Abstract
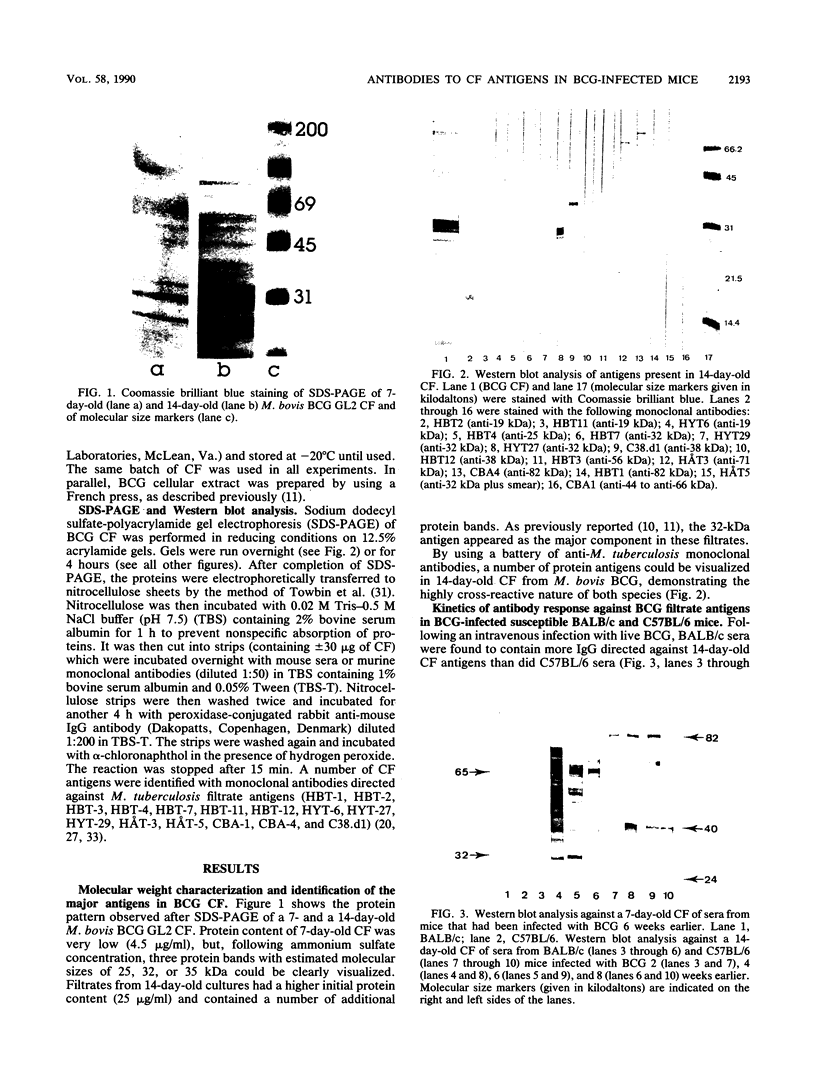
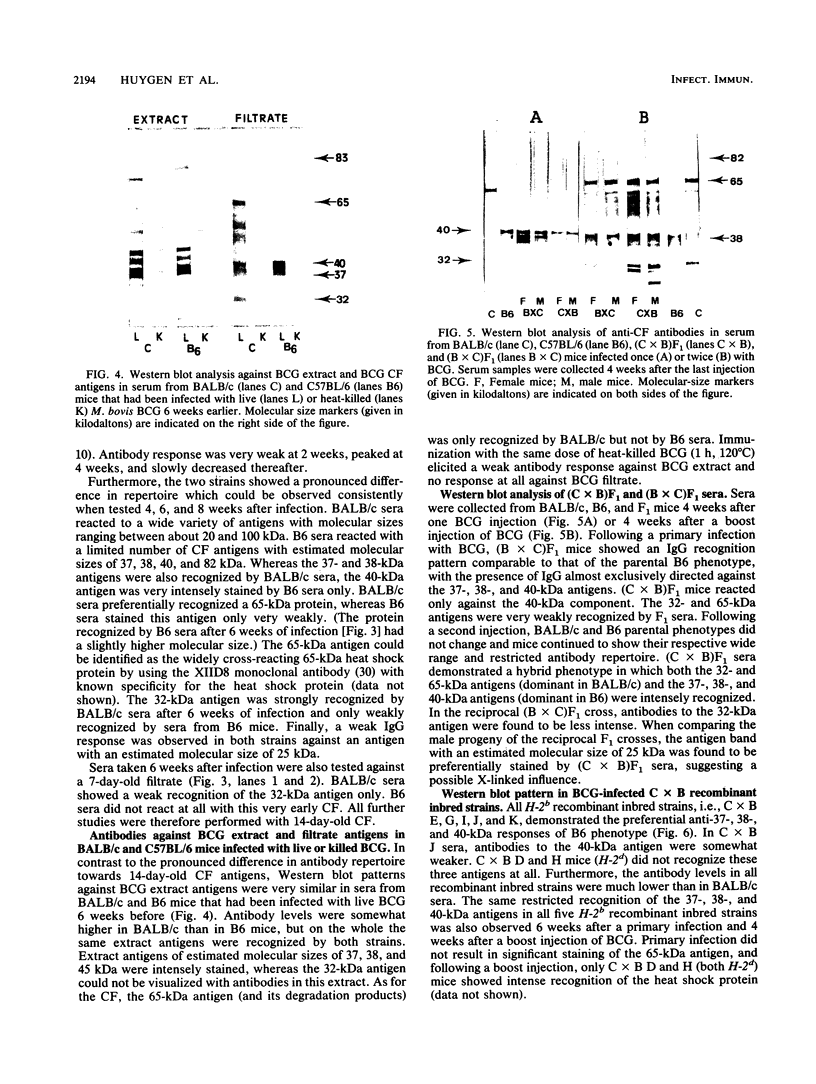
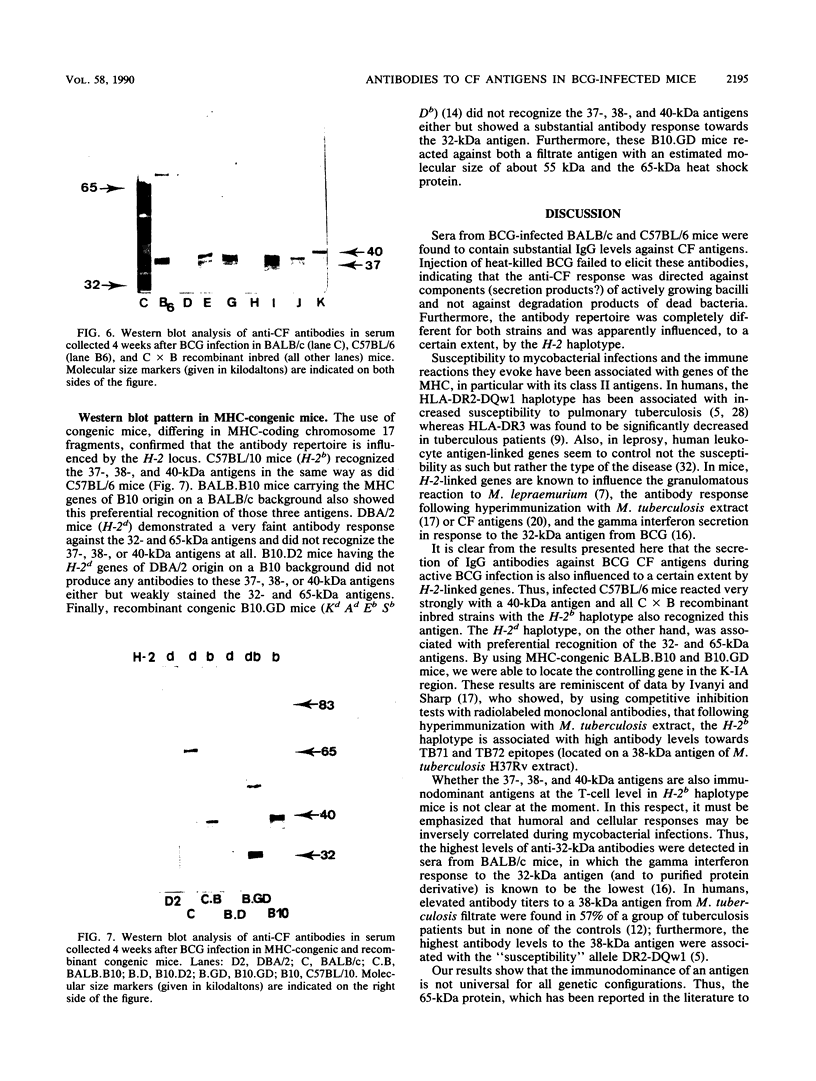
Two susceptible (Bcgs) mouse strains, BALB/c and C57BL/6, were compared by Western blot (immunoblot) analysis for their immunoglobulin G response to 14-day-old BCG culture filtrate (CF) following intravenous infection with live Mycobacterium bovis BCG. The two strains demonstrated a completely different antibody repertoire. BALB/c antibodies were directed against a wide range of CF antigens between 20 and about 100 kilodaltons (kDa), with a preferential recognition of the 65-kDa heat shock protein and the 32-kDa fibronectin-binding protein. C57BL/6 sera, on the other hand, showed a much more restricted antibody pattern, almost exclusively directed against three antigens with estimated molecular sizes of 37, 38, and 40 kDa. Whereas the 37- and 38-kDa antigens were also recognized by BALB/c mice, the 40-kDa antigen was very intensely stained by C57BL/6 sera only. F1 mice had the restricted antibody pattern of C57BL/6 after one injection of BCG and had a hybrid BALB/c-C57BL/6 phenotype following a boost injection of BCG 2 months after the initial infection. Analysis of seven recombinant inbred strains derived from the BALB/c x C57BL/6 cross and of congenic mice differing in major histocompatibility complex-coding chromosome 17 fragments suggests that a gene in the K-IA region of the H-2 locus is associated with the preferential recognition of certain CF antigens. Inoculation with the same dose of killed BCG failed to elicit an antibody response to these filtrate antigens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. F., Mehra V., Hirschfield G. R., Fong S. J., Abou-Zeid C., Rook G. A., Hunter S. W., Brennan P. J., Modlin R. L. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J Immunol. 1989 Oct 15;143(8):2656–2662. [PubMed] [Google Scholar]
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- Borremans M., Weckx M., Verhofstadt R. A comparison of the rate of oxygen uptake and the adenosine triphosphate content of routine and experimental BCG preparations. J Biol Stand. 1983 Jul;11(3):205–212. doi: 10.1016/s0092-1157(83)80007-9. [DOI] [PubMed] [Google Scholar]
