Abstract
Background
Many traditional risk factors for coronary artery disease (CAD) are associated with altered autonomic function. Inflammation may provide a link between risk factors, autonomic dysfunction, and CAD. We examined the association between heart rate variability (HRV), a measure of autonomic function, and inflammation, measured by C-reactive protein (CRP) and interleukin-6 (IL-6).
Methods
We examined 264 middle-aged male twins free of symptomatic CAD. All underwent ambulatory ECG monitoring, and 24-hour ultra-low, very-low, low, and high frequency power (ULF, VLF, LF, and HF) were calculated using power spectral analysis. CRP and IL-6 were measured and risk factors including age, smoking, hypertension, lipids, diabetes, body mass index (BMI), depression, and physical activity assessed.
Results
Physical activity, BMI, HDL cholesterol, smoking, depression, and hypertension were directly associated with CRP and IL-6 and inversely associated with one or more HRV variables. There was a graded inverse relationship between all HRV parameters (except HF) and CRP and IL-6. After adjustment for age, BMI, activity, HDL, smoking, hypertension, depression, and diabetes, ULF and VLF remained significant predictors of CRP (p<0.01.)
Conclusions
CRP is associated with decreased HRV, even after controlling for traditional CAD risk factors. Autonomic dysregulation leading to inflammation may represent one pathway through which traditional risk factors promote development of CAD.
Introduction
Physiological and behavioral risk factors for the development of coronary artery disease, (CAD) such as smoking, obesity, inactivity, hypertension, diabetes, hypercholesterolemia, and depression, are well known. The physiological pathways linking these factors to development of CAD, however, are incompletely understood. These risk factors alter autonomic function,1–3 4 which in turn is associated with both the progression of CAD5 and increased mortality in the general population.6, 7 Further, each of these well-known risk factors is associated with increased levels of inflammatory mediators,8 associated with atherogenesis.9–11 In laboratory experiments, alterations in both sympathetic and parasympathetic function activate pro-inflammatory cytokines.12–16 Thus, autonomic dysregulation resulting in inflammation could represent a pathway through which traditional risk factors lead to progression of CAD. Heart rate variability (HRV), a measure of beat-to-beat heart rate fluctuations over time, is an established measure of autonomic function.17 A relationship between HRV and inflammation, as measured by serum markers such as interleukin 6 (IL-6) and C reactive protein (CRP), has been demonstrated in patients with congestive heart failure and acute coronary syndromes.18–20 Studies of populations free of overt cardiac disease have suggested similar relationships.21–23
The Twins Heart Study (THS) is an investigation of psychological, behavioral and biological risk factors for subclinical cardiovascular disease using a twins design which includes comprehensive autonomic evaluation. In this population, we investigated the inter-relationships between 24-hour heart rate variability, sympathetic activity, (norepinephrine), inflammatory markers (CRP and IL-6), and traditional risk factors.
Methods
Subjects
Twins included in THS were selected from the Vietnam Era Twin Registry, which includes 7,369 middle-aged male-male twin pairs both of whom served in the United States military during the Vietnam War.24 THS included 180 monozygotic (MZ) and dizygotic (DZ) twin pairs (360 twins), all born between 1946 and 1956. The methods of construction of this sample have been described previously4, 25. Briefly, the twins were free of a self-reported previous diagnosis of cardiovascular disease based on survey data collected in 1990, including a previous diagnosis of myocardial infarction, coronary heart disease, angina, congestive heart failure or stroke, or previous coronary angioplasty or coronary bypass surgery. From this group, random samples of twins in two strata were selected: one stratum included twins discordant for a lifetime history of major depression and in a second stratum neither twin had a history of depression. Subjects who had since developed cardiovascular disease based on current medical history (N= 35) were excluded. All twins were examined in pairs at the Emory University General Clinical Research Center between March 2002 and March 2006. All data collection occurred during the 27 hour GCRC admission under controlled conditions. Activity was limited to the environs of the GCRC and kept similar for each pair. This protocol was approved by the Institutional Review Board at Emory University. Informed consent was obtained from all subjects.
