Abstract
The role of putative humoral factors, known as phosphatonins, in phosphate homeostasis and the relationship between phosphate handling by the kidney and gastrointestinal tract are incompletely understood. Matrix extracellular phosphoglycoprotein (MEPE), one of several candidate phosphatonins, promotes phosphaturia, but whether it also affects intestinal phosphate absorption is unknown. Here, using the in situ intestinal loop technique, we demonstrated that short-term infusion of MEPE inhibits phosphate absorption in the jejunum but not the duodenum. Simultaneous measurement of urinary phosphate excretion suggests that the phosphaturic action of MEPE correlates with a significant reduction in the protein levels of the renal sodium-phosphate co-transporter NaPi-IIa in the proximal convoluted tubules of the outer renal cortex, assessed by Western blotting and immunohistochemistry. This short-term inhibitory effect of MEPE on renal and intestinal phosphate handling occurred without any changes in circulating levels of parathyroid hormone, 1,25-dihydroxyvitamin D3, or fibroblast growth factor 23. Taken together, these findings suggest that MEPE is a candidate phosphatonin involved in phosphate homeostasis, acting in both the kidney and the gastrointestinal tract.
In the past 5 years, our understanding of the different mechanisms maintaining phosphate homeostasis has increased significantly, and recent attention has focused on the novel circulating factors called phosphatonins and their potential role in controlling renal phosphate excretion. These factors were originally identified in mesenchymal tumors from patients with tumor-induced osteomalacia, a disorder associated with renal phosphate wasting. So far they have been identified as fibroblast growth factor 23 (FGF-23), FGF-7, secreted frizzled related protein 4 (sFRP-4), and matrix extracellular phosphoglycoprotein (MEPE). All of them have been shown to inhibit renal phosphate absorption in vitro and in vivo; however, only FGF-23 and sFRP-4 are considered to play an important role in regulating (by inhibiting) the synthesis of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3; for comprehensive reviews, see references1–4]. In contrast to the kidney, the role that these phosphatonins might have in regulating intestinal phosphate absorption has received limited attention.
Recently, several groups, including our own, have begun to reevaluate the processes and regulatory mechanisms involved in intestinal phosphate absorption and the relationship of intestinal to renal phosphate handling.5 Using both in vivo and in vitro techniques, we and others have found clear differences in the regional profile of intestinal phosphate absorption in rats compared with mice.6,7 Indeed, the axial pattern of phosphate absorption in the rat is more similar to that reported in humans; therefore, the rat is perhaps a more appropriate animal model of phosphate absorption along the gastrointestinal tract in vivo.
Early studies using intestinal brush border membrane (BBM) vesicles to investigate phosphate uptake indicated that phosphate absorption across this membrane involved both sodium-dependent and sodium-independent pathways. The sodium-dependent transporter was later identified as NaPi-IIb,8,9 and it is now generally accepted that this protein is largely responsible for regulating phosphate transfer across the enterocyte BBM9,10 Indeed, it was shown recently that this pathway constitutes approximately 78% of inorganic phosphate absorption at physiological luminal phosphate concentrations.11
The importance of the small intestine in the regulation of phosphate homeostasis was highlighted recently by Berndt et al.,12 who performed elegant studies demonstrating the interplay between the small intestine and the kidney. They provided evidence for the existence of an “entero-renal axis” controlling phosphate balance, which may influence short-term renal phosphate excretion after ingestion of dietary phosphate, mediated by an as-yet-unidentified “intestinal phosphatonin.”
Of the classical candidate phosphatonins, only FGF-23 has been reported to influence intestinal phosphate transport. It has been shown that FGF-23 injection into normal mice causes a reduction in plasma 1,25(OH)2D3 with concomitant inhibition of intestinal (using BBM vesicles) phosphate transport and NaPi-IIb protein expression.13 Moreover, injection of FGF-23 into vitamin D receptor (VDR) null mice does not elicit any change in intestinal phosphate transport activity or in transporter expression, indicating that the action of FGF-23 depends on the VDR.13 Conversely, administration of 1,25(OH)2D3 to mice results in a dramatic increase in serum FGF-23 levels, and it has been proposed that these two hormones are part of a kidney-intestine-bone axis that controls phosphate balance.14
Our results demonstrate that MEPE can acutely inhibit both renal and intestinal phosphate transport and that MEPE might also play a role in normal phosphate balance; however, because MEPE fails to elicit changes in circulating parathyroid hormone (PTH), 1,25(OH)2D3, or FGF-23, its short-term action must occur via a mechanism that is independent of the classical modulators of phosphate homeostasis.
