Abstract
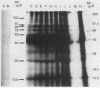
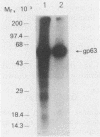
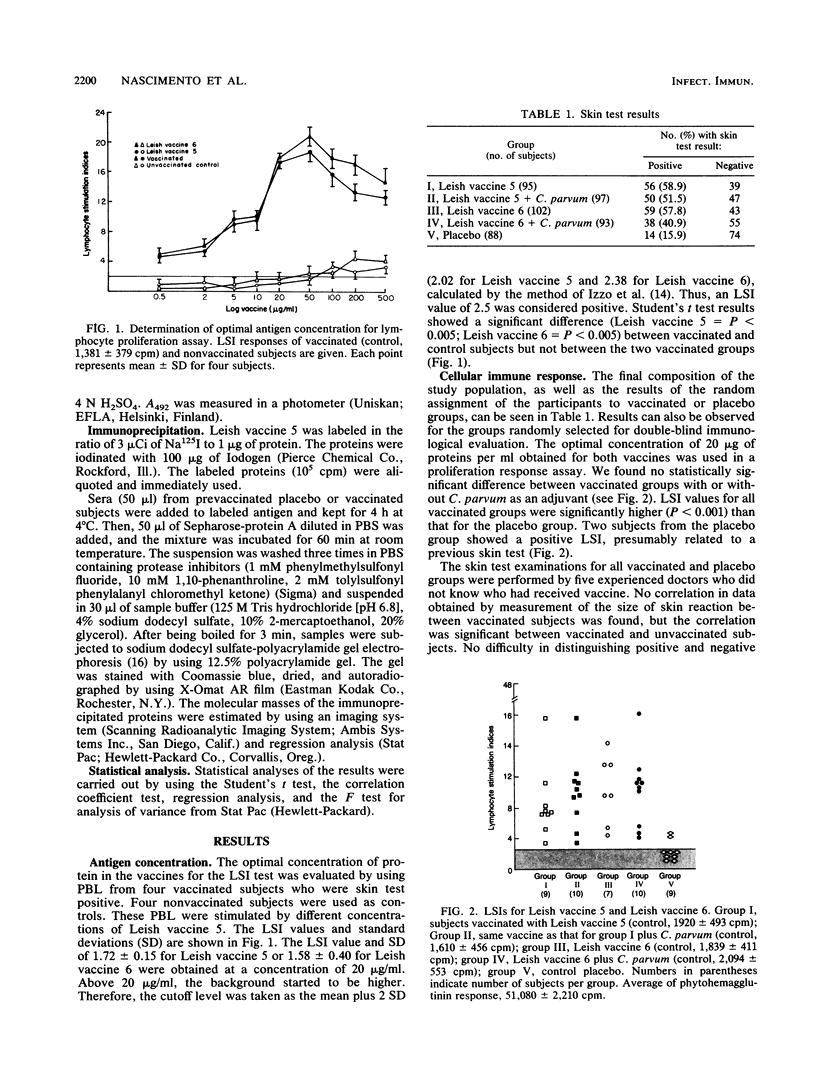
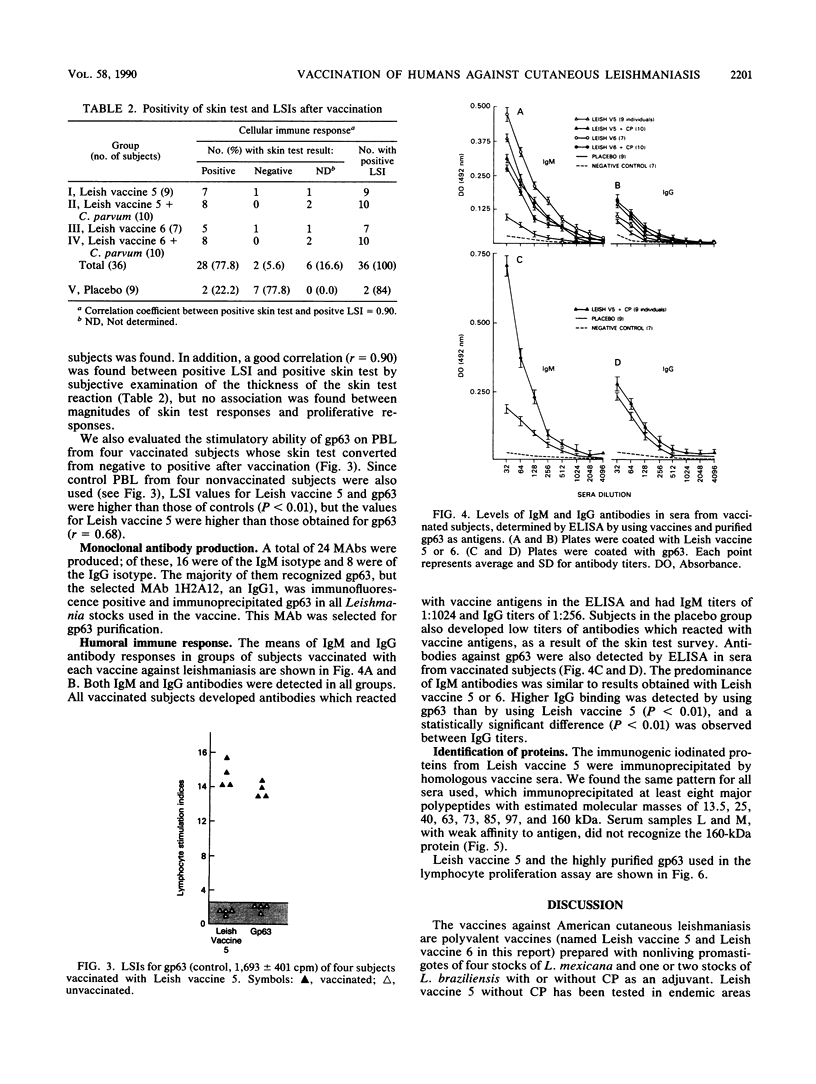
Brazilian army conscripts were vaccinated against American cutaneous leishmaniasis by using nonliving polyvalent promastigote Leish vaccine 5 or Leish vaccine 6 (vaccines with five or six Leishmania stocks) with or without Corynebacterium parvum. No statistically significant differences in lymphocyte stimulation indices were found between vaccinated groups with or without C. parvum, but lymphocyte stimulation indices of all vaccinees were significantly higher (P less than 0.001) than those of the placebo group. A correlation of 90% was found between positive skin test results and positive lymphocyte stimulation indices. Eight major antigens with estimated molecular masses of 13.5, 25, 40, 63, 73, 85, 97, and 160 kilodaltons were recognized by Leish vaccine 5 sera. Our finding also demonstrated the predominance of immunoglobulin M antibody in sera of vaccinated subjects and that a component of Leish vaccine 5, gp63, was immunogenic in humans both at the T-cell level and at the antibody level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antunes C. M., Mayrink W., Magalhaes P. A., Costa C. A., Melo M. N., Dias M., Michalick M. S., Williams P., Lima A. O., Vieira J. B. Controlled field trials of a vaccine against New World cutaneous leishmaniasis. Int J Epidemiol. 1986 Dec;15(4):572–580. doi: 10.1093/ije/15.4.572. [DOI] [PubMed] [Google Scholar]
- Barral-Netto M., Reed S. G., Sadigursky M., Sonnenfeld G. Specific immunization of mice against Leishmania mexicana amazonensis using solubilized promastigotes. Clin Exp Immunol. 1987 Jan;67(1):11–19. [PMC free article] [PubMed] [Google Scholar]
- Berman J. D., Edwards N., King M., Grogl M. Biochemistry of Pentostam resistant Leishmania. Am J Trop Med Hyg. 1989 Feb;40(2):159–164. doi: 10.4269/ajtmh.1989.40.159. [DOI] [PubMed] [Google Scholar]
- Bonfante-Garrido R. Leishmanias y leishmaniasis tegumentaria en América Latina. Bol Oficina Sanit Panam. 1983 Nov;95(5):418–426. [PubMed] [Google Scholar]
- Bouvier J., Etges R. J., Bordier C. Identification and purification of membrane and soluble forms of the major surface protein of Leishmania promastigotes. J Biol Chem. 1985 Dec 15;260(29):15504–15509. [PubMed] [Google Scholar]
- Bouvier J., Etges R., Bordier C. Identification of the promastigote surface protease in seven species of Leishmania. Mol Biochem Parasitol. 1987 May;24(1):73–79. doi: 10.1016/0166-6851(87)90117-4. [DOI] [PubMed] [Google Scholar]
