Abstract
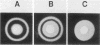
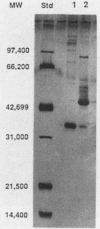
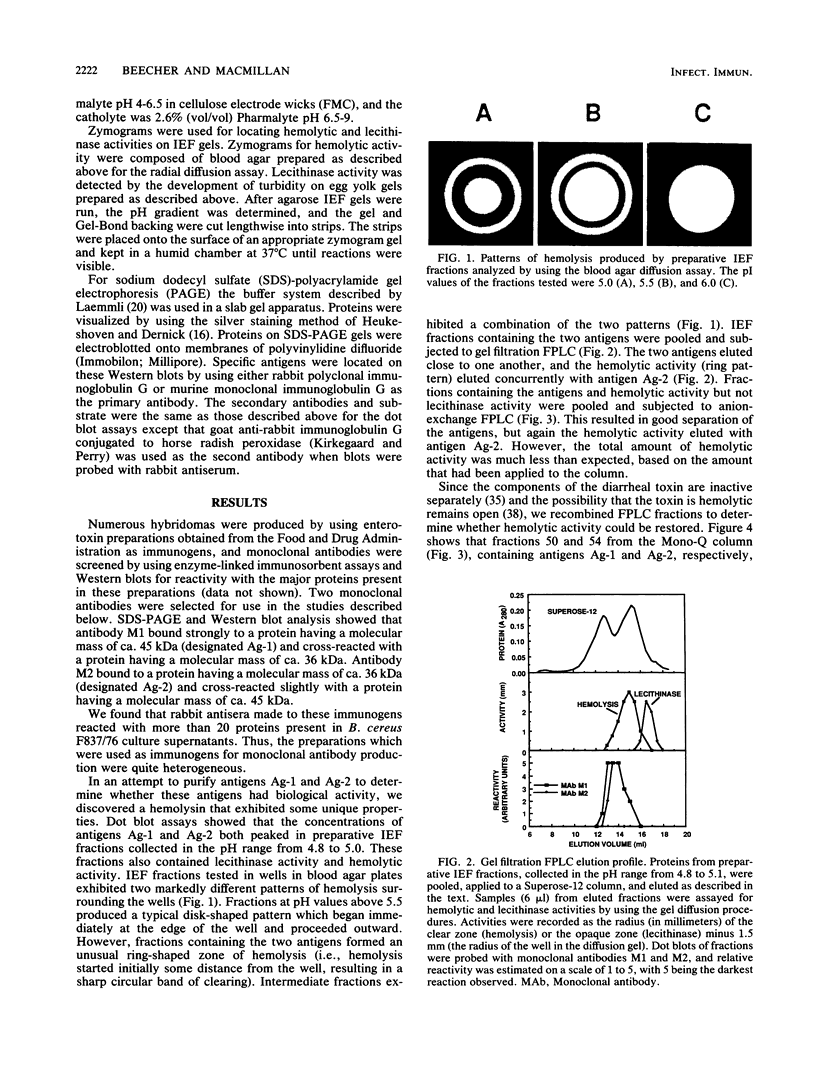
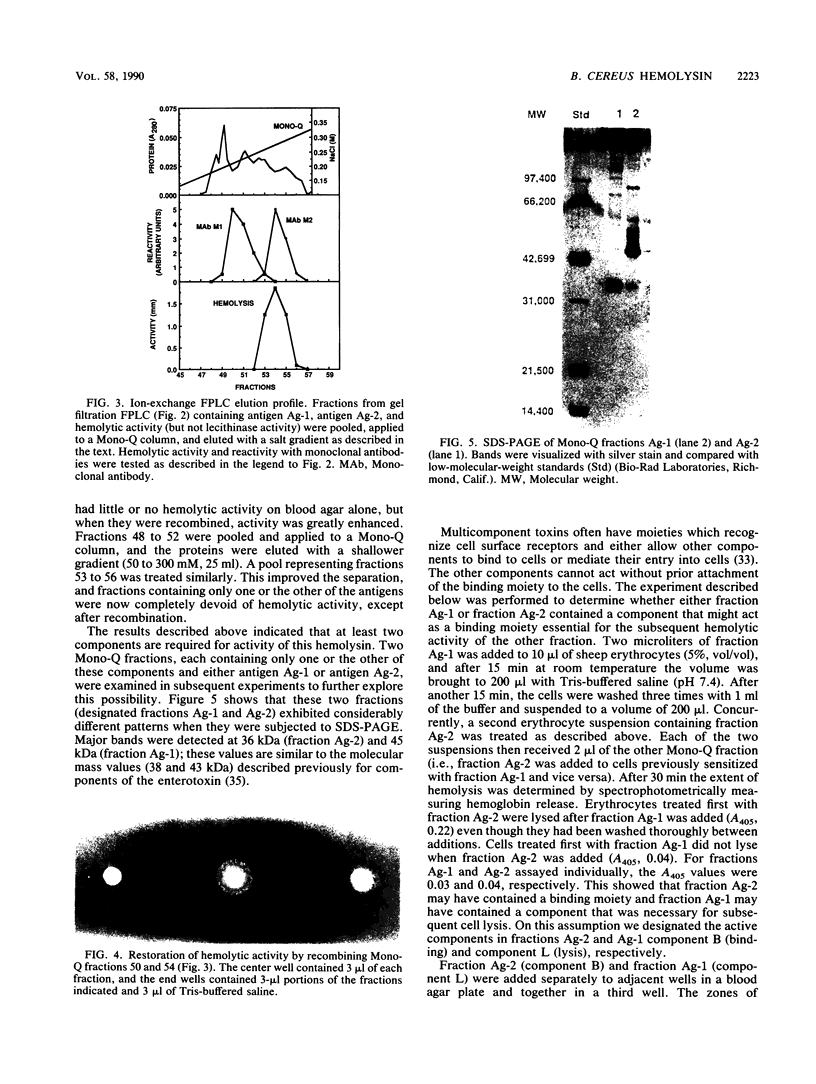
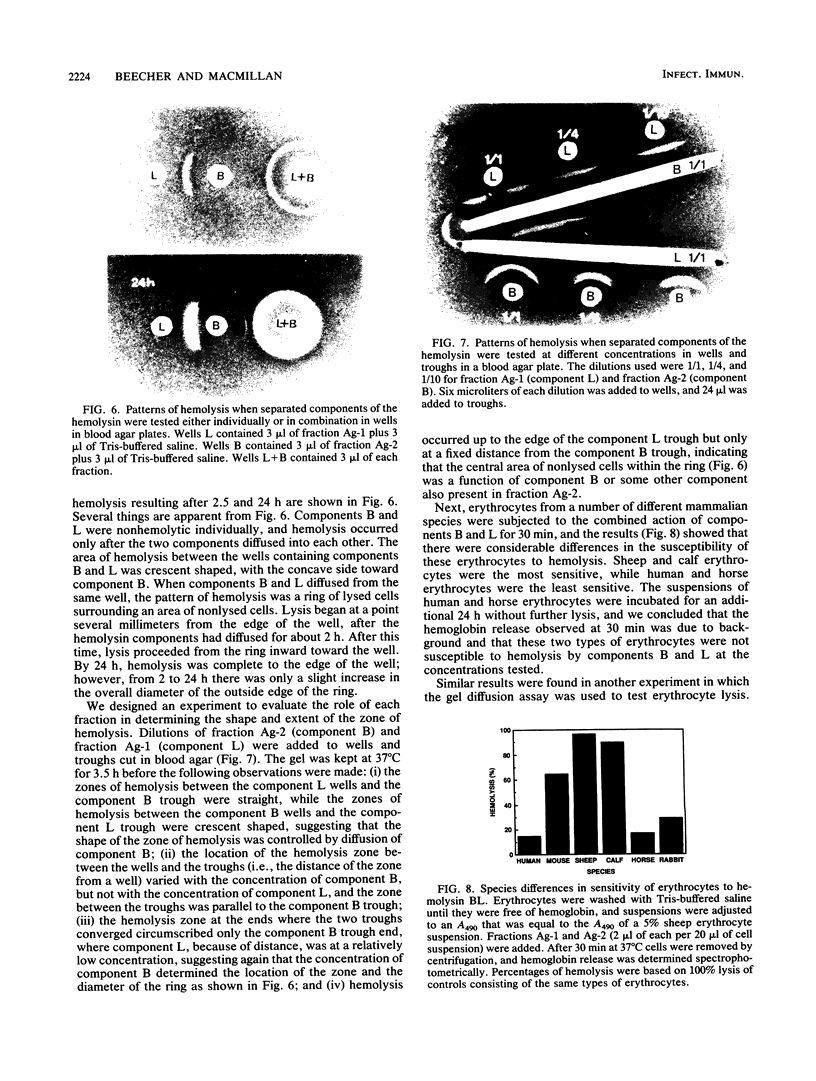
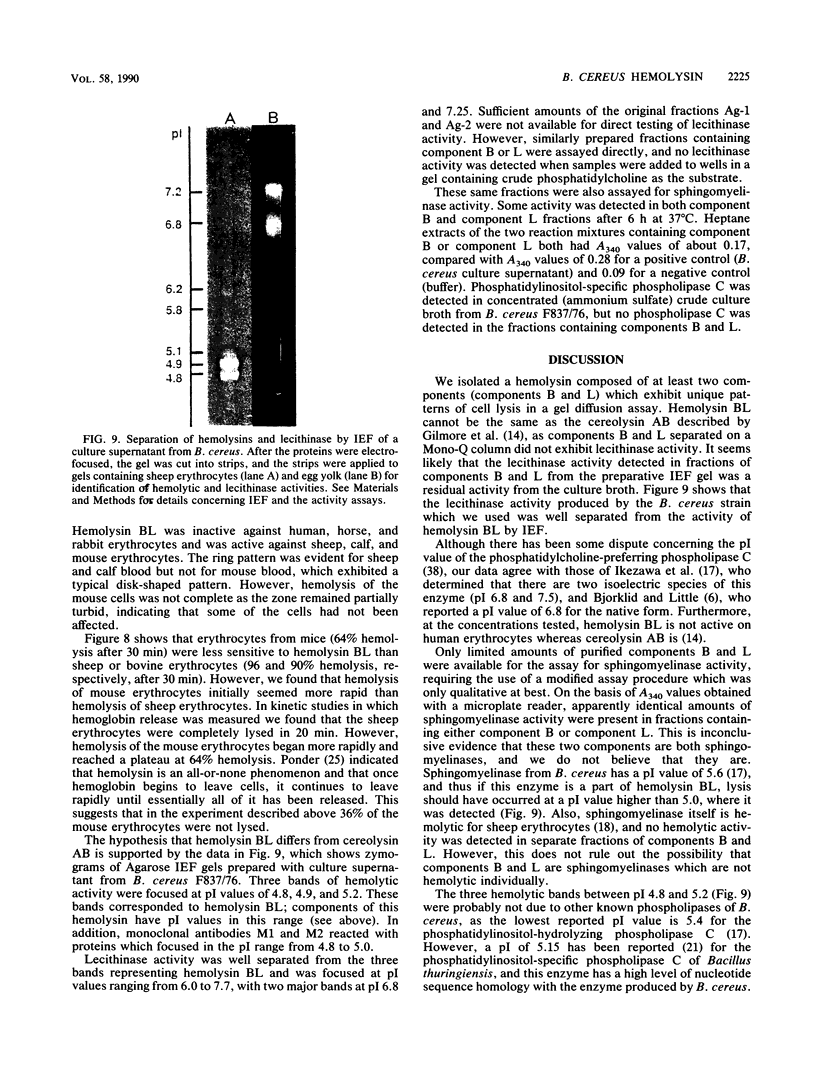
A procedure combining isoelectric focusing (Sephadex IEF) and fast protein liquid chromatography (Superose 12; Mono-Q) removed hemolytic activity (presumably a contaminant) from partially purified preparations of the multicomponent diarrheal enterotoxin produced by Bacillus cereus. However, when the separated fractions were recombined, hemolytic activity was restored, suggesting that hemolysis is a property of the enterotoxin components. Combined fractions exhibited a unique ring pattern in gel diffusion assays in blood agar. During diffusion of the hemolysin from an agar well, the erythrocytes closest to the well were not lysed initially. After diffusion, hemolysis was observed as a sharp ring beginning several millimeters away from the edge of the well. With time the cells closer to the well were also lysed. This novel hemolysin consists of a protein (component B) which binds to or alters cells, allowing subsequent lysis by a second protein (component L). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, isoelectric focusing, and Western blot analysis showed that hemolysin BL has properties similar to those described previously for the enterotoxin and that both components are distinct from cereolysin and cereolysin AB.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernheimer A. W. Assay of hemolytic toxins. Methods Enzymol. 1988;165:213–217. doi: 10.1016/s0076-6879(88)65033-6. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Grushoff P. Cereolysin: production, purification and partial characterization. J Gen Microbiol. 1967 Jan;46(1):143–150. doi: 10.1099/00221287-46-1-143. [DOI] [PubMed] [Google Scholar]
