0457 | 2.95 | 0.016 |
| error | 48 | 0.7427 | 0.0155 | ||
| total | 59 | 2.0582 | |||
| d | |||||
| pH | 3 | 1489.8 | 496.593 | 1.40 | n.s. |
| arms regenerating | 1 | 23.0 | 23.014 | 0.06 | n.s. |
| interaction | 3 | 432.4 | 144.121 | 0.41 | n.s. |
| error | 32 | 11361.3 | 355.039 | ||
| total | 39 | 13306.4 | |||
Figure 1.
Impact of seawater pH on (a) oxygen uptake (μmol per day per gram animal), (b) length of arm regeneration (mm), (c) calcium content of established and regenerated arms (%; hatched bars, established; dotted bars, regenerated) and (d) egg feret diameter (μm) following a 40-day exposure. All values are means ±95% CI.
The sediment pH profiles from another acidification study using sediment cores (S. Widdicombe 2005, 2007, unpublished data) show that the pH of the sediment is lower than that of the overlying water even under normocapnic conditions; the pH is 7.64 at a depth of 5 cm, the depth at which A. filiformis is typically found. However, after a four-week exposure to mild hypercapnic conditions (overlying water pH 7.7) the sediment pH at 5 cm deep was still 7.64, while more severe hypercapnia (pH 7.3 and 6.5) only reduced sediment pH at 5 cm depth by 0.16 and 0.22 pH units, respectively. In a study by Widdicombe et al. (in preparation), cores of both muddy and sandy sediment were exposed to acidified seawater (pH 7.8, 7.4 and 6.8) for 60 days. After this time oxygen profiles were measured through the sediment. It was demonstrated that seawater acidification had no significant impact on the sediment oxygen profiles in either the sand or the mud, indicating no increase in sediment anoxia. pH imaging of Nereis succinea burrows showed that the porewater pH was dependent on the burrow profile, animal size and rate of irrigation, with high porewater pH associated with periods of irrigation (Zhu et al. 2006). Amphiura filiformis continually ventilate their burrows by arm undulation; therefore, the pH of their burrow porewater is expected to be related to surface water pH rather than the surrounding sediment. As such, the burrowing lifestyle of this study species is not counteracting or altering the experimental pH conditions created for the purposes of this study and the results shown are as a result of altering seawater pH.
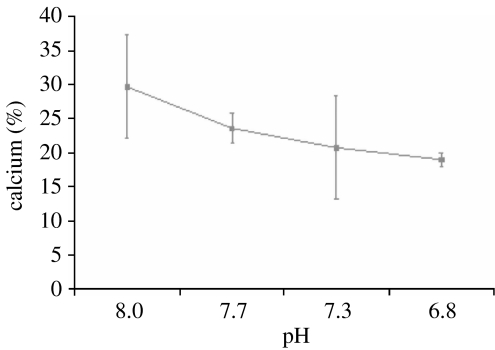
To disentangle the direct chemical effect of pH on the calcium carbonate within A. filiformis arms from the active biological processes used by the species to maintain calcium carbonate structures, a separate 7-day exposure at all four pH treatments was carried out on ‘dead’ arms. The arms were removed from the animal, frozen for a period in excess of 7 days to −80°C to kill, and then brought back to seawater temperature. In this experiment, the dead arms were placed in small pots with no sediment and supplied continuously with seawater of appropriate pH. Under these conditions calcium levels decreased with pH (figure 2). As these arms were detached from the individual and therefore could not replenish the calcium carbonate skeleton, the decrease in calcium indicates that this structure is susceptible to dissolution at lowered pH. Therefore, in live (attached) arms, an increased rate of calcification is required merely to maintain calcium carbonate structures in their original condition. In regenerated arms, calcium levels were greater in those organisms exposed to acidified seawater than in those held in untreated seawater (figure 1c). This was true for all three levels of acidified seawater. The data from the detached (dead) arms (figure 2) showed that lowered pH caused dissolution of arm calcium carbonate. Therefore, where these three lowered pH treatments appear to have had a similar response, there was actually an increasing rate of calcification with lowered pH. Calcium carbonate in established arms was also affected by lowered pH. At pH 6.8, calcium levels increased and at pH 7.7 and pH 7.3, calcium levels were equal to the control indicating that A. filiformis actively replaced calcium carbonate lost by dissolution.
Figure 2.
Calcium content (%) of arms which had been exposed to lowered pH after being removed from animal. All values are means ±95% CI.