Measurement of Heart Rate Variability
Twins wore a Holter monitor (GE Medical SEER digital system) for 24 hours. HRV data were analyzed in the frequency domain following published methodology.26, 27 Holter recordings were digitally sampled and analyzed. Each tape was manually processed and edited for accurate identification of QRS complexes. A list of R-R intervals with annotations denoting normal beats, ectopics, and artifact was saved and later transferred to a computer workstation for further processing and analysis with customized software. The file was first edited to remove ectopics and artifact. Gaps in the time series were filled by interpolation with linear splines. The RR interval file was then resampled at 3.41 Hz (1024 samples per 5 minutes) to create a uniformly spaced time series. The power spectrum was computed from the fast Fourier transform (FFT) of the time series modified by a Parzen window to reduce spectral leakage and corrected for window attenuation and boxcar sampling. Because long-term autonomic function was the goal of this study, the FFT was performed on the 24-hour R-R interval file. The power spectrum was integrated over four discrete frequency bands:27 ultra low frequency (ULF) <0.0033 Hz; very low frequency (VLF) 0.0033 to <0.04 Hz; low frequency (LF) 0.04 to <0.15 Hz; high frequency (HF) 0.15 to <0.40 Hz. Subjects with >20% interpolated RR intervals or <18 recorded hours were excluded. Our main measures of interest were ULF and VLF, which reflect overall autonomic balance,28 and were the measures previously most closely correlated with cardiovascular outcomes.27
Markers of Inflammation
Plasma IL-6 was assessed using commercially available ELISA kits (R and D Systems) and all samples run in duplicate. Inter- and intra-assay variability of this assay is reliably <10%. Plasma CRP was measured with the Beckman Coulter High Sensitivity C-Reactive Protein assay on the Synchron LX-20 analyzer. Subjects with values of IL-6 above mean plus 3 times the SD were excluded, as correction for outliers.
Other Measurements
A medical history and a physical exam were obtained from all twins. Blood pressure was measured in sitting position after 10 minutes of rest, using the average of two measurements 5-minute apart. Venous blood samples were drawn after an overnight fast. Total triglycerides were determined by enzymatic methods (Beckman Coulter Diagnostics, Fullerton, CA). Direct high-density lipoprotein (HDL) and low density lipoprotein cholesterol were measured with homogeneous assays (Equal Diagnostics, Exton, PA). Glucose levels were measured on the Beckman CX7 chemistry autoanalyzer. Norepinephrine concentration was measured in 24-hour urine collections, reflecting long-term sympathetic responses,29 during the period of holter monitoring, using high-performance liquid chromatography coupled with dual-electrode Coulometric electrochemical detection (reductive mode.) Physical activity was assessed by the Baecke Questionnaire of Habitual Physical Activity (global physical activity score.) Cigarette smoking was classified into current versus never or past smoker. Diabetes mellitus was defined as fasting glucose level > 126 mg/dl or taking anti-diabetic medications. Depression was measured by the Beck Depression Inventory.
Statistical Analyses
Correlations between HRV parameters, inflammatory markers and CAD risk factors as listed in Table 1 and Table 2 were assessed using Pearson correlations for continuous variables and Spearman correlations for categorical variables. The association of inflammation with HRV, adjusting for age, BMI, physical activity, HDL-C, smoking, hypertension, diabetes, and depression, was tested using generalized estimating equations (GEE) to account for the dependency between siblings (SAS PROC GENMOD). To improve the distributional properties of the HRV parameters and inflammatory markers, data were transformed to the logarithmic scale. Analyses were repeated controlling for use of anti-hypertensive, cholesterol-lowering, and diabetic medications.
Table 1.
