Abstract
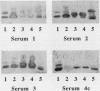
We developed a human inhibition monoclonal enzyme-linked immunosorbent assay (HIMELISA) to investigate the human immune response to the lipooligosaccharides (LOS) of Neisseria meningitidis. Monoclonal antibodies (MAb) were used to define seven epitopes on four LOS molecules of a meningococcal strain (126E) previously shown to express immunogenic LOS epitopes. The assay could distinguish epitope-specific antibody within whole sera. Neither the specificity nor the amount of the antibody measured by HIMELISA in sera of vaccinates changed during the immune response to meningococcal capsular polysaccharides, a chemically unrelated antigen. By using the HIMELISA, it was determined that sera from adults convalescing from meningococcal disease strongly inhibited MAb binding to two of the seven defined epitopes. The 3.6-kilodalton LOS of strain 126E expressed both of these epitopes. In addition, one of the inhibited epitopes was also expressed on the 4.0-kilodalton LOS of strain 126E. The convalescent-phase sera inhibited MAb binding to these two epitopes when they were expressed on LOS of diverse meningococcal strains. An acute-phase serum blocked MAb to the two epitopes to a lesser degree than did a convalescent-phase serum from the same patient. Immunoblotting the sodium dodecyl sulfate-polyacrylamide gel electrophoresis-separated LOS with convalescent-phase sera confirmed the specificity of the human anti-LOS antibody identified by HIMELISA.
Full text
PDF


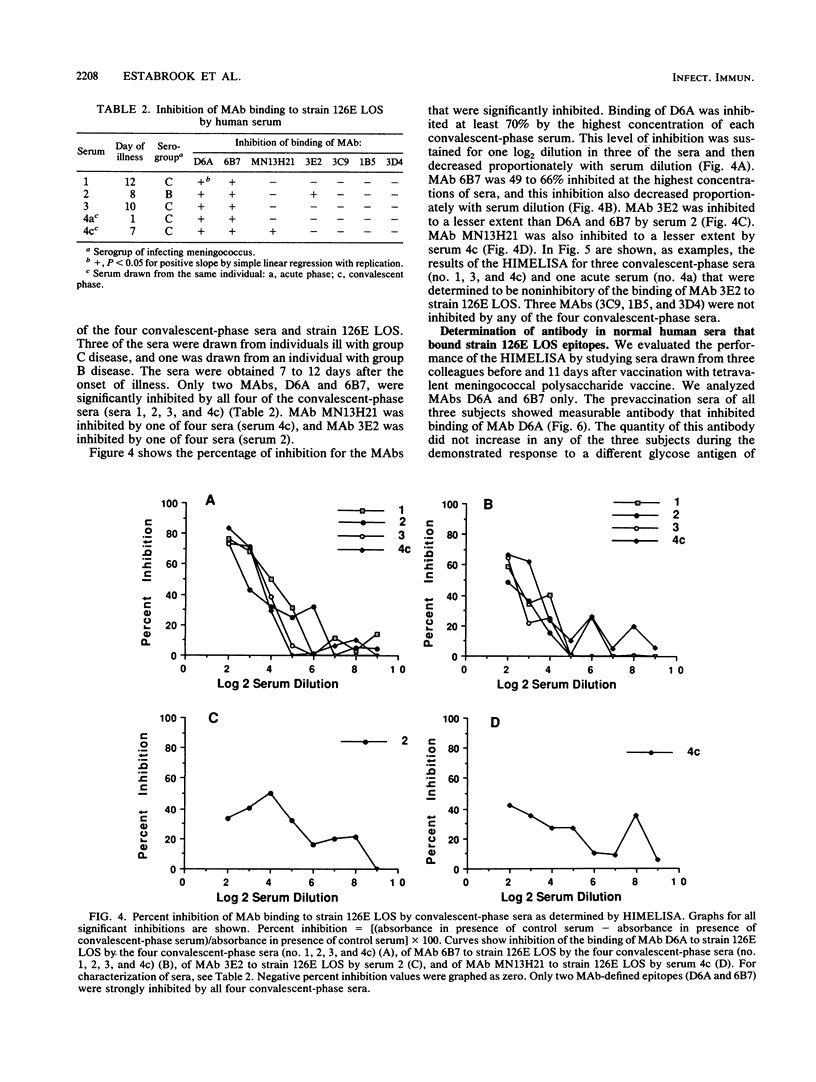
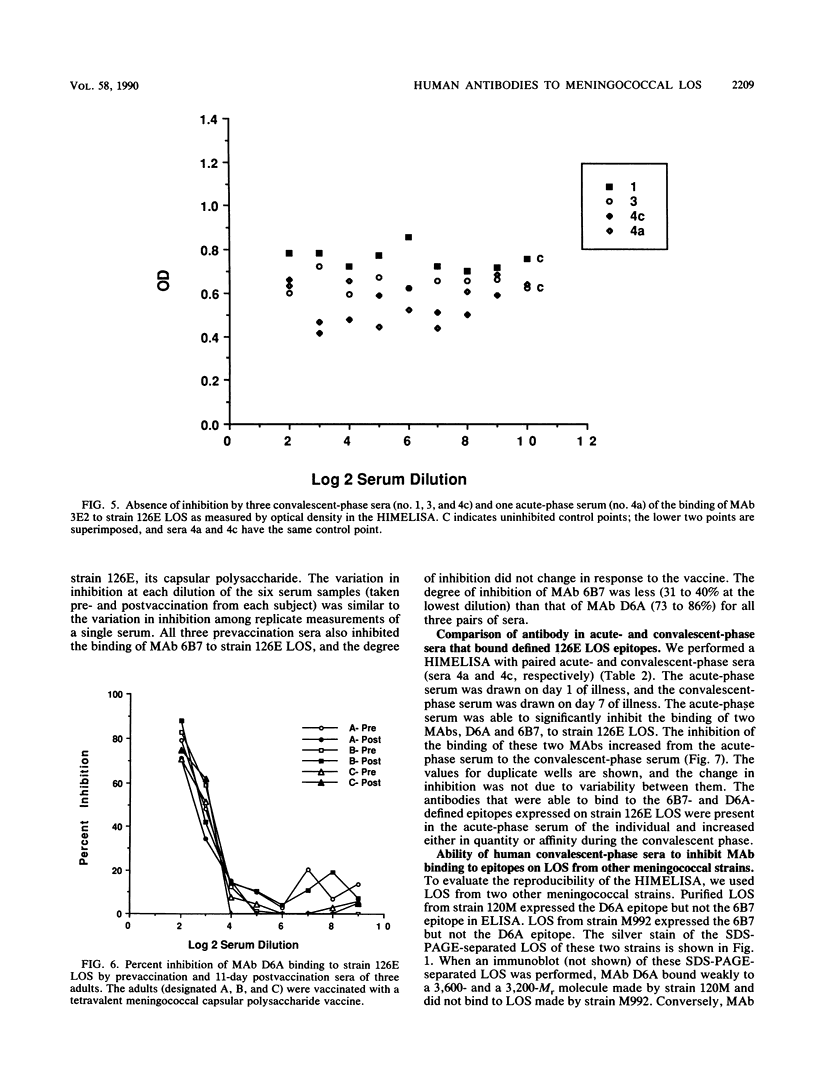
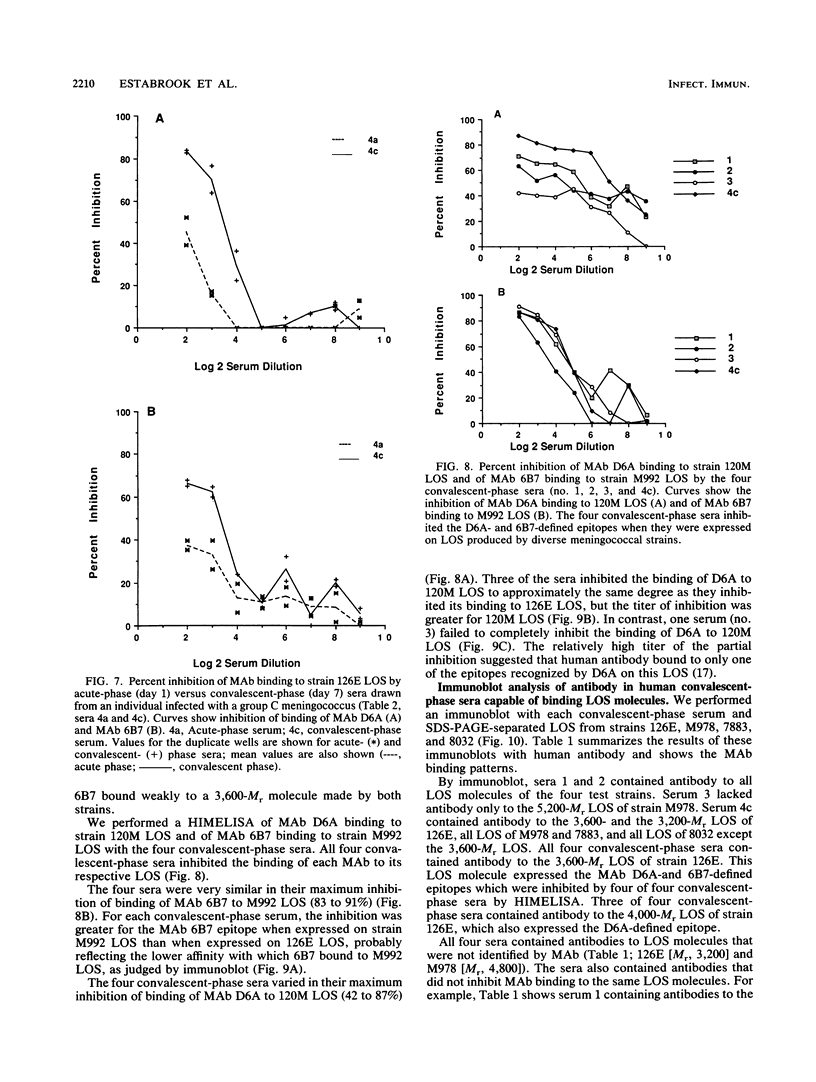
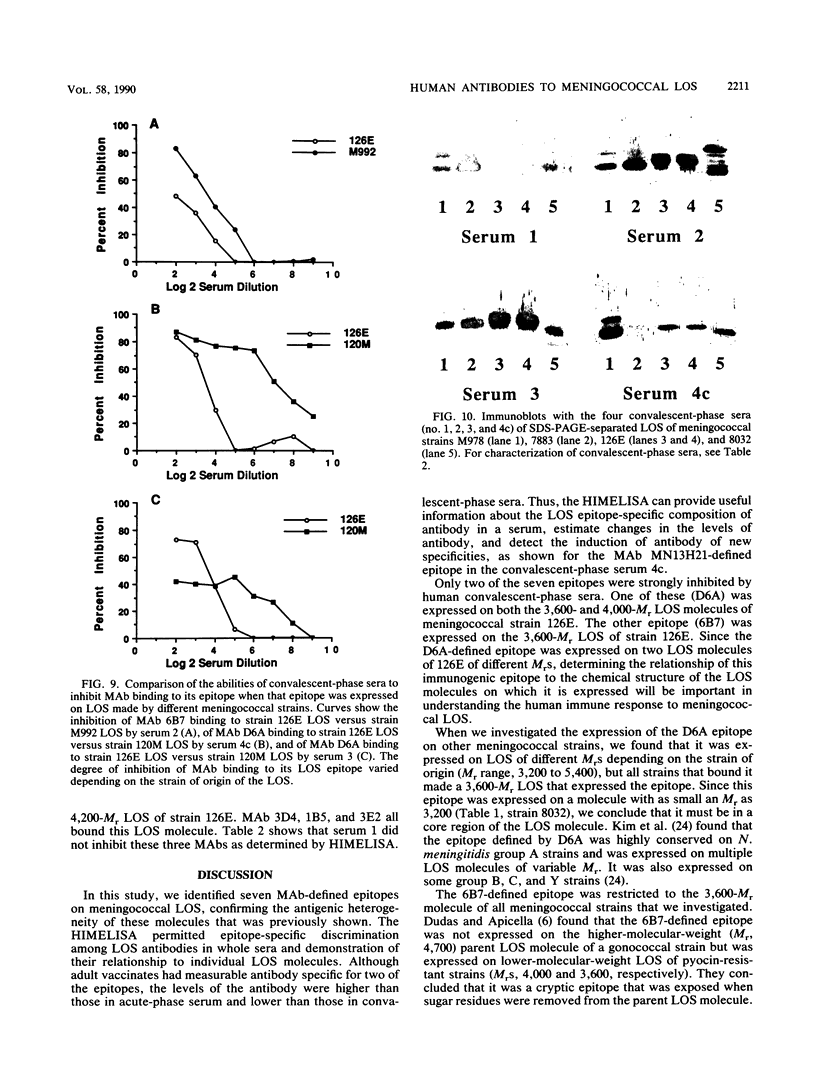


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Shero M., Jarvis G. A., Griffiss J. M., Mandrell R. E., Schneider H. Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide. Infect Immun. 1987 Aug;55(8):1755–1761. doi: 10.1128/iai.55.8.1755-1761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram M. A., Griffiss J. M., Broud D. D. Response to antigenic determinants of Neisseria meningitidis lipopolysaccharide investigated with a new radioactive antigen-binding assay. J Immunol. 