- CAMARGO E. P. GROWTH AND DIFFERENTIATION IN TRYPANOSOMA CRUZI. I. ORIGIN OF METACYCLIC TRYPANOSOMES IN LIQUID MEDIA. Rev Inst Med Trop Sao Paulo. 1964 May-Jun;6:93–100. [PubMed] [Google Scholar]
- Figueiredo Y. P., da Costa C. A., Mayrink W., Araujo F. G., Dias M., Melo M. N., Magalhaes P., Williams P., Batista S. M., Coelho M. V. Nutriço e metabolismo de formas de cultura de Leishmania. Rev Inst Med Trop Sao Paulo. 1976 Sep-Oct;18(5):306–314. [PubMed] [Google Scholar]
- Frommel D., Ogunkolade B. W., Vouldoukis I., Monjour L. Vaccine-induced immunity against cutaneous leishmaniasis in BALB/c mice. Infect Immun. 1988 Apr;56(4):843–848. doi: 10.1128/iai.56.4.843-848.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. M. Immunization with Leishmania-specific T cell not B cell lines or hybridomas can modulate the response of susceptible mice infected with viable parasites. J Immunol. 1987 Nov 1;139(9):3070–3075. [PubMed] [Google Scholar]
- Grögl M., Oduola A. M., Cordero L. D., Kyle D. E. Leishmania spp.: development of pentostam-resistant clones in vitro by discontinuous drug exposure. Exp Parasitol. 1989 Jul;69(1):78–90. doi: 10.1016/0014-4894(89)90173-2. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo A. A., Marmion B. P., Worswick D. A. Markers of cell-mediated immunity after vaccination with an inactivated, whole-cell Q fever vaccine. J Infect Dis. 1988 Apr;157(4):781–789. doi: 10.1093/infdis/157.4.781. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lepay D. A., Nogueira N., Cohn Z. Surface antigens of Leishmania donovani promastigotes. J Exp Med. 1983 May 1;157(5):1562–1572. doi: 10.1084/jem.157.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Hodson K., Lelchuk R. Prophylactic immunization against experimental leishmaniasis. VI. Comparison of protective and disease-promoting T cells. J Immunol. 1987 Nov 1;139(9):3112–3117. [PubMed] [Google Scholar]
- Mayrink W., Magalhães P. A., Dias M., Da Costa C. A., Melo M. N., Lima A. O. Responses to Montenegro antigen after immunization with killed Leishmania promastigotes. Trans R Soc Trop Med Hyg. 1978;72(6):676–676. doi: 10.1016/0035-9203(78)90041-x. [DOI] [PubMed] [Google Scholar]
- Mayrink W., Williams P., da Costa C. A., Magalhães P. A., Melo M. N., Dias M., Oliveira Lima A., Michalick M. S., Ferreira Carvalho E., Barros G. C. An experimental vaccine against American dermal leishmaniasis: experience in the State of Espírito Santo, Brazil. Ann Trop Med Parasitol. 1985 Jun;79(3):259–269. doi: 10.1080/00034983.1985.11811917. [DOI] [PubMed] [Google Scholar]