- Bitsaev A. R., Ezepchuk Iu V. Molekuliarnaia priroda patogennogo deistviia, vyzyvaemogo B. cereus. Mol Gen Mikrobiol Virusol. 1987 Jul;(7):18–23. [PubMed] [Google Scholar]
- Bjørklid E., Little C. The isoelectric point of phospholipase C from Bacillus cereus. FEBS Lett. 1980 May 5;113(2):161–163. doi: 10.1016/0014-5793(80)80582-5. [DOI] [PubMed] [Google Scholar]
- Coolbaugh J. C., Williams R. P. Production and characterization of two hemolysins of Bacillus cereus. Can J Microbiol. 1978 Nov;24(11):1289–1295. doi: 10.1139/m78-209. [DOI] [PubMed] [Google Scholar]
- Cowell J. L., Grushoff-Kosyk P. S., Bernheimer A. W. Purification of cereolysin and the electrophoretic separation of the active (reduced) and inactive (oxidized) forms of the purified toxin. Infect Immun. 1976 Jul;14(1):144–154. doi: 10.1128/iai.14.1.144-154.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell K. M., Lutz F. Pseudomonas aeruginosa cytotoxin: the influence of sphingomyelin on binding and cation permeability increase in mammalian erythrocytes. Toxicon. 1989;27(5):531–540. doi: 10.1016/0041-0101(89)90114-1. [DOI] [PubMed] [Google Scholar]
- FOSSUM K. SEPARATION OF HEMOLYSIN AND EGG YOLK TURBIDITY FACTOR IN CELL-FREE EXTRACTS OF BACILLUS CEREUS. Acta Pathol Microbiol Scand. 1963;59:400–406. doi: 10.1111/j.1699-0463.1963.tb01810.x. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- García Arribas M. L., Plaza C. J., de la Rosa M. C., Mosso M. A. Characterization of Bacillus cereus strains isolated from drugs and evaluation of their toxins. J Appl Bacteriol. 1988 Mar;64(3):257–264. doi: 10.1111/j.1365-2672.1988.tb03383.x. [DOI] [PubMed] [Google Scholar]