- Borremans M., de Wit L., Volckaert G., Ooms J., de Bruyn J., Huygen K., van Vooren J. P., Stelandre M., Verhofstadt R., Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun. 1989 Oct;57(10):3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothamley G. H., Beck J. S., Schreuder G. M., D'Amaro J., de Vries R. R., Kardjito T., Ivanyi J. Association of tuberculosis and M. tuberculosis-specific antibody levels with HLA. J Infect Dis. 1989 Mar;159(3):549–555. doi: 10.1093/infdis/159.3.549. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Lamb J. R., Cox J. H., Rothbard J. B., Mehlert A., Ivanyi J. Differential pattern of T cell recognition of the 65-kDa mycobacterial antigen following immunization with the whole protein or peptides. Eur J Immunol. 1989 Jul;19(7):1303–1310. doi: 10.1002/eji.1830190723. [DOI] [PubMed] [Google Scholar]
- Closs O., Løvik M., Wigzell H., Taylor B. A. H-2-linked gene(s) influence the granulomatous reaction to viable Mycobacterium lepraemurium in the mouse. Scand J Immunol. 1983 Jul;18(1):59–63. doi: 10.1111/j.1365-3083.1983.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Lamb J. R., Young D. B. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect Immun. 1988 May;56(5):1260–1266. doi: 10.1128/iai.56.5.1260-1266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Downs M., Neimes R. E., Ognibene A. J., Yamashita T. S., Ellner J. J. Immunogenetic analysis of human tuberculosis. J Infect Dis. 1988 Dec;158(6):1302–1308. doi: 10.1093/infdis/158.6.1302. [DOI] [PubMed] [Google Scholar]
- De Bruyn J., Bosmans R., Nyabenda J., Van Vooren J. P. Effect of zinc deficiency on the appearance of two immunodominant protein antigens (32 kDa and 65 kDa) in culture filtrates of mycobacteria. J Gen Microbiol. 1989 Jan;135(1):79–84. doi: 10.1099/00221287-135-1-79. [DOI] [PubMed] [Google Scholar]
- De Bruyn J., Huygen K., Bosmans R., Fauville M., Lippens R., Van Vooren J. P., Falmagne P., Weckx M., Wiker H. G., Harboe M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987 May;2(5):351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- Espitia C., Cervera I., González R., Mancilla R. A 38-kD Mycobacterium tuberculosis antigen associated with infection. Its isolation and serologic evaluation. Clin Exp Immunol. 1989 Sep;77(3):373–377. [PMC free article] [PubMed] [Google Scholar]
