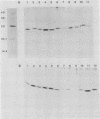
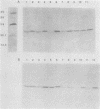
Abstract
We investigated the binding of antibodies raised against four different Haemophilus influenzae type b (Hib) plus antigen preparations to the native pili and denatured pilins of 21 Hib isolates. Antibodies against live piliated Hib M43p+, adsorbed with a nonpiliated variant to remove nonpilus antibodies, bound to 18 of the 21 piliated Hib isolates in immunodot assays but failed to recognize the denatured pilins from any of the strains in Western immunoblot assays. Similarly, antibodies against purified native pili of strain E1ap+ bound to 11 of 21 piliated strains in immunodot assays but to only 2 of 21 piliated strains in Western blot assays. The native pili of all 21 strains were recognized by one or both of the antisera. These observations suggest that the immunodominant epitopes of native Hib pili are dependent on conformation and are moderately conserved. In contrast, antibodies against denatured M43p+ pilin or against a peptide derived from amino acids 5 through 17 of M43p+ pilin failed to bind to native pili from any of the 21 piliated isolates on immunodot assay. However, both sera recognized the denatured pilins from all the piliated strains on Western blot assay. These data indicate that the immunodominant epitopes of denatured pilins are highly conserved among different strains of Hib but are unavailable on intact pili for antibody binding.
Full text
PDF

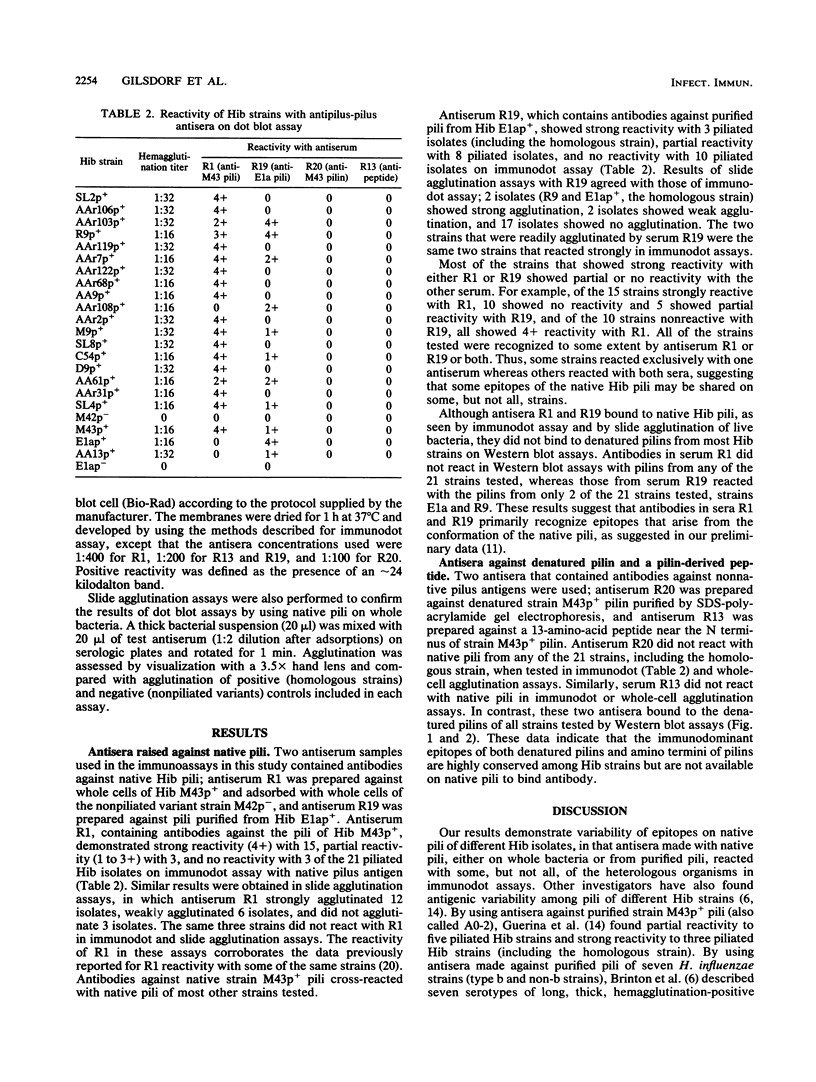
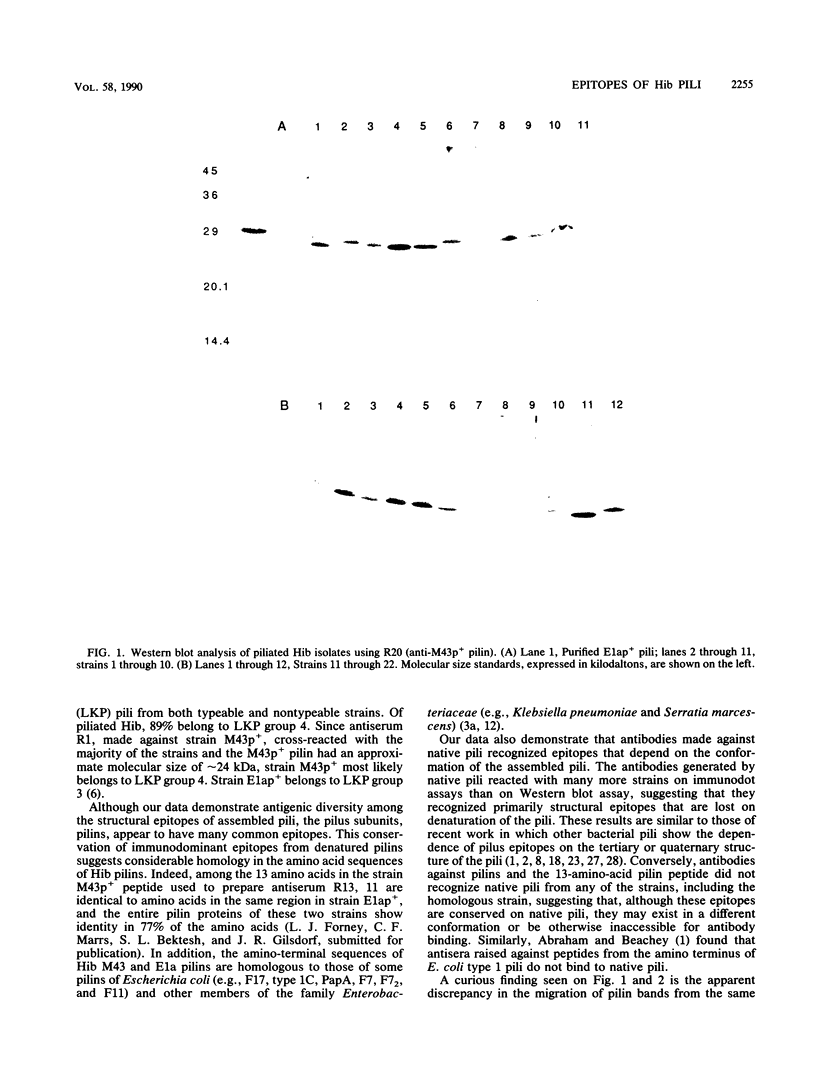


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Beachey E. H. Assembly of a chemically synthesized peptide of Escherichia coli type 1 fimbriae into fimbria-like antigenic structures. J Bacteriol. 1987 Jun;169(6):2460–2465. doi: 10.1128/jb.169.6.2460-2465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. J., Mattick J. S., Cox P. T., Kristo C. L., Egerton J. R. Western blot (immunoblot) analysis of the fimbrial antigens of Bacteroides nodosus. J Bacteriol. 1987 Sep;169(9):4018–4023. doi: 10.1128/jb.169.9.4018-4023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Smith D. H., Ingram D. L., Wilkins J., Wehrle P. F., Howie V. M. Antibody of polyribophate of Haemophilus influenzae type b in infants and children: effect of immunization with polyribophosphate. J Infect Dis. 1977 Aug;136 (Suppl):S57–S62. doi: 10.1093/infdis/136.supplement.s57. [DOI] [PubMed] [Google Scholar]
- Armes L. G., Forney L. J. The complete primary structure of pilin from Haemophilus influenzae type b strain Eagan. J Protein Chem. 1990 Feb;9(1):45–52. doi: 10.1007/BF01024983. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Bessen D., Fischetti V. A. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect Immun. 1988 Oct;56(10):2666–2672. doi: 10.1128/iai.56.10.2666-2672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr, Carter M. J., Derber D. B., Kar S., Kramarik J. A., To A. C., To S. C., Wood S. W. Design and development of pilus vaccines for Haemophilus influenzae diseases. Pediatr Infect Dis J. 1989 Jan;8(1 Suppl):S54–S61. [PubMed] [Google Scholar]