4. Effect of ocean acidification on A. filiformis metabolism
Rates of oxygen (O2) uptake (as a measure of metabolic rate), or MO2, were significantly greater at reduced pHs (7.7, 7.3 and 6.8) than in controls (pH 8; figure 1a). However, MO2 was not significantly different between the three lowered pH treatments (figure 1a). Increased rates of physiological processes that require energy are paralleled by an increase in metabolism; this relationship is seen with growth and metabolism here in our results.
5. Effect of ocean acidification on A. filiformis growth and regrowth
Seawater acidification stimulated arm regeneration. After the 40-day exposure, the length of the regenerated arm was greater in acidified treatments than in the controls (two-way ANOVA, table 1b; figure 1b). This increased rate of growth coincided with increased metabolism. Regeneration was not affected by the number of arms removed, nor was there a significant difference in any of the physiological parameters measured as a result of having two arms regenerating instead of one. The ability to regenerate lost arms faster meant a reduction in the length of time animal function (e.g. burrow ventilation and feeding) was compromised by reduced arm length.
6. The biological cost of ocean acidification
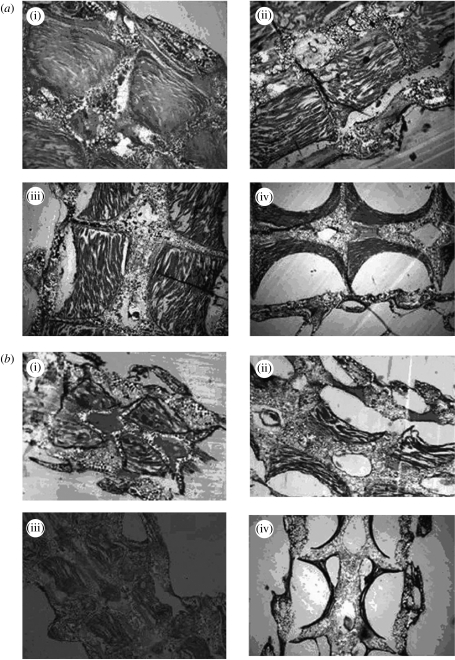
The internal structure of A. filiformis arms was affected by pH (figure 3); muscle wastage occurred at lowered pH. Longitudinal sections of the arm showed distinct loss of muscle mass as pH decreased. In each arm segment there are four sections separated by the calcium carbonate skeleton. In the control individuals, these sections were filled with muscle. As pH decreased, large empty spaces were clearly visible (figure 3). Candia Carnevali et al. (2001) found that muscle de-differentiation occurred in regenerating arms as a result of PCBs, which appeared visually similar to muscle loss. However, the de-differentiated muscle showed a change in structure not seen in our study; the arm muscle from lowered pH samples has the same visual structure as the controls, with just less present. In addition, our results showed muscle loss in established arms as well as regenerating, whereas Candia Carnevali et al. (2001) found de-differentiation in the regeneration process. The absence of muscle as a result of lowered pH is not de-differentiation or an inability to synthesize muscle tissue under hypercapnia, but rather muscle loss. In conclusion, arms can be regenerated under hypercapnic conditions but they are unlikely to function as well as arms regenerated under normal conditions. Amphiura filiformis uses its arms to collect food particles and irrigate its burrow. Therefore, muscle loss can be expected to result in a loss of arm movement which in turn will affect both feeding and respiration and ultimately survival. This species is also predated on by the commercial flatfish dab, Limanda limanda (Bowmer & Keegan 1983), which crop the arms extended into the water column. If the muscle mass in these arms is significantly reduced, so too is the nutritional value, indicating the effects of ocean acidification could be transferred between trophic levels.
Figure 3.
Longitudinal cross sections of (a) established and (b) regenerated arms (×10 mag) mounted in methacrylate resin and stained with Lee's basic blue fuchin. ((i) pH 8, (ii) pH 7.7, (iii) pH 7.3 and (iv) pH 6.8).
7. Effect of ocean acidification on A. filiformis reproduction and mortality
Egg size (feret diameter) and structure were not affected by seawater acidification (two-way ANOVA, table 1d; figure 1d). However, the timing of this study (December–January) falls in a latent period of egg growth; development of eggs laid down the previous autumn typically begins in March (Bowmer 1982). Therefore, while no degeneration of eggs was found in this study, egg development may still be affected by hypercapnia. A further experiment encompassing the egg growth phase is required to assess the impact of ocean acidification on egg development. A study by Lowe et al. (in preparation) has found that the process of vitellogenesis in the surface dwelling ophiuroid Ophiura ophiura was disrupted by lowered pH, highlighting the potential for disruption in the growth phase. Spermatogonia were not investigated in the current study as all individuals sampled were female. While the sex ratio of A. filiformis is thought to be 1 : 1, patchiness in the distribution of sexes has been documented (Bowmer 1982), which may explain the reason for the absence of males from the samples fixed for gonadal assessment.