RESULTS
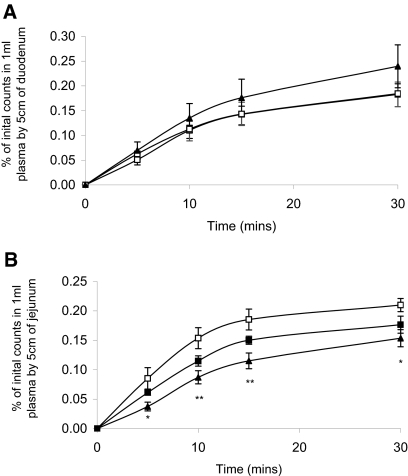
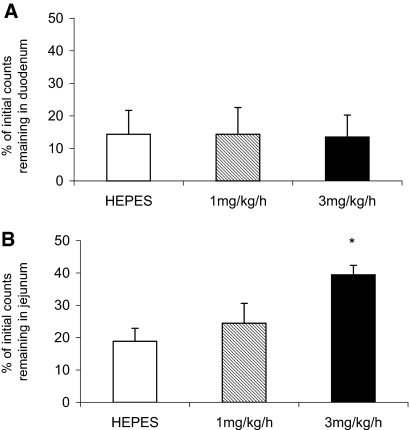
Using the in situ intestinal loop technique, we demonstrated for the first time that infusion of recombinant human MEPE significantly and dosage-dependently inhibits transepithelial phosphate absorption by the small intestine (Figure 1). The inhibitory action of MEPE on intestinal phosphate absorption seems to be confined to the jejunum (Figure 1B), because infusion of MEPE for 3 h had no significant effect on transepithelial phosphate transfer by the duodenum (Figure 1A). This is confirmed by the finding of a dosage-dependent increase in the percentage of phosphate remaining in the lumen of the jejunal but not duodenal segment after MEPE infusion (Figure 2).
Figure 1.
(A and B) In vivo uptake of phosphate by the duodenum (A) and jejunum (B) in rats infused with HEPES (□) or 1 mg/kg per h MEPE (▪) or 3 mg/kg per h MEPE (▴) for 3 h. Data are means ± SEM of six experiments per region, as a percentage of the initial counts transferred from 5 cm of small intestine into 1 ml of blood. *P < 0.05 and **P < 0.01 compared with HEPES control using an ANOVA with post hoc comparisons performed using the Bonferroni multiple comparisons test.
Figure 2.
(A and B) Retention of phosphate in the duodenum (A) and jejunum (B) in rats infused with HEPES, 1 mg/kg per h MEPE or 3 mg/kg per h MEPE for 3 h. Data are means ± SEM of six experiments per region, as a percentage of the initial counts remaining in the small intestine segment. *P < 0.05 compared with HEPES control using an ANOVA with post hoc comparisons performed using the Bonferroni multiple comparisons test.
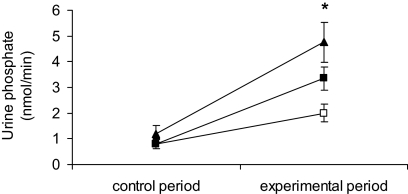
Urinary phosphate excretion did not differ among the three experimental groups during the control period; however, there was a dosage-dependent effect of MEPE on urinary phosphate excretion (Figure 3). Infusion of the low dosage of MEPE tended to increase excretion, but this was not statistically significant, whereas the higher dosage of 3 mg/kg per h significantly increased urinary phosphate excretion.
Figure 3.
Urinary phosphate excretion in rats infused with HEPES (□) or 1 mg/kg per h MEPE (▪) or 3 mg/kg per h MEPE (▴). Data are means ± SEM; n = 6. Comparison between the changes in phosphate excretion in the MEPE-treated groups with that in the vehicle group was made using an ANOVA with post hoc comparisons performed using the Bonferroni multiple comparisons test. *P < 0.05.