Subject Characteristics (n = 260)
| Variables | Values |
|---|---|
| Age, years | 54.4 ± 2.85 |
| Smoker, current/former/never | 43 / 111 / 106 |
| Hypertension, yes/no | 114 / 146 |
| Diabetes, yes/no | 21 / 239 |
| Physical Activity | 7.51 ± 1.58 |
| BMI, kg/m2 | 29.1 ± 4.61 |
| SBP, mmHg | 128.4 ± 16.03 |
| DBP, mmHg | 80.6 ± 10.64 |
| TC, mg/dl | 189.1 ± 37.96 |
| HDL-C, mg/dl | 38.6 ± 9.49 |
| LDL-C, mg/dl | 125.2 ± 33.72 |
| Glucose, mg/dl | 100.0 ± 16.26 |
| Norepinephrine, ug/24H | 23.6 ± 16.9 |
| BDI scores | 4.88 ± 6.72 |
| IL-6, pg/ml | 2.29 ± 2.04 |
| CRP, mg/l | 2.32 ± 3.12 |
| HR, beat/min | 66.4 ± 8.75 |
| Ln ULF | 9.14 ± 0.56 |
| Ln VLF | 7.63 ± 0.57 |
| Ln LF | 6.69 ± 0.72 |
| Ln HF | 5.41 ± 0.88 |
Mean ± SD for continuous variables and count number for categorical variables. SD, standard deviation; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; GLU, glucose; BDI, Beck depression inventory; IL-6, interleukin-6; CRP, C-reactive protein; HR, heart rate; ULF, ultra low frequency; VLF, very low frequency; LF, low frequency; HF, high frequency.
Table 2.
Correlation Coefficients between Heart Rate Variability, Inflammatory Markers and Covariates
| Variables | Ln ULF | Ln VLF | Ln LF | Ln HF | Ln IL-6 | Ln CRP |
|---|---|---|---|---|---|---|
| Age | 0.02 | 0.09 | −0.05 | −0.10 | 0.07 | 0.09 |
| Smoking | −0.23† | −0.24‡ | −0.20† | −0.09 | 0.18† | 0.21† |
| Hypertension | −0.14 | −0.15 | −0.18† | 0.19† | 0.03 | 0.12* |
| Diabetes | −0.12 | −0.12 | −0.13 | −0.04 | 0.12 | 0.12 |
| Physical activity | 0.12 | 0.23† | 0.18† | 0.06 | −0.25† | −0.28† |
| BMI | −0.05 | −0.06 | −0.14* | −0.05 | 0.10 | 0.29† |
| HR | −0.59† | −0.66† | −0.41* | 0.35† | 0.17† | 0.29† |
| SBP | −0.03 | 0.04 | 0.01 | −0.04 | −0.01 | 0.07 |
| DBP | −0.09† | 0.02 | 0.06 | −0.03 | −0.14‡ | 0.02 |
| TC | 0.04 | 0.08 | 0.06 | 0.08 | −0.06 | 0.002 |
| HDL-C | 0.10 | 0.19† | 0.11 | 0.09* | −0.23† | −0.24† |
| LDL-C | 0.11 | 0.15 | 0.16 | 0.13 | −0.01 | 0.03 |
| Glucose | −0.01 | −0.08 | −0.13 | −0.07 | 0.04 | 0.07 |
| Norepinephrine | −0.16* | −0.23† | −0.16* | −0.02 | 0.20† | 0.21† |
| BDI scores | −0.22† | −0.24† | −0.15* | −0.08 | 0.21* | 0.14 |
| Ln IL-6 | −0.21* | −0.23† | −0.19 | −0.02 | - | |
| Ln CRP | −0.29† | −0.26† | −0.22† | −0.04 | 0.55† | - |
P < 0.05
P < 0.01
P value is adjusted for correlations within twin pairs. Abbreviations as defined in Table 1
Twins share maternal factors, familial and childhood/adolescent environment such as socioeconomic, lifestyle and other factors shared by individuals of similar family background. The matched nature of the co-twin control design minimizes confounding by these factors. If the paired effects are smaller than the effects seen when twins are analyzed as separate individuals, this is evidence that there is confounding by factors shared by co-twins. In addition, daily activities and other environmental factors during the ambulatory ECG recording are controlled in paired analyses since co-twins were examined at the same time and under identical conditions. Therefore, we also conducted a matched-pair analysis in which CRP levels were compared within twin-pairs discordant for HRV-tertile for ULF (tertile cutoffs 7.49–8.90/8.90–9.41/9.42–10.56) and VLF (tertile cutoffs 5.17–7.43/7.44–7.87/7.89–9.19) i.e., pairs whose members had HRV values which fell into different tertiles of the HRV sample distribution, for example, one twin had ULF in the highest tertile, and his brother in a lower (N=49 twin-pairs). Analyses were performed using the statistical software SAS, version 9.0 (SAS, Inc., Cary, NC).