- Gatt S., Dinur T., Barenholz Y. A spectrophotometric method for determination of sphingomyelinase. Biochim Biophys Acta. 1978 Sep 28;530(3):503–507. doi: 10.1016/0005-2760(78)90169-8. [DOI] [PubMed] [Google Scholar]
- Gilmore M. S., Cruz-Rodz A. L., Leimeister-Wächter M., Kreft J., Goebel W. A Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: nucleotide sequence and genetic linkage. J Bacteriol. 1989 Feb;171(2):744–753. doi: 10.1128/jb.171.2.744-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezawa H., Mori M., Ohyabu T., Taguchi R. Studies on sphingomyelinase of Bacillus cereus. I. Purification and properties. Biochim Biophys Acta. 1978 Feb 27;528(2):247–256. [PubMed] [Google Scholar]
- Ikezawa H., Mori M., Taguchi R. Studies on sphingomyelinase of Bacillus cereus: hydrolytic and hemolytic actions on erythrocyte membranes. Arch Biochem Biophys. 1980 Feb;199(2):572–578. doi: 10.1016/0003-9861(80)90315-x. [DOI] [PubMed] [Google Scholar]
- Kennett R. H. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lechner M., Kupke T., Stefanovic S., Götz F. Molecular characterization and sequence of phosphatidylinositol-specific phospholipase C of Bacillus thuringiensis. Mol Microbiol. 1989 May;3(5):621–626. doi: 10.1111/j.1365-2958.1989.tb00209.x. [DOI] [PubMed] [Google Scholar]
- Linder R. Alteration of mammalian membranes by the cooperative and antagonistic actions of bacterial proteins. Biochim Biophys Acta. 1984 Dec 4;779(4):423–435. doi: 10.1016/0304-4157(84)90019-4. [DOI] [PubMed] [Google Scholar]