- Forget A., Benoit J. C., Turcotte R., Gusew-Chartrand N. Enhancement activity of anti-mycobacterial sera in experimental Mycobacterium bovis (BCG) infection in mice. Infect Immun. 1976 May;13(5):1301–1306. doi: 10.1128/iai.13.5.1301-1306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., McNeil M., Modlin R. L., Mehra V., Bloom B. R., Brennan P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J Immunol. 1989 Apr 15;142(8):2864–2872. [PubMed] [Google Scholar]
- Huygen K., Palfliet K., Jurion F., Hilgers J., ten Berg R., Van Vooren J. P., De Bruyn J. H-2-linked control of in vitro gamma interferon production in response to a 32-kilodalton antigen (P32) of Mycobacterium bovis bacillus Calmette-Guérin. Infect Immun. 1988 Dec;56(12):3196–3200. doi: 10.1128/iai.56.12.3196-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J., Sharp K. Control by H-2 genes of murine antibody responses to protein antigens of Mycobacterium tuberculosis. Immunology. 1986 Nov;59(3):329–332. [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Flesch I. E. The role of T cell--macrophage interactions in tuberculosis. Springer Semin Immunopathol. 1988;10(4):337–358. doi: 10.1007/BF02053845. [DOI] [PubMed] [Google Scholar]
- Lenzini L., Rottoli P., Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977 Feb;27(2):230–237. [PMC free article] [PubMed] [Google Scholar]
- Ljungqvist L., Worsaae A., Heron I. Antibody responses against Mycobacterium tuberculosis in 11 strains of inbred mice: novel monoclonal antibody specificities generated by fusions, using spleens from BALB.B10 and CBA/J mice. Infect Immun. 1988 Aug;56(8):1994–1998. doi: 10.1128/iai.56.8.1994-1998.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon-Kaplan J., Hunter S. W., McNeil M., Stewart C., Modlin R. L., Rea T. H., Convit J., Salgame P., Mehra V., Bloom B. R. Immunological significance of Mycobacterium leprae cell walls. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1917–1921. doi: 10.1073/pnas.85.6.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mutis T., van Schooten W. C., de Vries R. R. A peptidoglycan protein complex purified from M. leprae cell walls contains most or all immunodominant M. leprae T-cell antigens. Int J Lepr Other Mycobact Dis. 1989 Dec;57(4):788–793. [PubMed] [Google Scholar]