Results
Study Population
Of the 325 twins free of cardiovascular disease, 264 had ambulatory ECG data adequate for analysis. Two subjects and their co-twins were excluded due to IL-6 levels above the outlier threshold. The remaining 260 subjects comprise the study population. The mean age was 54 years (range 47–60). Eight percent had diabetes, 44% hypertension, and 17% were current smokers. Subject characteristics are listed in Table 1.
Associations between HRV, inflammatory markers, and clinical factors
Bivariate correlations between HRV, inflammatory markers, and clinical factors are shown in Table 2. Physical activity, BMI, HDL cholesterol, smoking, hypertension and depression were directly associated with CRP and/or IL-6 and inversely associated with one or more HRV variables.
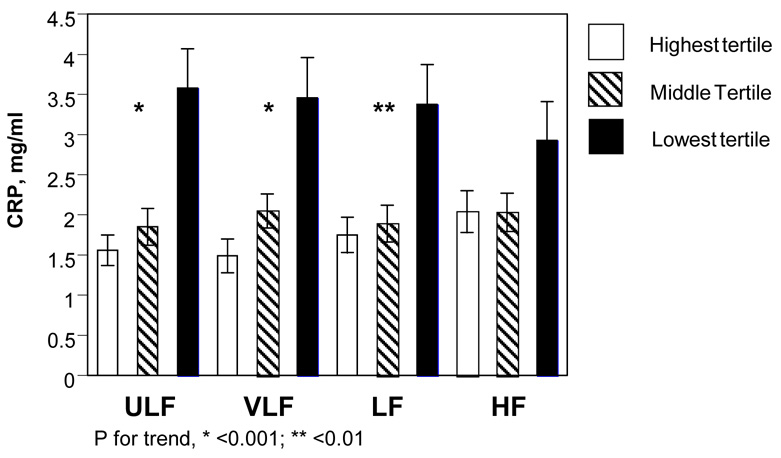
Both CRP and IL-6 were correlated with all HRV variables except HF, most strongly with ULF and VLF. When the group was categorized into tertiles based on HRV variables (Figure 1), CRP increased as HRV decreased. Plasma concentrations of CRP of those in the lowest tertile of ULF and VLF were more than twice that of those in the highest tertile. A similar pattern was seen for IL-6.
Figure.
Levels of CRP for each HRV variable, with grouping by tertile. CRP, C-reactive protein; HRV, heart rate variability; ULF, VLF, LF, and HF, ultra-low, very-low, low, and high frequency power.
Inter-relationships among HRV, inflammatory markers, and clinical factors
In GEE regression models, after adjustment for age, BMI, physical activity, HDL-C, smoking, hypertension, diabetes, and depression, which were the CAD risk factors found significantly associated with HRV and/or inflammation in bivariate analyses, ULF and VLF remained significant predictors of CRP, as was HR (all p<0.01, Table 3). In a subsequent model, inclusion of use of anti-hypertensive, cholesterol-lowering, and diabetic medications did not alter any associations, nor did inclusion of beta-blocker use (data not shown). In a model adjusting for HR, ULF remained an independent predictor of CRP.
Table 3.
Multivariate-adjusted Generalized Estimating Equation Regression Models for the Association of HRV and Heart Rate with CRP and IL-6
| HRV Index* | Dependent Variable: Ln CRP | Dependent Variable: Ln IL-6 | ||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| β | Lower | Upper | P Values | β | Lower | Upper | P Values | |
| Ln ULF | −0.43 | −0.69 | −0.17 | 0.001 | −0.12 | −0.30 | 0.05 | 0.16 |
| Ln VLF | −0.38 | −0.63 | −0.13 | 0.003 | −0.15 | −0.34 | 0.04 | 0.12 |
| Ln LF | −0.17 | −0.37 | 0.03 | 0.10 | −0.08 | −0.24 | 0.07 | 0.30 |
| Ln HF | 0.03 | −0.13 | 0.19 | 0.72 | 0.03 | −0.08 | 0.15 | 0.54 |
| HR | 0.02 | 0.01 | 0.04 | 0.003 | 0.01 | −0.01 | 0.02 | 0.34 |
Each HRV parameter and HR was included in a separate model. All models were adjusted for age, BMI, physical activity, HDL-C, depressive symptoms, smoking, hypertension, and diabetes. Abbreviations as defined in Table 1.