- Connor E. M., Loeb M. R. A hemadsorption method for detection of colonies of Haemophilus influenzae type b expressing fimbriae. J Infect Dis. 1983 Nov;148(5):855–860. doi: 10.1093/infdis/148.5.855. [DOI] [PubMed] [Google Scholar]
- Emery D. L., Stewart D. J., Clark B. L. The structural integrity of pili from Bacteroides nodosus is required to elicit protective immunity against foot-rot in sheep. Aust Vet J. 1984 Jul;61(7):237–238. doi: 10.1111/j.1751-0813.1984.tb05999.x. [DOI] [PubMed] [Google Scholar]
- Eskola J., Peltola H., Takala A. K., Käyhty H., Hakulinen M., Karanko V., Kela E., Rekola P., Rönnberg P. R., Samuelson J. S. Efficacy of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine in infancy. N Engl J Med. 1987 Sep 17;317(12):717–722. doi: 10.1056/NEJM198709173171201. [DOI] [PubMed] [Google Scholar]
- Gilsdorf J. R., Ferrieri P. Adherence of Haemophilus influenzae to human epithelial cells. Scand J Infect Dis. 1984;16(3):271–278. doi: 10.3109/00365548409070400. [DOI] [PubMed] [Google Scholar]
- Gilsdorf J. R., Forney L. J., LiPuma J. J. Reactivity of Haemophilus influenzae type b anti-pili antibodies. Microb Pathog. 1989 Oct;7(4):311–316. doi: 10.1016/0882-4010(89)90049-1. [DOI] [PubMed] [Google Scholar]
- Gilsdorf J. R., Marrs C. F., McCrea K. W., Forney L. J. Cloning, expression, and sequence analysis of the Haemophilus influenzae type b strain M43p+ pilin gene. Infect Immun. 1990 Apr;58(4):1065–1072. doi: 10.1128/iai.58.4.1065-1072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerina N. G., Langermann S., Clegg H. W., Kessler T. W., Goldman D. A., Gilsdorf J. R. Adherence of piliated Haemophilus influenzae type b to human oropharyngeal cells. J Infect Dis. 1982 Oct;146(4):564–564. doi: 10.1093/infdis/146.4.564. [DOI] [PubMed] [Google Scholar]
- Guerina N. G., Langermann S., Schoolnik G. K., Kessler T. W., Goldmann D. A. Purification and characterization of Haemophilus influenzae pili, and their structural and serological relatedness to Escherichia coli P and mannose-sensitive pili. J Exp Med. 1985 Jan 1;161(1):145–159. doi: 10.1084/jem.161.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Robertson S. M., Gulig P. A., Frisch C. F., Haanes E. J. Immunoprotection of rats against Haemophilus influenzae type B disease mediated by monoclonal antibody against a haemophilus outer-membrane protein. Lancet. 1982 Feb 13;1(8268):366–368. doi: 10.1016/s0140-6736(82)91394-0. [DOI] [PubMed] [Google Scholar]