1976 Mar;116(3):842–846. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Counts G. W., Gregory D. F., Spearman J. G., Lee B. A., Filice G. A., Holmes K. K., Griffiss J. M. Group A meningococcal disease in the U.S. Pacific Northwest: epidemiology, clinical features, and effect of a vaccination control program. Rev Infect Dis. 1984 Sep-Oct;6(5):640–648. doi: 10.1093/clinids/6.5.640. [DOI] [PubMed] [Google Scholar]
- Crowe B. A., Wall R. A., Kusecek B., Neumann B., Olyhoek T., Abdillahi H., Hassan-King M., Greenwood B. M., Poolman J. T., Achtman M. Clonal and variable properties of Neisseria meningitidis isolated from cases and carriers during and after an epidemic in The Gambia, West Africa. J Infect Dis. 1989 Apr;159(4):686–700. doi: 10.1093/infdis/159.4.686. [DOI] [PubMed] [Google Scholar]
- Dudas K. C., Apicella M. A. Selection and immunochemical analysis of lipooligosaccharide mutants of Neisseria gonorrhoeae. Infect Immun. 1988 Feb;56(2):499–504. doi: 10.1128/iai.56.2.499-504.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Zollinger W. D., Poolman J. T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985 Jul-Aug;7(4):504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- Gold R., Lepow M. L., Goldschneider I., Draper T. F., Gotshlich E. C. Kinetics of antibody production to group A and group C meningococcal polysaccharide vaccines administered during the first six years of life: prospects for routine immunization of infants and children. J Infect Dis. 1979 Nov;140(5):690–697. doi: 10.1093/infdis/140.5.690. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Brandt B. L., Broud D. D., Altieri P. L., Berman S. L. Relationship of dose to the reactogenicity and immunogenicity of meningococcal polysaccharide vaccines in adults. Mil Med. 1985 Oct;150(10):529–533. [PubMed] [Google Scholar]