The co-twin matched pair analysis confirmed the above results. As shown in Table 4, the twin with VLF in a higher tertile of the sample distribution had significantly lower CRP than his brother whose VLF fell in a lower tertile, with a similar trend seen for ULF. These associations were similar in monozygotic and dizygotic twin pairs, with no significant interaction between zygosity and HRV tertile.
Table 4.
Within-pair comparison of CRP plasma levels in twin pairs discordant for ULF or VLF tertile.
| CRP, mg/L | ||
|---|---|---|
| ln ULF | ln VLF | |
| Twin with lower HRV tertile | 2.66 ± 4.69 | 2.51 ± 2.41 |
| Twin with higher HRV tertile | 1.63 ± 1.41 | 1.61 ± 1.65 |
| Within-pair difference | 1.03 ± 4.35 | 0.89 ± 2.15 |
| Wilcoxon Test | p = 0.15 | p = 0.003 |
Means ± standard deviation
Sympathetic activity, HRV, and inflammation
Twenty-four hour urine norepinephrine concentration was correlated in bivariate analysis with both HRV variables and inflammatory markers. (Table 2) In multivariable analysis, however, HRV variables remained independently associated with CRP, while norepinephrine did not (β=0.007, p=0.15).
Discussion
In middle-aged men free of cardiovascular disease, autonomic dysfunction, as demonstrated by decreased HRV, was associated with higher levels of the inflammatory biomarkers CRP and IL-6. Decreased long-term HRV (ULF and VLF) remained an independent predictor of plasma concentration of CRP after adjustment for CAD risk factors associated with both autonomic dysfunction and inflammation.
Clinical Implications
While the role of physiological and behavioral risk factors in the pathogenesis of CAD is well established, the pathways through which these factors exert their atherogenic effect remain incompletely understood. These risk factors all alter autonomic regulation,1–3 which in turn is linked to progression of atherosclerosis5 as well as mortality6, 7. Further, these factors all increase inflammatory processes,8 which promote many phases of atherogenesis, from T-cells entering the intima, to smooth muscle proliferation, to growth and finally rupture of the atherosclerotic plaque.9 CRP, an acute phase reactant produced in the liver in response to IL-6 and other inflammatory cytokines,9 was strongly associated with decreased HRV in this study. Although the exact role of CRP in the atherosclerotic process is debated, CRP may actively promote atherogenesis.30 Our findings suggest that inflammatory and autonomic processes may be linked, and that many CAD-risk factors may be pro-inflammatory in part due to autonomic dysregulation. Further investigation is needed to evaluate whether therapy which improves HRV, such beta-blockers,26 may be beneficial in decreasing inflammatory processes, as suggested by basic studies.16 Whether autonomic dysfunction, as measured by HRV, is a mediator of the inflammatory effects of traditional risk factors, or rather, is a marker for the overall physiologic impact of risk factors on the individual including their inflammatory effects, requires further study.
The inflammatory process is complex, and only two markers were examined in this study. While the association between HRV and CRP remained significant after controlling for other factors, that between HRV and IL-6 did not. IL-6 has a short half-life,31 and varies throughout the day, showing circadian variation,31 whereas CRP levels remain stable over 24 hours.32 This may explain why HRV, measured over 24-hours, showed a stronger association with CRP than IL-6.