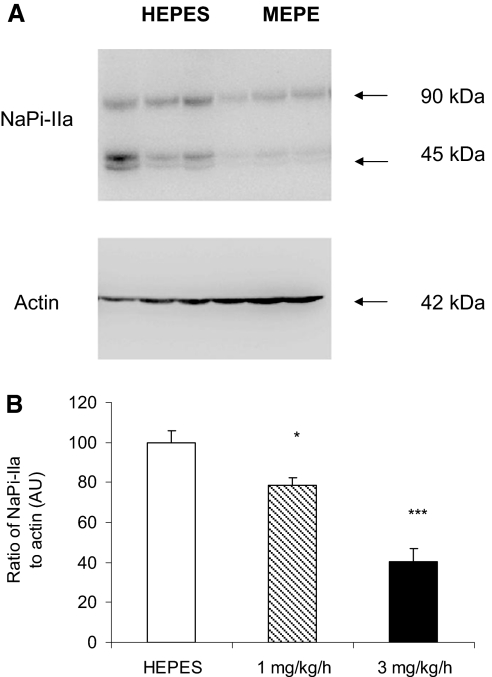
Western blotting revealed that MEPE infusion significantly reduced the levels of NaPi-IIa protein in BBM vesicles prepared from the renal cortex. As with transepithelial phosphate absorption by the small intestine and urinary phosphate excretion, the effect on NaPi-IIa occurred in a dosage-dependent manner (Figure 4). Although low-dosage MEPE significantly decreased NaPi-IIa protein levels, this effect was not sufficient to influence phosphate excretion significantly. In contrast, the increase in phosphate excretion seen with the higher dosage used in this study can be attributed to the significant decrease in NaPi-IIa protein levels.
Figure 4.
Western blot analysis of NaPi-IIa protein in the kidneys of rats infused with HEPES or MEPE. (A) Representative Western blot of NaPi-IIa in BBM vesicles prepared from HEPES and 3 mg/kg per h MEPE-treated rats. (B) Quantification of NaPi-IIa protein relative to β-actin. Data are means ± SEM of Western blots performed on six individual BBM vesicle samples from each experimental group. The abundance of NaPi-IIa is given as a ratio of NaPi-IIa protein to β-actin protein in arbitrary units (AU). *P < 0.05 and ***P < 0.001 compared with HEPES control using an ANOVA with post hoc comparisons performed using the Bonferroni multiple comparisons test.
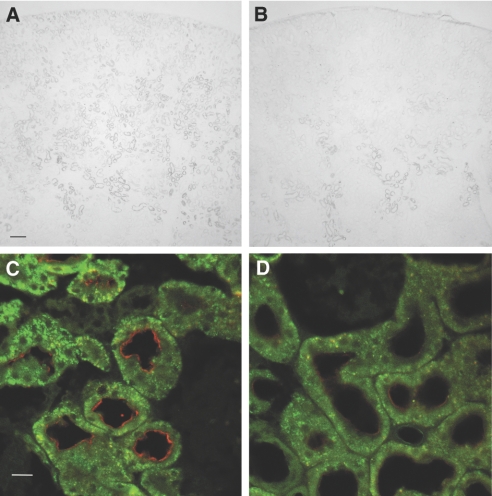
Immunohistochemistry using kidneys from HEPES-infused animals showed the expected brush border staining of NaPi-IIa, which was apparent throughout the proximal tubules of the cortex (Figure 5A). In contrast, in the kidneys of high-dosage MEPE–treated animals, the protein was visibly reduced in the proximal tubules of the outer cortex, and it was detectable in any abundance only in the tubules within the medullary rays (Figure 5B). High-power confocal images, taken in regions of the outer cortex where glomeruli were within the field of view, revealed the presence of sparse positive staining for NaPi-IIa in proximal tubules of the MEPE-treated animals. In addition, in the small number of tubules in which the protein was detectable, the levels were visibly reduced (Figure 5D) when compared with that seen in the control, HEPES-infused animals (Figure 5C).
Figure 5.
(A through D) Immunohistochemistry of NaPi-IIa in the kidneys of HEPES-infused (A and C) and 3 mg/kg per h MEPE–infused (B and D) rats. (A and B) Cortical NaPi-IIa localization using 3,3′-diaminobenzidine tetrahydrochloride staining and light microscopy. Bar = 200 μm. (C and D) Protein levels at the BBM of proximal tubules using Texas red staining and confocal imaging. Bar = 20 μm.