- Pendleton I. R., Bernheimer A. W., Grushoff P. Purification and partial characterization of hemolysins from Bacillus thuringiensis. J Invertebr Pathol. 1973 Mar;21(2):131–135. doi: 10.1016/0022-2011(73)90192-4. [DOI] [PubMed] [Google Scholar]
- Plommet M. Preparation and purification of gamma-hemolysin of staphylococci. Methods Enzymol. 1988;165:8–16. doi: 10.1016/s0076-6879(88)65005-1. [DOI] [PubMed] [Google Scholar]
- Priest F. G., Goodfellow M., Todd C. A numerical classification of the genus Bacillus. J Gen Microbiol. 1988 Jul;134(7):1847–1882. doi: 10.1099/00221287-134-7-1847. [DOI] [PubMed] [Google Scholar]
- Radola B. J. High-resolution preparative isoelectric focusing. Methods Enzymol. 1984;104:256–275. doi: 10.1016/s0076-6879(84)04094-5. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P., Fox P. C., Berenstein E. H. Methods of enhancing the frequency of antigen-specific hybridomas. Methods Enzymol. 1983;92:17–26. doi: 10.1016/0076-6879(83)92005-0. [DOI] [PubMed] [Google Scholar]
- Slein M. W., Logan G. F. Characterization of the Phospholipases of Bacillus cereus and Their Effects on Erythrocytes, Bone, and Kidney Cells. J Bacteriol. 1965 Jul;90(1):69–81. doi: 10.1128/jb.90.1.69-81.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira W. M., Goepfert J. M. Biological characteristics of an enterotoxin produced by Bacillus cereus. Can J Microbiol. 1975 Aug;21(8):1236–1246. doi: 10.1139/m75-185. [DOI] [PubMed] [Google Scholar]
- Stähli C., Staehelin T., Miggiano V. Spleen cell analysis and optimal immunization for high-frequency production of specific hybridomas. Methods Enzymol. 1983;92:26–36. doi: 10.1016/0076-6879(83)92006-2. [DOI] [PubMed] [Google Scholar]
- Taggart R. T., Samloff I. M. Stable antibody-producing murine hybridomas. Science. 1983 Mar 11;219(4589):1228–1230. doi: 10.1126/science.6402815. [DOI] [PubMed] [Google Scholar]