Previous studies
Group means for HRV variables here are similar to those previously reported for middle-aged individuals free of cardiovascular disease, although those in the lowest tertiles for HRV, who also displayed the highest levels of inflammatory markers, had HRV in the ranges reported for individuals with CAD.33 Measured IL-6 was somewhat higher than that reported for healthy individuals,11 as was CRP.10 BMI, cholesterol, and blood pressure were similar to those in the ARIC study in subjects free of cardiovascular disease.6 Mean BDI score for the group was within the normal range.4
Previous studies have demonstrated associations between inflammatory markers and HRV in patients with CHF,18 and in stable,19 and unstable CAD.20 In studies of individuals without cardiovascular disease,21–23 HRV, assessed by varying methods, is inversely associated with CRP and/or IL-6. The current findings confirm these associations with a rigorous study design, through controlling for activity, known to influence long-term HRV,34 and through the use of the twins design, which allowed us to control for potential unmeasured confounders, such as socioeconomic, lifestyle and other factors shared by individuals of similar family background. This study further expands prior findings, through inclusion of ULF, the HRV variable most predictive of mortality in some studies.27
Many of these studies hypothesized that inflammation altered HRV. We hypothesized the opposite directional relationship: that autonomic changes would be pro-inflammatory. As described below, data from basic science supports both possibilities.
Potential biological mechanisms
Experiments at the cellular, animal, and human levels support the link between autonomic and inflammatory processes. Experimentally, both exposure to acetylcholine and direct vagal stimulation inhibits release of cytokines by macrophages, termed the “cholinergic anti-inflammatory pathway”.12 Sympathetic activation, conversely, is pro-inflammatory. In isolated adipocytes, β-stimulation increases IL-6, and in humans, IL-6 levels increase with isoproterenol infusion.15 Beta-blockers dampen the IL-6 increase normally seen in response to stress in rats.16
ULF and VLF, the HRV variables most closely linked with inflammation in the current study, as well as being most predictive of mortality in previous studies,27 may be markers for many processes contributing to overall autonomic dysregulation.28 Heart rate variability in the VLF, LF, and HF band are all influenced in large part by parasympathetic activity17, 28, while VLF may also be influenced by other neurohormonal influences such as the renin-angiotensin-aldosterone system28, and LF by sympathetic influences in addition28. ULF may be influenced by day-night changes, especially when activity is controlled,34, 35 which in turn are influenced by autonomic activity.
In this study, both urinary norepinephrine and HRV were associated with inflammation. In multivariable analysis, HRV appeared a more important predictor of inflammation than norepinephrine, suggesting that parasympathetic dysregulation bore greater responsibility for our findings than did sympathetic excess. However, urinary norepinephrine may be an imprecise measure of sympathetic activity, and while studies show a good correlation between plasma and cardiac norepinephrine 36, the correlation between plasma and central nervous system measures of norepinephrine, is less clear (r=0.48 in one study)37. Sympathetic stimulation inhibits vagal output38, and it is also possible that the relationships seen here between HRV and inflammation were a reflection of sympathetic effects (ie, that low HRV was a marker for increased sympathetic activity) or that the two may have independent effects.
Other experimental studies suggest the opposite directional relationship, with inflammation causing autonomic changes. For example, IL-6 deactivates nitric oxide, which augments vagal activity as measured by HRV.13 Marz has shown that rat sympathetic neurons both respond to and produce IL-6,14 suggesting that the association between inflammation and autonomic regulation is bidirectional.
Limitations
As in any cross-sectional study, the directionality of the association seen between HRV and inflammation in this study cannot be determined. Further, the possibility that a third, unmeasured variable may be responsible for the relationship between inflammation and autonomic dysfunction can not be excluded. Also, female twin pairs were not included in the VET Registry given their extremely low representation among Vietnam era military personnel. Gender influences HRV in some33 (although not all) studies, and similarly, gender may effect inflammation.39 Whether the relationship between inflammation and the autonomic nervous system differs in women is an important area of future investigation.
Conclusions
Markers of inflammation are associated with decreased HRV, even after controlling for clinical CAD risk factors. Autonomic dysregulation leading to inflammation may represent one pathway through which traditional risk factors promote the development of CAD.
Acknowledgements
We gratefully acknowledge the continued cooperation of the members of the Vietnam Era Twin Registry.