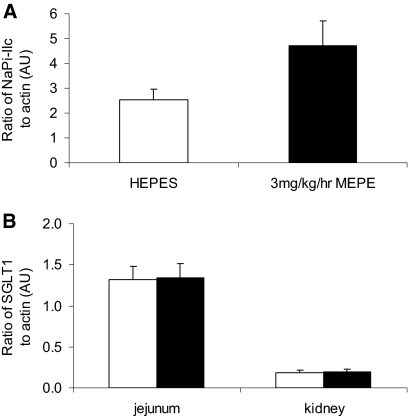
In contrast to the reduction in NaPi-IIa protein levels, there was a trend for increased protein levels of NaPi-IIc after MEPE infusion; however, this did not reach significance (Figure 6A). In addition, Western blotting of both renal and intestinal BBM vesicles for SGLT1 showed that infusion of MEPE had no significant effect on protein levels of this sodium-dependent glucose transporter (Figure 6B).
Figure 6.
(A) Western blot analysis of NaPi-IIc in renal BBM vesicles prepared from HEPES and 3 mg/kg per h MEPE–treated rats. (B) Western blot analysis of SGLT1 protein in renal and intestinal (jejunal) BBM vesicles prepared from HEPES-treated (□) and 3 mg/kg per h MEPE–treated (▪) rats. Data are means ± SEM of six individual BBM vesicle samples from each experimental group. The abundance of NaPi-IIc or SGLT1 is given as a ratio to β-actin protein in AU.
Although infusion of MEPE decreased transepithelial phosphate absorption across the small intestine and increased urinary phosphate excretion, there was no significant change in plasma phosphate concentration during the 3-h infusion period (Table 1). Measurement of the classical endocrine modulators of phosphate homeostasis revealed that the effect of MEPE on renal and intestinal phosphate handling was independent of changes in PTH and 1,25(OH)2D3 (Table 1). Moreover, circulating levels of the phosphatonin FGF-23 were unaffected by MEPE infusion (Table 1).
Table 1.
Effect of MEPE infusion on plasma concentrations of phosphate, PTH, 1,25(OH)2D3, and FGF-23a
| Parameter | HEPES | MEPE
|
|
|---|---|---|---|
| 1 mg/kg per h | 3 mg/kg per h | ||
| Phosphate (mg/dl) | 7.58 ± 0.24 | 7.74 ± 0.21 | 7.66 ± 0.23 |
| PTH (pg/ml) | 154.5 ± 28.2 | 176.4 ± 20.2 | 139.4 ± 23.7 |
| 1,25(OH)2D3 (pg/ml) | 234.5 ± 34.8 | 183.8 ± 14.6 | 194.0 ± 17.6 |
| FGF-23 (pg/ml) | 151.0 ± 16.7 | 126.4 ± 12.9 | 170.4 ± 25.3 |
Data are means ± SEM; n = 6.
DISCUSSION
In this study, we examined the effect of the phosphatonin MEPE on transepithelial phosphate absorption across the small intestine and on phosphate excretion and transporter expression by the kidney. Our results showed that MEPE inhibited intestinal phosphate absorption and significantly reduced the protein levels of the renal sodium-phosphate co-transporter NaPi-IIa but not NaPi-IIc, which resulted in increased urinary phosphate excretion. In contrast, MEPE had no effect on protein levels of the sodium-dependent glucose transporter SGLT1 in the kidney or small intestine, indicating that this peptide influences specifically phosphate handling in these tissues. Interestingly, these effects occurred without any associated changes in circulating PTH, 1,25(OH)2D3, or FGF-23 levels during this period.
MEPE is a member of the small integrin-binding ligand-interacting glycoprotein (SIBLING) family of proteins15; it was originally identified in tumors from patients with oncogenic hypophosphatemic osteomalacia16 and from cultured bone marrow cells during osteoblast differentiation.17 Messenger RNA for this protein has been found at high levels in healthy individuals in bone marrow and brain, with significantly lower levels in the lungs, kidneys, and placenta,16 but it is now thought that the major physiological sources of MEPE are osteoblasts, osteocytes, and odontoblasts1 and that the protein is involved mainly in bone and tooth mineralization (comprehensively reviewed in references1,4).