- Karch H., Leying H., Goroncy-Bermes P., Kroll H. P., Opferkuch W. Three-dimensional structure of fimbriae determines specificity of immune response. Infect Immun. 1985 Nov;50(2):517–522. doi: 10.1128/iai.50.2.517-522.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Gilsdorf J. R. Structural and serological relatedness of Haemophilus influenzae type b pili. Infect Immun. 1988 May;56(5):1051–1056. doi: 10.1128/iai.56.5.1051-1056.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Zachary A. L., Smith D. H. Isolation and partial characterization of outer and inner membranes from encapsulated Haemophilus influenzae type b. J Bacteriol. 1981 Jan;145(1):596–604. doi: 10.1128/jb.145.1.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels R. H., Stonebraker F. E., Robbins J. B. Use of antiserum agar for detection of Haemophilus influenzae type b in the pharynx. Pediatr Res. 1975 May;9(5):513–516. doi: 10.1203/00006450-197505000-00010. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., van der Heide H. G., ter Avest A. R., Welinder K. G., Livey I., van der Zeijst B. A., Gaastra W. Characterization of fimbrial subunits from Bordetella species. Microb Pathog. 1987 Jun;2(6):473–484. doi: 10.1016/0882-4010(87)90054-4. [DOI] [PubMed] [Google Scholar]
- Murphy T. F., Nelson M. B., Dudas K. C., Mylotte J. M., Apicella M. A. Identification of a specific epitope of Haemophilus influenzae on a 16,600-dalton outer membrane protein. J Infect Dis. 1985 Dec;152(6):1300–1307. doi: 10.1093/infdis/152.6.1300. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pichichero M. E., Loeb M., Anderson, Smith D. H. Do pili play a role in pathogenicity of Haemophilus influenzae type B? Lancet. 1982 Oct 30;2(8305):960–962. doi: 10.1016/s0140-6736(82)90161-1. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Hanley J., Clubb L., Fanning S. Detection of pilus subunits (pilins) and filaments by using anti-P pilin antisera. Infect Immun. 1988 Sep;56(9):2330–2335. doi: 10.1128/iai.56.9.2330-2335.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferli D. M., Abraham S. N., Beachey E. H. Use of monoclonal antibodies to probe subunit- and polymer-specific epitopes of 987P fimbriae of Escherichia coli. Infect Immun. 1987 Apr;55(4):923–930. doi: 10.1128/iai.55.4.923-930.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Granoff D. M. Human antibody responses to lipopolysaccharide after meningitis due to Haemophilus influenzae type b. J Infect Dis. 1982 Feb;145(2):181–190. doi: 10.1093/infdis/145.2.181. [DOI] [PubMed] [Google Scholar]
- Stull T. L., Mendelman P. M., Haas J. E., Schoenborn M. A., Mack K. D., Smith A. L. Characterization of Haemophilus influenzae type b fimbriae. Infect Immun. 1984 Dec;46(3):787–796. doi: 10.1128/iai.46.3.787-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]