Financial Support
National Institutes of Health: K24HL077506, R01 HL68630 and R01 AG026255; Emory General Clinical Research Center MO1-RR00039; American Heart Association: 0245115N. United States Department of Veterans Affairs has supported the development and maintenance of the Vietnam Era Twin Registry. Invaluable assistance provided by: VA Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; NIH; National Opinion Research Center; National Research Council, National Academy of Sciences; Institute for Survey Research, Temple University.
Ambulatory ECG analysis software provided by GE Medical, Milwaukee, WI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Minami J, Ishimitsu T, Matsuoka H. Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension. 1999;33:586–590. doi: 10.1161/01.hyp.33.1.586. [DOI] [PubMed] [Google Scholar]
- 2.Mussalo H, Vanninen E, Ikaheimo R, et al. Heart rate variability and its determinants in patients with severe or mild essential hypertension. Clinical Physiology. 2001;21:594–604. doi: 10.1046/j.1365-2281.2001.00359.x. [DOI] [PubMed] [Google Scholar]
- 3.Pehlivanidis AN, Athyros VG, Demitriadis DS, et al. Heart rate variability after long-term treatment with atorvastatin in hypercholesterolaemic patients with or without coronary artery disease. Atherosclerosis. 2001;157:463–469. doi: 10.1016/s0021-9150(00)00746-2. [DOI] [PubMed] [Google Scholar]
- 4.Vaccarino V, Lampert R, Bremner JD, et al. Depressive symptoms and heart rate variability: Evidence for a shared genetic substrate in a study of twins. Psychosom Med. doi: 10.1097/PSY.0b013e31817bcc9e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huikuri HV, Jokinen V, Syvanne M, et al. Heart rate variability and progression of coronary atherosclerosis. Arteriosclerosis, Thrombosis & Vascular Biology. 1999;19:1979–1985. doi: 10.1161/01.atv.19.8.1979. [DOI] [PubMed] [Google Scholar]
- 6.Liao D, Cai J, Rosamond JC, et al. Cardiac autonomic function and incident coronary heart disease: A population-based case-cohort study. Am J Epidemiol. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events: The Framingham heart study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 8.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. Journal of the American College of Cardiology. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 9.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. New England Journal of Medicine. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Rifai N, Stampfer MJ, et al. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 12.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhary S, Vaile JC, Fletcher J, et al. Nitric oxide and cardiac autonomic control in humans. Hypertension. 2000;36:264–269. doi: 10.1161/01.hyp.36.2.264. [DOI] [PubMed] [Google Scholar]
- 14.Marz P, Cheng JG, Gadient RA, et al. Sympathetic neurons can produce and respond to interleukin 6. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3251–3256. doi: 10.1073/pnas.95.6.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed-Ali V, Flower L, Sethi J, et al. beta-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. Journal of Clinical Endocrinology & Metabolism. 2001;86:5864–5869. doi: 10.1210/jcem.86.12.8104. [DOI] [PubMed] [Google Scholar]
- 16.Soszynski D, Kozak W, Conn CA, et al. Beta-adrenoceptor antagonists suppress elevation in body temperature and increase in plasma IL-6 in rats exposed to open field. Neuroendocrinology. 1996;63:459–467. doi: 10.1159/000127072. [DOI] [PubMed] [Google Scholar]
- 17.Akselrod S, Gordon D, Ubel FA, et al. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 18.Aronson D, Mittleman MA, Burger AJ. Interleukin-6 levels are inversely correlated with heart rate variability in patients with decompensated heart failure. Journal of Cardiovascular Electrophysiology. 2001;12:294–300. doi: 10.1046/j.1540-8167.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- 19.Janszky I, Ericson M, Lekander M, et al. Inflammatory markers and heart rate variability in women with coronary heart disease. Journal of Internal Medicine. 2004;256:421–428. doi: 10.1111/j.1365-2796.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 20.Lanza GA, Sgueglia GA, Cianflone D, et al. Relation of heart rate variability to serum levels of C-reactive protein in patients with unstable angina pectoris. American Journal of Cardiology. 