Jain et al.18 demonstrated serum levels of MEPE in the micromolar range in normal healthy adults, as well as an age-related decline in concentrations and correlation of MEPE levels with serum phosphate and PTH concentrations. Studies of rodents showed that 1,25(OH)2D3 inhibits MEPE production by osteoblasts19 and that MEPE stimulates FGF-23 production in bone marrow stromal cells.20 Indeed, a model of the complex interplay among FGF-23, 1,25(OH)2D3, and MEPE was described by Rowe and colleagues.1,21
Increased serum concentrations of MEPE have been reported in patients with X-linked hypophosphatemic rickets and in the Hyp mouse model of this disorder,22 and MEPE was originally proposed as the factor responsible for hypophosphatemia, phosphaturia, and defective mineralization characteristic of X-linked hypophosphatemic rickets; however, a study of Hyp mice crossed onto a MEPE null background showed that MEPE is not the phosphaturic factor in this mouse model, because hypophosphatemia persisted.23 In contrast to this finding, there is good evidence that MEPE is involved in the regulation of renal phosphate handling in vivo,24,25 and it has been suggested that MEPE is a negative regulator of renal phosphate transport working in conjunction with other factors to maintain normal phosphate balance.25 In keeping with this concept, our study is the first to demonstrate that MEPE is also a potential negative regulator of intestinal phosphate absorption. MEPE dosage-dependently inhibits phosphate absorption by the jejunum but has no effect on phosphate handling in the duodenum. Unfortunately, no commercial antibody against rat NaPi-IIb is available, so we were unable to compare our uptake data with protein levels for this transporter. Using BBM vesicles, FGF-23 was previously shown to affect intestinal phosphate absorption; however, a regional effect has not been described.13 Interestingly, our previous studies demonstrated that although there are striking differences in the regional profile of phosphate handling by the rat and mouse small intestine, in both rodent species, only the jejunum shows an increase in phosphate absorption in response to 1,25(OH)2D3.6 This finding, together with the results of this study, suggests that the jejunum, at least in the rat, is of particular importance in the regulation of phosphate absorption.
It is unclear why the jejunum is the only region that seems to be capable of regulating the rate of phosphate absorption. 1,25(OH)2D3 is deemed to be the most important physiologic regulator of intestinal phosphate absorption,10,26 with its action occurring via the VDR27; however, the VDR is expressed throughout the small intestine, although expression levels in different regions of the intestine have not been examined, and its activation might be expected to enhance phosphate absorption in regions expressing NaPi-IIb. Interestingly, previous studies showed that there is a distinct and tissue-specific regulation of VDR by dietary phosphate, calcium, and 1,25(OH)2D3 in the kidney and small intestine,28,29 and that cells derived from different regions of the small intestine respond to 1,25(OH)2D3 in different ways, linked to activation of protein kinase C.30 Thus, that regulation of phosphate absorption is confined to the jejunum might be owing to differences in VDR content or events that take place at postreceptor sites.
Further experiments are needed to clarify more precisely the mechanisms involved in the regulation of intestinal phosphate absorption and to investigate the role of novel regulatory factors. Of importance is the suggestion that unique intestinal factors, termed ′intestinal phosphatonins,” are released by increases in luminal phosphate concentration, inducing short-term alterations in renal phosphate excretion.12 Whether these intestinal phosphatonins can also evoke short- and long-term changes in intestinal phosphate handling has yet to be fully explored.
Another potential and novel regulator of intestinal phosphate absorption is the calcium-sensing receptor (CaSR). Although its function in the parathyroid gland is now well established, its function in the kidney is just beginning to emerge, as is its function in the gastrointestinal tract.31 The CaSR was first identified in the parathyroid gland32 and later in the kidney and intestinal epithelium.32–34 Recent studies demonstrated that changes in dietary phosphate alter expression of both renal NaPi-IIa and the CaSR,35 and it has been suggested that co-regulation of CaSR and NaPi-IIa may be involved in the “fine tuning” of phosphate reabsorption in the proximal tubule.35 Moreover, 1,25(OH)2D3 has been shown to regulate the renal CaSR,36 and activation of this receptor can blunt PTH-sensitive (inhibition of) renal phosphate absorption.37 Because the CaSR is present in the intestinal epithelium, it may also be involved in the regulation of enterocyte phosphate uptake.