- Orme I. M. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988 May 15;140(10):3589–3593. [PubMed] [Google Scholar]
- Reggiardo Z., Middlebrook G. Failure of passive serum transfer of immunity against aerogenic tuberculosis in rabbits. Proc Soc Exp Biol Med. 1974 Jan;145(1):173–175. doi: 10.3181/00379727-145-37771. [DOI] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Shigekane V. M., Fisher W. L., Locksley R. M. Cellular and humoral immunity to Leishmania major in genetically susceptible mice after in vivo depletion of L3T4+ T cells. J Immunol. 1987 Aug 15;139(4):1303–1309. [PubMed] [Google Scholar]
- Schou C., Yuan Z. L., Andersen A. B., Bennedsen J. Production and partial characterization of monoclonal hybridoma antibodies to Mycobacterium tuberculosis. Acta Pathol Microbiol Immunol Scand C. 1985 Dec;93(6):265–272. doi: 10.1111/j.1699-0463.1985.tb02955.x. [DOI] [PubMed] [Google Scholar]
- Singh S. P., Mehra N. K., Dingley H. B., Pande J. N., Vaidya M. C. Human leukocyte antigen (HLA)-linked control of susceptibility to pulmonary tuberculosis and association with HLA-DR types. J Infect Dis. 1983 Oct;148(4):676–681. doi: 10.1093/infdis/148.4.676. [DOI] [PubMed] [Google Scholar]
- Skamene E. Genetic control of resistance to mycobacterial infection. Curr Top Microbiol Immunol. 1986;124:49–66. doi: 10.1007/978-3-642-70986-9_4. [DOI] [PubMed] [Google Scholar]
- Thole J. E., van Schooten W. C., Keulen W. J., Hermans P. W., Janson A. A., de Vries R. R., Kolk A. H., van Embden J. D. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect Immun. 1988 Jun;56(6):1633–1640. doi: 10.1128/iai.56.6.1633-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsaae A., Ljungqvist L., Heron I. Monoclonal antibodies produced in BALB.B10 mice define new antigenic determinants in culture filtrate preparations of Mycobacterium tuberculosis. J Clin Microbiol. 1988 Dec;26(12):2608–2614. doi: 10.1128/jcm.26.12.2608-2614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Gonzalez N. M., de Vries R. R., Convit J., van Rood J. J. HLA-linked control of predisposition to lepromatous leprosy. J Infect Dis. 1985 Jan;151(1):9–14. doi: 10.1093/infdis/151.1.9. [DOI] [PubMed] [Google Scholar]