While some ophiuroids reallocate energy from gonadal to somatic growth, and a decrease of egg size is seen when arm regeneration is undertaken, this was not seen in A. filiformis (figure 1d). Ocean acidification has the potential to also affect reproductive success indirectly; as a broadcast spawner, A. filiformis must come to the sediment surface to spawn. This behaviour requires the arms to move the individual through the sediment and should arm muscle wastage reduce motility then individuals may release gametes within their burrows and far fewer gametes would enter the water column; significantly reducing reproductive success.
The duration of this experiment (40 days) was chosen to investigate long-term physiological responses to hypercapnia. Shirayama & Thornton (2005) have elegantly demonstrated with echinoids that mortality as a result of a 0.05 pH decrease (560 ppm) may only occur after several months. Interestingly, even at high levels of hypercapnia (the 6.8 pH treatment crosses the threshold into acidic water, i.e. pH<7.0) investigated here, no mortality was observed. In light of the results regarding the trade-off between calcification and muscle mass it is probable that mortality at low pH will occur as an indirect result of lowered pH, and this may take longer than the experimental duration. Any loss, or impairment, of an important ecosystem engineer (Jones et al. 1994) will profoundly affect the biotic and abiotic environments where they occur; therefore, the potential for loss of this species would alter ecosystems on a large geographical scale.
8. Discussion
All previous ocean acidification studies on benthic marine invertebrates have reported reduced calcification rates (Gazeau et al. 2007) and hypometabolism (Michaelidis et al. 2007) as common outcomes. Here we have shown the opposite; that in some species at least, ocean acidification can increase both the rate of calcification and metabolism. These results change the face of predictions for future marine assemblages with respect to ocean acidification. Whereas it was previously assumed that all calcifiers would be unable to construct shells or skeletons, and inevitably succumb to dissolution as carbonate became undersaturated, we now know that this is not the case for every species. However, by investigating the functional consequences of hypercapnia and lowered pH at an organism level rather than focusing on a single process, we have also detected a cost to these increased activities. Arm muscle mass decreased with pH, i.e. as calcification and metabolism increase. There was a trade-off between maintaining skeletal integrity and arm function. pH decreased the arm muscle mass by causing the brittlestar to use the muscle as an energy source. As muscle loss was seen in established as well as regenerated arms, it is clearly not just a failure to synthesize muscle tissue under hypercapnic conditions. For this particular ophiuroid species, the loss of muscle mass experienced at low pH has implications for survival and ecosystem function; arm movement is necessary for feeding (Loo et al. 1996), burrow aeration (Woodley 1975) and predator avoidance (O'Reilly et al. 2006). In areas where this animal is present, burrow creation and irrigation by A. filiformis is responsible for up to 80% of all bioturbation (Vopel et al. 2003); therefore, the effects of ocean acidification will also alter the surrounding environment. Results of a previous study indicate that this trade-off of increased calcification against reduced muscle mass is occurring in other species; Shirayama & Thornton (2005) found that the decrease in test thickness did not account for total mass loss of the echinoderms Hemicentrotus pulcherrimus and Echinometra mathaei exposed to hypercapnic conditions. These species may also be decreasing muscle mass as a cost of increasing calcification and metabolism. Here we show that A. filiformis, and almost certainly other species, will attempt to cope with changes in seawater acid–base balance. Unfortunately, it appears that the physiological responses to combat the effects of ocean acidification may themselves reduce survival and fitness as much as acidification itself.
The Intergovernmental Panel on Climate Change predicts that under their worst case scenario of carbon dioxide emissions, seawater pH will reach our experimental level of 7.7 by 2100. Here we show that some species at least can modulate their biological processes in response to ocean acidification and while calcified structures are affected by ocean acidification, so too is the rest of the animal. Such trade-offs are likely to be present in other species but to identify these, future studies need to work at the organism rather than the process level. To place the importance of calcification above other factors without empirical evidence leads to false assumptions and therein the capacity of some species to respond effectively may be overlooked.
