Abstract
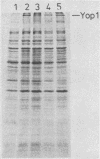
HEp-2 cell monolayers were challenged with genetic variants of Yersinia pseudotuberculosis YPIII(pIB1) and Yersinia enterocolitica W22708(pYL4). Both strains were represented by (i) variants harboring the 70-kilobase virulence plasmid, (ii) their isogenic plasmid-cured derivatives, and (iii) two transposon mutants constructed by insertional inactivation of the plasmid genes encoding outer membrane protein Yop1 and Ca2+ dependency in strains YPIII(pIB1) and W22708(pYL4). When the HEp-2 cells were pretreated with recombinant alpha interferon subtype A, all invasive variants of Y. enterocolitica and Y. pseudotuberculosis, except those variants which expressed Yop1, showed a significantly reduced ability to localize intracellularly. The anti-invasive effect of interferon was abolished when the gene was expressed or when a sterile filtered sonic extract of a Yop1-producing strain was added to the cell cultures. To obtain further evidence of a potential role of Yop1, a DNA fragment encoding Yop1 cloned into the vector pBR322 was used. After introduction of the resultant recombinant plasmid pYMS2 into the plasmid-cured variant YPIII and the Yop1-negative transposon mutant YPIII(pIB102) of Y. pseudotuberculosis, both transformants regained the ability to produce Yop1 and showed complete inhibition of the interferon effect. Moreover, the sterile sonic extract of an Escherichia coli strain, which carried pYMS2, neutralized the anti-invasive effect of interferon. The results provide direct genetic evidence that Yop1 mediates inhibition of the anti-invasive effect of interferon in HEp-2 cell cultures. The results also demonstrated that Yop1 itself reduces the ability of Yersinia spp. to localize intracellularly in HEp-2 cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balligand G., Laroche Y., Cornelis G. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect Immun. 1985 Jun;48(3):782–786. doi: 10.1128/iai.48.3.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S., Dianzani F., Stanton G. J. General considerations of the interferon system. Tex Rep Biol Med. 1981;41:1–12. [PubMed] [Google Scholar]
- Bukholm G., Berdal B. P., Haug C., Degré M. Mouse fibroblast interferon modifies Salmonella typhimurium infection in infant mice. Infect Immun. 1984 Jul;45(1):62–66. doi: 10.1128/iai.45.1.62-66.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukholm G., Bergh M., Degré M. Interferon-induced protein synthesis inhibits endocytosis of bacteria in epithelial cells. J Interferon Res. 1987 Aug;7(4):409–417. doi: 10.1089/jir.1987.7.409. [DOI] [PubMed] [Google Scholar]
- Bukholm G., Degré M. Effect of human gamma interferon on invasiveness of Salmonella typhimurium in HEp-2 cell cultures. J Interferon Res. 1985 Winter;5(1):45–53. doi: 10.1089/jir.1985.5.45. [DOI] [PubMed] [Google Scholar]
- Bukholm G., Degré M. Effect of human leukocyte interferon on invasiveness of Salmonella species in HEp-2 cell cultures. Infect Immun. 1983 Dec;42(3):1198–1202. doi: 10.1128/iai.42.3.1198-1202.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukholm G., Degré M. Shiga toxin inhibits the anti-invasive effect of interferons. J Infect Dis. 1988 Apr;157(4):849–850. doi: 10.1093/infdis/157.4.849. [DOI] [PubMed] [Google Scholar]
- Bukholm G., Degré M., Whitaker-Dowling P. Interferon treatment reduces endocytosis of virus and facultatively intracellular bacteria in various cell lines. J Interferon Res. 1990 Feb;10(1):83–89. doi: 10.1089/jir.1990.10.83. [DOI] [PubMed] [Google Scholar]
- Bukholm G., Johansen B. V., Namork E., Lassen J. Bacterial adhesiveness and invasiveness in cell culture monolayer. Acta Pathol Microbiol Immunol Scand B. 1982 Dec;90(6):403–408. doi: 10.1111/j.1699-0463.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Bölin I., Forsberg A., Norlander L., Skurnik M., Wolf-Watz H. Identification and mapping of the temperature-inducible, plasmid-encoded proteins of Yersinia spp. Infect Immun. 1988 Feb;56(2):343–348. doi: 10.1128/iai.56.2.343-348.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Portnoy D. A., Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985 Apr;48(1):234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect Immun. 1984 Jan;43(1):72–78. doi: 10.1128/iai.43.1.72-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Laroche Y., Balligand G., Sory M. P., Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987 Jan-Feb;9(1):64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Sory M. P., Laroche Y., Derclaye I. Genetic analysis of the plasmid region controlling virulence in Yersinia enterocolitica 0:9 by Mini-Mu insertions and lac gene fusions. Microb Pathog. 1986 Aug;1(4):349–359. doi: 10.1016/0882-4010(86)90067-7. [DOI] [PubMed] [Google Scholar]
- Dahl H., Degré M. A micro assay for mouse and human interferon. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):863–870. doi: 10.1111/j.0365-5563.1973.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Degré M., Bukholm G., Czarniecki C. W. In vitro treatment of HEp-2 cells with human tumor necrosis factor-alpha and human interferons reduces invasiveness of Salmonella typhimurium. J Biol Regul Homeost Agents. 1989 Jan-Mar;3(1):1–7. [PubMed] [Google Scholar]