2006;97:1702–1706. doi: 10.1016/j.amjcard.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Araujo F, Antelmi I, Pereira AC, et al. Lower heart rate variability is associated with higher serum high-sensitivity C-reactive protein concentration in healthy individuals aged 46 years or more. International Journal of Cardiology. 2006;107:333–337. doi: 10.1016/j.ijcard.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 22.Sajadieh A, Nielsen OW, Rasmussen V, et al. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. European Heart Journal. 2004;25:363–370. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Stein PK, Barzilay JI, Chaves PH, et al. Higher levels of inflammation factors and greater insulin resistance are independently associated with higher heart rate and lower heart rate variability in normoglycemic older individuals: the Cardiovascular Health Study. Journal of the American Geriatrics Society. 2008;56:315–321. doi: 10.1111/j.1532-5415.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg J, Curran B, Vitek ME, et al. The Vietnam Era Twin Registry. Twin Research. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 25.Dai J, Miller AH, Bremner JD, et al. Adherence to the mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: a twin study. Circulation. 2008;117:169–175. doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lampert R, Ickovics J, Viscoli C, et al. Effects of propranolol on recovery of heart rate variability following acute myocardial infarction and relation to outcome in the Beta Blocker Heart Attack Trial. Am J Cardiol. 2003;91:137–142. doi: 10.1016/s0002-9149(02)03098-9. [DOI] [PubMed] [Google Scholar]
- 27.Bigger JT, Fleiss JL, Steinman RC, et al. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 28.Taylor J, Carr D, Myers C, et al. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- 29.Dimsdale JE, Ziegler JE. What do plasma and urinary measures of catecholamines tell us about human response to stressors? Circulaton. 1991;83:II36–II42. [PubMed] [Google Scholar]
- 30.Bisoendial RJ, Kastelein JJ, Levels JH, et al. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circulation Research. 2005;96:714–716. doi: 10.1161/01.RES.0000163015.67711.AB. [DOI] [PubMed] [Google Scholar]
- 31.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Eng J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 32.Meier-Ewert HK, Ridker PM, Rifal N, et al. Absence of diurnal variation of C-reactive protein concentrations ins healthy human subjects. Clinical Chemistry. 2001;47:426–430. [PubMed] [Google Scholar]
- 33.Bigger JT, Fleiss JL, Steinman RC, et al. RR variability in healthy, middle-aged persons compared with patients with chronic coronary heart disease or recent acute myocardial infarction. Circulation. 1995;91:1936–1943. doi: 10.1161/01.cir.91.7.1936. [DOI] [PubMed] [Google Scholar]
- 34.Roach D, Wilson W, Ritchie D, et al. Dissection of long-range heart rate variability; controlled induction of prognostic measures by activity in the laboratory. J Am Coll Cardiol. 2004;43:2271–2277. doi: 10.1016/j.jacc.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 35.Roach D, Sheldon A, Wilson W, et al. Temporally localized contributions to measures of large-scale heart rate variability. American Journal of Physiology. 1998;274:H1465–H1471. doi: 10.1152/ajpheart.1998.274.5.H1465. [DOI] [PubMed] [Google Scholar]
- 36.Wallin BG, Esler M, Dorward P, et al. Simultaneous Measurements of cardiac Noradrenaline Spillover and Sympathetic Outflow to Skeletal Muscle in Humans. Journal of Physiology. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gold PW, Wong ML, Goldstein DS, et al. Cardiac implications of increased arterial entry and reversible 24-h central and peripheral norepinephrine levels in melancholia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8303–8308. doi: 10.1073/pnas.0503069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerati D, Schwartz PJ. Single cardiac vagal fiber activity, acute myocardial ischemia, and risk for sudden death. Circ Res. 1991;69:1389–1401. doi: 10.1161/01.res.69.5.1389. [DOI] [PubMed] [Google Scholar]
- 39.Worns MA, Victor A, Galle PR, et al. Genetic and environmental contributions to plasma C-reactive protein and interleukin-6 levels--a study in twins. Genes & Immunity. 2006;7:600–605. doi: 10.1038/sj.gene.6364330. [DOI] [PubMed] [Google Scholar]