- Griffiss J. M., Brandt B. L., Broud D. D., Goroff D. K., Baker C. J. Immune response of infants and children to disseminated infections with Neisseria meningitidis. J Infect Dis. 1984 Jul;150(1):71–79. doi: 10.1093/infdis/150.1.71. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Broud D. D., Silver C. A., Artenstein M. S. Immunoepidemiology of meningococcal disease in military recruits. I. A model for serogroup independency of epidemic potential as determined by serotyping. J Infect Dis. 1977 Aug;136(2):176–186. doi: 10.1093/infdis/136.2.176. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M. Epidemic meningococcal disease: synthesis of a hypothetical immunoepidemiologic model. Rev Infect Dis. 1982 Jan-Feb;4(1):159–172. doi: 10.1093/clinids/4.1.159. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Goroff D. K. Immunological cross-reaction between a naturally occurring galactan, agarose, and an LPS locus for immune lysis of Neisseria meningitidis by human sera. Clin Exp Immunol. 1981 Jan;43(1):20–27. [PMC free article] [PubMed] [Google Scholar]
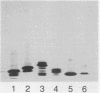
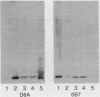
- Griffiss J. M., O'Brien J. P., Yamasaki R., Williams G. D., Rice P. A., Schneider H. Physical heterogeneity of neisserial lipooligosaccharides reflects oligosaccharides that differ in apparent molecular weight, chemical composition, and antigenic expression. Infect Immun. 1987 Aug;55(8):1792–1800. doi: 10.1128/iai.55.8.1792-1800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Schneider H., Mandrell R. E., Yamasaki R., Jarvis G. A., Kim J. J., Gibson B. W., Hamadeh R., Apicella M. A. Lipooligosaccharides: the principal glycolipids of the neisserial outer membrane. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S287–S295. doi: 10.1093/cid/10.supplement_2.s287. [DOI] [PubMed] [Google Scholar]
- Hicks C. B., Boslego J. W., Brandt B. Evidence of serum antibodies to Neisseria gonorrhoeae before gonococcal infection. J Infect Dis. 1987 Jun;155(6):1276–1281. doi: 10.1093/infdis/155.6.1276. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Bhattacharjee A. K., Kenne L., Kenny C. P., Calver G. The R-type lipopolysaccharides of Neisseria meningitidis. Can J Biochem. 1980 Feb;58(2):128–136. doi: 10.1139/o80-018. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Hawes G. B., Adams G. A., Kenny C. P. The chemical composition and serological reactions of lipopolysaccharides from serogroups A,B,X, and Y Neisseria meningitidis. Can J Biochem. 