- Thompson N. E., Ketterhagen M. J., Bergdoll M. S., Schantz E. J. Isolation and some properties of an enterotoxin produced by Bacillus cereus. Infect Immun. 1984 Mar;43(3):887–894. doi: 10.1128/iai.43.3.887-894.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull P. C. Bacillus cereus toxins. Pharmacol Ther. 1981;13(3):453–505. doi: 10.1016/0163-7258(81)90026-7. [DOI] [PubMed] [Google Scholar]
- Turnbull P. C., Jørgensen K., Kramer J. M., Gilbert R. J., Parry J. M. Severe clinical conditions associated with Bacillus cereus and the apparent involvement of exotoxins. J Clin Pathol. 1979 Mar;32(3):289–293. doi: 10.1136/jcp.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull P. C., Kramer J. M., Jørgensen K., Gilbert R. J., Melling J. Properties and production characteristics of vomiting, diarrheal, and necrotizing toxins of Bacillus cereus. Am J Clin Nutr. 1979 Jan;32(1):219–228. doi: 10.1093/ajcn/32.1.219. [DOI] [PubMed] [Google Scholar]
- Turnbull P. C., Kramer J. M. Non-gastrointestinal Bacillus cereus infections: an analysis of exotoxin production by strains isolated over a two-year period. J Clin Pathol. 1983 Oct;36(10):1091–1096. doi: 10.1136/jcp.36.10.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull P. C. Studies on the production of enterotoxins by Bacillus cereus. J Clin Pathol. 1976 Oct;29(10):941–948. doi: 10.1136/jcp.29.10.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. C., Chang M. H., Fan J. Y. Incidence and characterization of Bacillus cereus isolates contaminating dairy products. Appl Environ Microbiol. 1988 Mar;54(3):699–702. doi: 10.1128/aem.54.3.699-702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]