- Ferber D. M., Brubaker R. R. Plasmids in Yersinia pestis. Infect Immun. 1981 Feb;31(2):839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg A., Wolf-Watz H. The virulence protein Yop5 of Yersinia pseudotuberculosis is regulated at transcriptional level by plasmid-plB1-encoded trans-acting elements controlled by temperature and calcium. Mol Microbiol. 1988 Jan;2(1):121–133. [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T., Wohlhieter J. A. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect Immun. 1980 Jun;28(3):1044–1047. doi: 10.1128/iai.28.3.1044-1047.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goguen J. D., Yother J., Straley S. C. Genetic analysis of the low calcium response in Yersinia pestis mu d1(Ap lac) insertion mutants. J Bacteriol. 1984 Dec;160(3):842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Algermissen B., Laufs R. Genetically manipulated virulence of Yersinia enterocolitica. Infect Immun. 1984 Oct;46(1):105–110. doi: 10.1128/iai.46.1.105-110.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R. Determinants for thermoinducible cell binding and plasmid-encoded cellular penetration detected in the absence of the Yersinia pseudotuberculosis invasin protein. Infect Immun. 1989 Jul;57(7):1998–2005. doi: 10.1128/iai.57.7.1998-2005.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Izadkhah Z., Mandel A. D., Sonnenfeld G. Effect of treatment of mice with sera containing gamma interferon on the course of infection with Salmonella typhimurium strain LT-2. J Interferon Res. 1980 Fall;1(1):137–145. doi: 10.1089/jir.1980.1.137. [DOI] [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skarpeid H. J. Temperature-inducible surface fibrillae associated with the virulence plasmid of Yersinia enterocolitica and Yersinia pseudotuberculosis. Infect Immun. 1985 Feb;47(2):561–566. doi: 10.1128/iai.47.2.561-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skurnik M., Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987 Sep;55(9):2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen J., Kapperud G. Serotype-related HEp-2 cell interaction of Yersinia enterocolitica. Infect Immun. 1986 Apr;52(1):85–89. doi: 10.1128/iai.52.1.85-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C. J., Hwang W. S., Pai C. H. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect Immun. 1987 May;55(5):1176–1183. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesel D. W., Hess C. B., Cho Y. J., Klimpel K. D., Klimpel G. R. Natural and recombinant interferons inhibit epithelial cell invasion by Shigella spp. Infect Immun. 1986 Jun;52(3):828–833. doi: 10.1128/iai.52.3.828-833.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Vwa+ phenotype of Yersinia enterocolitica. Infect Immun. 1983 Apr;40(1):166–171. doi: 10.1128/iai.40.1.166-171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Falkow S. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J Bacteriol. 1981 Dec;148(3):877–883. doi: 10.1128/jb.148.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Martinez R. J. Role of a plasmid in the pathogenicity of Yersinia species. Curr Top Microbiol Immunol. 1985;118:29–51. doi: 10.1007/978-3-642-70586-1_3. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Wolf-Watz H. Virulence plasmid-associated HeLa cell induced cytotoxicity of Yersinia pseudotuberculosis. Microb Pathog. 1986 Jun;1(3):229–240. doi: 10.1016/0882-4010(86)90047-1. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Hale T. L., Dammin G. J., Kapfer C., Collins H. H., Jr, Formal S. B. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun. 1983 Mar;39(3):1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann D. A., Devenish J. A. Relationship of HeLa cell infectivity to biochemical, serological, and virulence characteristics of Yersinia enterocolitica. Infect Immun. 1982 Feb;35(2):497–506. doi: 10.1128/iai.35.2.497-506.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M., Fauchère J. L., Berche P. Role of virulence-associated plasmid in the uptake and killing of Yersinia pseudotuberculosis by resident macrophages. Ann Inst Pasteur Microbiol. 1985 Nov-Dec;136B(3):283–294. doi: 10.1016/s0769-2609(85)80074-0. [DOI] [PubMed] [Google Scholar]
- Skurnik M., Bölin I., Heikkinen H., Piha S., Wolf-Watz H. Virulence plasmid-associated autoagglutination in Yersinia spp. J Bacteriol. 1984 Jun;158(3):1033–1036. doi: 10.1128/jb.158.3.1033-1036.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M., Wolf-Watz H. Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp. Mol Microbiol. 1989 Apr;3(4):517–529. doi: 10.1111/j.1365-2958.1989.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Bowmer W. S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986 Feb;51(2):445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T., Nurmi T., Mäki M., Skurnik M., Sundqvist C., Granfors K., Grönroos P. Plasmids in Yersinia enterocolitica serotypes O:3 and O:9: correlation with epithelial cell adherence in vitro. Infect Immun. 1981 Sep;33(3):870–876. doi: 10.1128/iai.33.3.870-876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Portnoy D. A., Bölin I., Falkow S. Transfer of the virulence plasmid of Yersinia pestis to Yersinia pseudotuberculosis. Infect Immun. 1985 Apr;48(1):241–243. doi: 10.1128/iai.48.1.241-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]