1973 Oct;51(10):1347–1354. doi: 10.1139/o73-178. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Johnson K. G., Kenne L. The structure of an R-type oligosaccharide core obtained from some lipopolysaccharides of Neisseria meningitidis. Carbohydr Res. 1983 Sep 16;121:233–241. doi: 10.1016/0008-6215(83)84020-8. [DOI] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Griffiss J. M. Neisseria lactamica and Neisseria meningitidis share lipooligosaccharide epitopes but lack common capsular and class 1, 2, and 3 protein epitopes. Infect Immun. 1989 Feb;57(2):602–608. doi: 10.1128/iai.57.2.602-608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Hu Z., Westerink M. A., Poolman J. T., Griffiss J. M. Electromorphic characterization and description of conserved epitopes of the lipooligosaccharides of group A Neisseria meningitidis. Infect Immun. 1988 Oct;56(10):2631–2638. doi: 10.1128/iai.56.10.2631-2638.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käyhty H., Karanko V., Peltola H., Sarna S., Mäkelä P. H. Serum antibodies to capsular polysaccharide vaccine of group A Neissera meningitidis followed for three years in infants and children. J Infect Dis. 1980 Dec;142(6):861–868. doi: 10.1093/infdis/142.6.861. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Lipopolysaccharide serotyping of Neisseria meningitidis by hemagglutination inhibition. Infect Immun. 1977 May;16(2):471–475. doi: 10.1128/iai.16.2.471-475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz C. S., Apicella M. A., Morse S. A. Electrophoretic and serological characterization of the lipopolysaccharide produced by Neisseria gonorrhoeae. J Infect Dis. 1984 Apr;149(4):544–552. doi: 10.1093/infdis/149.4.544. [DOI] [PubMed] [Google Scholar]
- Peltola H., Mäkelä H., Käyhty H., Jousimies H., Herva E., Hällström K., Sivonen A., Renkonen O. V., Pettay O., Karanko V. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977 Sep 29;297(13):686–691. doi: 10.1056/NEJM197709292971302. [DOI] [PubMed] [Google Scholar]