- Mayrink W., da Costa C. A., Magalhães P. A., Melo M. N., Dias M., Lima A. O., Michalick M. S., Williams P. A field trial of a vaccine against American dermal leishmaniasis. Trans R Soc Trop Med Hyg. 1979;73(4):385–387. doi: 10.1016/0035-9203(79)90159-7. [DOI] [PubMed] [Google Scholar]
- Melo M. N., Mayrink W., da Costa C. A., Magalhaes P. A., Dias M., Williams P., Araujo F. G., Coelho M. V., Batista S. M. Padronizaço do antigeno de Montenegro. Rev Inst Med Trop Sao Paulo. 1977 May-Jun;19(3):161–164. [PubMed] [Google Scholar]
- Mitchell G. F., Handman E., Spithill T. W. Examination of variables in the vaccination of mice against cutaneous leishmaniasis using living avirulent cloned lines and killed promastigotes of Leishmania major. Int J Parasitol. 1985 Dec;15(6):677–684. doi: 10.1016/0020-7519(85)90015-3. [DOI] [PubMed] [Google Scholar]
- Modabber F. Experiences with vaccines against cutaneous leishmaniasis: of men and mice. Parasitology. 1989;98 (Suppl):S49–S60. doi: 10.1017/s0031182000072243. [DOI] [PubMed] [Google Scholar]
- Pappas M. G., McGreevy P. B., Hajkowski R., Hendricks L. D., Oster C. N., Hockmeyer W. T. Evaluation of promastigote and amastigote antigens in the indirect fluorescent antibody test for American cutaneous leishmaniasis. Am J Trop Med Hyg. 1983 Nov;32(6):1260–1267. doi: 10.4269/ajtmh.1983.32.1260. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Russell D. G., Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol. 1986 Apr 1;136(7):2613–2620. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Pearce E., Natovitz P., Sher A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987 Jul 1;139(1):221–227. [PubMed] [Google Scholar]
- Scott P., Pearce E., Natovitz P., Sher A. Vaccination against cutaneous leishmaniasis in a murine model. II. Immunologic properties of protective and nonprotective subfractions of soluble promastigote extract. J Immunol. 1987 Nov 1;139(9):3118–3125. [PubMed] [Google Scholar]
- Scott P. The role of TH1 and TH2 cells in experimental cutaneous leishmaniasis. Exp Parasitol. 1989 Apr;68(3):369–372. doi: 10.1016/0014-4894(89)90120-3. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Hardin K. K. The major concanavalin A-binding surface glycoprotein of Leishmania donovani chagasi promastigotes is involved in attachment to human macrophages. J Immunol. 1988 Jul 1;141(1):265–272. [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]