Acknowledgments
This study was funded by a NERC Blue Skies PhD studentship awarded to H.L.W. The study used the minimum number of animals necessary to ensure scientific robustness. Under the Animals (Scientific Procedures) Act 1986, work with echinoderms is not a licensable activity.
We thank H. Findlay for critical discussions and assistance in the laboratory, D Lowe & C. Pascoe for advice on using methacrylate and sectioning technique and A. Beesley for assistance with collecting water data.
Footnotes
Research was conceived by H.L.W., S.W. and J.I.S., performed and analysed by H.L.W. The paper was written by H.L.W. with contributions and advice from J.I.S. and S.W.
Supplementary Material
Full methodology of experiments carried out in ‘Ocean acidification may increase calcification-but at a cost’
References
- Bannister R, McGonnell I.M, Graham A, Thorndyke M.C, Beesley P.W. Afuni, a novel transforming growth factor-β gene is involved in arm regeneration by the brittle star Amphiura filiformis. Dev. Genes Evol. 2005;215:393–401. doi: 10.1007/s00427-005-0487-8. doi:10.1007/s00427-005-0487-8 [DOI] [PubMed] [Google Scholar]
- Bowmer T. Reproduction in Amphiura filiformis (Echinodermata, Ophiuroidea)—seasonality in gonad development. Mar. Biol. (Berl.) 1982;69:281–290. doi:10.1007/BF00397493 [Google Scholar]
- Bowmer T, Keegan B.F. Field survey of the occurrence and significance of regeneration in Amphiura filiformis (Echinodermata, Ophiuroidea) from Galway Bay, West-Coast of Ireland. Mar. Biol. (Berl.) 1983;74:65–71. doi:10.1007/BF00394276 [Google Scholar]
- Caldeira K, Wickett M.E. Anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. doi:10.1038/425365a [DOI] [PubMed] [Google Scholar]
- Candia Carnevali M.D, Galassi S, Bonasoro F, Patruno M, Thorndyke M.C. Regenerative response and endocrine disrupters in crinoid echinoderms: arm regeneration in Antedon mediterranea after experimental exposure to polychlorinated biphenyls. J. Exp. Biol. 2001;204:835–842. doi: 10.1242/jeb.204.5.835. [DOI] [PubMed] [Google Scholar]
- Feely R.A, Sabine C.L, Lee K, Berelson W, Kleypas J, Fabry V.J, Millero F.J. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science. 2004;305:362–366. doi: 10.1126/science.1097329. doi:10.1126/science.1097329 [DOI] [PubMed] [Google Scholar]
- Gattuso J.P, Frankignoulle M, Smith S.V. Measurement of community metabolism and significance in the coral reef CO2 source–sink debate. Proc. Natl Acad. Sci. USA. 1999;96:13 017–13 022. doi: 10.1073/pnas.96.23.13017. doi:10.1073/pnas.96.23.13017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazeau F, Quiblier C, Jansen J.M, Gattuso J.-P, Middelburg J.J, Heip C.H.R. Impact of elevated CO2 on shellfish calcification. Geophys. Res. Lett. 2007;34:L07603. doi:10.1029/2006GL028554 [Google Scholar]
- Jones C.G, Lawton J.H, Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69:373–386. doi:10.2307/3545850 [Google Scholar]
- Lewis G.H.J, Bowen I.D. A methacrylate embedding technique for combined autoradiography and acid-phosphatase histochemistry. Histochem. J. 1985;17:467–475. doi: 10.1007/BF01003206. doi:10.1007/BF01003206 [DOI] [PubMed] [Google Scholar]
- Loo L.O, Jonsson P.R, Skold M, Karlsson I. Passive suspension feeding in Amphiura filiformis (Echinodermata: Ophiuroidea): feeding behaviour in flume flow and potential feeding rate of field populations. Mar. Ecol. Prog. Ser. 1996;139:143–155. doi:10.3354/meps139143 [Google Scholar]
- Lowe, D. M., Widdicombe, S., Beesley, A., Pascoe, C., McNeill, C. L., Oexnevad, S. & Berge, J. A. In preparation. Impact of CO2 induced seawater acidification on the reproductive and digestive tissues of Ophiura ophiura and Amphiura filiformis
- Michaelidis B, Ouzounis C, Paleras A, Pörtner H.O. Effects of long-term moderate hypercapnia on acid–base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 2005;293:109–118. doi:10.3354/meps293109 [Google Scholar]