- Peltola H., Safary A., Käyhty H., Karanko V., André F. E. Evaluation of two tetravalent (ACYW135) meningococcal vaccines in infants and small children: a clinical study comparing immunogenicity of O-acetyl-negative and O-acetyl-positive group C polysaccharides. Pediatrics. 1985 Jul;76(1):91–96. [PubMed] [Google Scholar]
- Sarafian S. K., Tam M. R., Morse S. A. Gonococcal protein I-specific opsonic IgG in normal human serum. J Infect Dis. 1983 Dec;148(6):1025–1032. doi: 10.1093/infdis/148.6.1025. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Mandrell R. E., Jarvis G. A. Elaboration of a 3.6-kilodalton lipooligosaccharide, antibody against which is absent from human sera, is associated with serum resistance of Neisseria gonorrhoeae. Infect Immun. 1985 Dec;50(3):672–677. doi: 10.1128/iai.50.3.672-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Williams G. D., Pier G. B. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982 Jan;128(1):13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawara R. J., Prato C., Sippel J. E. Monoclonal antibodies against Neisseria meningitidis lipopolysaccharide. Infect Immun. 1983 Dec;42(3):863–868. doi: 10.1128/iai.42.3.863-868.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawara R. J. Recognition of serogroup A Neisseria meningitidis serotype antigens by human antisera. Infect Immun. 1985 Apr;48(1):23–28. doi: 10.1128/iai.48.1.23-28.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Boykins R., Frasch C. E. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983 Aug;155(2):498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977 Nov;18(2):424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Type-specific antigens of group A Neisseria meningitidis: lipopolysaccharide and heat-modifiable outer membrane proteins. Infect Immun. 1980 May;28(2):451–458. doi: 10.1128/iai.28.2.451-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Pennington C. L., Artenstein M. S. Human antibody response to three meningococcal outer membrane antigens: comparison by specific hemagglutination assays. Infect Immun. 1974 Nov;10(5):975–984. doi: 10.1128/iai.10.5.975-984.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]