- Michaelidis B, Spring A, Pörtner H.O. Effects of long-term acclimation to environmental hypercapnia on extracellular acid–base status and metabolic capacity in Mediterranean fish Sparus aurata. Mar. Biol. (Berl.) 2007;150:1417–1429. doi:10.1007/s00227-006-0436-8 [Google Scholar]
- Miles H, Widdicombe S, Spicer J.I, Hall-Spencer J. Effects of anthropogenic seawater acidification on acid–base balance in the sea urchin Psammechinus miliaris. Mar. Poll. Bull. 2007;54:89–96. doi: 10.1016/j.marpolbul.2006.09.021. doi:10.1016/j.marpolbul.2006.09.021 [DOI] [PubMed] [Google Scholar]
- O'Reilly R, Kennedy R, Patterson A. Destruction of conspecific bioturbation structures by Amphiura filiformis (Ophiuroida): evidence from luminophore tracers and in situ time-lapse sediment-profile imagery. Mar. Ecol. Prog. Ser. 2006;315:99–111. doi:10.3354/meps315099 [Google Scholar]
- Orr J.C, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–686. doi: 10.1038/nature04095. doi:10.1038/nature04095 [DOI] [PubMed] [Google Scholar]
- Pomory C.M, Lawrence J.M. Effect of arm regeneration on energy storage and gonad production in Ophiocoma echinata (Echinodermata: Ophiuroidea) Mar. Biol. (Berl.) 1999;135:57–63. doi:10.1007/s002270050601 [Google Scholar]
- Pörtner H.O, Langenbuch M, Reipschläger A. Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J. Oceanogr. 2004;60:705–718. doi:10.1007/s10872-004-5763-0 [Google Scholar]
- Pörtner H.O, Langenbuch M, Michaelidis B. Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: from Earth history to global change. J. Geophys. Res. Oceans. 2005;110:C09S10. doi:10.1029/2004JC002561 [Google Scholar]
- Shirayama Y, Thornton H. Effect of increased atmospheric CO2 on shallow water marine benthos. J. Geophys. Res. Oceans. 2005;110:C09S08. doi:10.1029/2004JC002618 [Google Scholar]
- Siegenthaler U, Sarmiento J.L. Atmospheric carbon dioxide and the ocean. Nature. 1993;365:119–125. doi:10.1038/365119a0 [Google Scholar]
- Spicer J.I, Eriksson S.P. Does the development of respiratory regulation always accompany the transition from pelagic larvae to benthic fossorial postlarvae in the Norway lobster Nephrops norvegicus (L.)? J. Exp. Mar. Biol. Ecol. 2003;295:219–243. doi:10.1016/S0022-0981(03)00296-X [Google Scholar]
- Vopel K, Thistle D, Rosenberg R. Effect of the brittle star Amphiura filiformis (Amphiuridae, Echinodermata) on oxygen flux into the sediment. Limnol. Oceanogr. 2003;48:2034–2045. [Google Scholar]
- Widdicombe S, Needham H.R. Impact of CO2-induced seawater acidification on the burrowing activity of Nereis virens and sediment nutrient flux. Mar. Ecol. Prog. Ser. 2007;341:111–122. doi:10.3354/meps341111 [Google Scholar]
- Widdicombe, S., Dashfield, S. L., McNeill, C. L., Needham, H. R., Beesley, A., McEvoy, A., Oexnevad, S., Clarke, K. R. & Berge, J. A. Submitted. Impact of CO2 induced seawater acidification on sediment diversity and nutrient flux.
- Widdicombe, S., Beesley, A., Berge, J. A., Dashfield, S. L., Lowe, D. M., Needham, H. R., McNeill, C. L. & Oexnevad, S. In preparation. Impact of burrowing heart urchins on sediment nutrient fluxes under elevated levels of CO2 [DOI] [PubMed]
- Woodley J.D. Behaviour of some amphiurid brittle-stars. J. Exp. Mar. Biol. Ecol. 1975;18:29–46. doi:10.1016/0022-0981(75)90014-3 [Google Scholar]
- Zeebe, R. E. & Wolf-Gladrow, D. 2001 CO2 in seawater: equilibrium, kinetics, isotopes Oceanography series, vol. 65, pp. 1–346. Amsterdam, The Netherlands: Elsevier.
- Zhu Q.Z, Aller R.C, Fan Y.Z. A new ratiometric, planar fluorosensor for measuring high resolution, two-dimensional pCO2 distributions in marine sediments. Mar. Chem. 2006;101:40–53. doi:10.1016/j.marchem.2006.01.002 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full methodology of experiments carried out in ‘Ocean acidification may increase calcification-but at a cost’