The only other phosphatonin that has been reported to influence intestinal phosphate absorption is FGF-23. Studies have provided evidence for FGF-induced suppression of renal 1αOHase activity and, therefore, 1,25(OH)2D3 production by the kidney, leading to decreased expression of the intestinal sodium phosphate co-transporter NaPi-IIb with reduced intestinal phosphate absorption.13 In our present study, no significant change in 1,25(OH)2D3 was detected after acute MEPE infusion, suggesting that in contrast to FGF-23, MEPE-induced inhibition of intestinal phosphate transport occurs independent of changes in 1,25(OH)2D3. This finding contrasts with previous reports of increased serum 1,25(OH)2D3 levels after administration of Hu-MEPE to mice.25 This discrepancy with our results could arise from differences in the exposure time to MEPE; it is unlikely to result from differences in posttranslational modification of the different sources of MEPE, because in our hands insect-, CHO-, and Escherichia coli–derived recombinant Hu-MEPE all elicited the same biologic response, albeit at slightly different dosages (unpublished observations).
Our results demonstrating a dosage-dependent effect of MEPE on urinary phosphate excretion are in keeping with previous reports. Injection of MEPE into mice resulted in an increase in fractional phosphate excretion at 7 and 31 h after treatment.25 A recently published renal clearance study in our laboratory showed that acute infusion of MEPE in normal rats had no effect on GFR and caused a rapid and dosage-dependent increase in absolute and fractional phosphate excretion, attributable to reduced renal phosphate reabsorption.24 Indeed, a preliminary in vivo micropuncture study confirmed that the effect of MEPE occurs in the proximal tubule and that renal clearance of sodium is also unaffected by MEPE infusion (Shirley DG, Dobbie H, unpublished observations). The results of this study confirm and extend those observations by demonstrating that MEPE dosage-dependently inhibits protein levels of the sodium-phosphate co-transporter NaPi-IIa in proximal convoluted tubules of the outer cortex. Our finding that there are no associated changes in circulating levels of PTH or FGF-23 demonstrates that established regulators of renal phosphate handling are not involved in the MEPE-induced increase in phosphate excretion and suggests that MEPE may have a direct effect on both renal and intestinal phosphate handling in vivo. In support of a direct effect of MEPE is the finding that short-term incubation of proximal tubule cells in vitro with insect-derived MEPE can inhibit phosphate uptake.25
MEPE mRNA and immunoreactivity have been detected in normal kidney, with highest levels present in the BBM of proximal convoluted tubules.38 This has led to the suggestion that MEPE could be secreted into the tubular lumen, where it may bind to cell surface receptors and act in an autocrine or paracrine manner; however, in contrast to the kidney, the presence of MEPE mRNA or immunoreactivity or a direct effect on the small intestine in vitro has not been described.
Although MEPE is generally thought to be involved in bone and tooth mineralization,4 this study provides evidence that this peptide can affect both renal and intestinal phosphate transport in vivo. Up to now, the effect of putative phosphatonins on intestinal phosphate transport has been neglected, but it is becoming apparent that combined hormonal regulation of intestinal and renal phosphate transport, as well as an interaction with bone (and perhaps also with the vasculature), is critical to normal phosphate balance and (potentially) its pathophysiology in renal failure.
CONCISE METHODS
In Situ Intestinal Loop Experiments
Male Sprague-Dawley rats (250 g) were bred at the Royal Free Comparative Biology Unit and allowed ad libitum access to water and a standard rat chow containing 0.52% phosphorous, 0.73% calcium, and 0.62 IU/g vitamin D (Diet RM1; SDS Ltd., Witham, Essex, UK). Rats were anesthetized with an intraperitoneal injection of 100 mg/kg sodium thiopentone (Link Pharmaceuticals, Horsham, Sussex, UK), and the femoral artery, jugular vein, and bladder were cannulated. Rats were then maintained at 37°C using a thermostatically controlled heating blanket (Harvard Apparatus Ltd., Kent, UK) and infused with HEPES buffer, containing 10 HEPES mmol/L in 154 NaCl mmol/L, for 30 min at an infusion rate of 2.6 ml/h, during which a baseline urine sample was collected.
Rats were then randomly divided into three groups that received HEPES buffer alone or 1 or 3 mg/kg per h E. coli–derived MEPE in HEPES buffer for an additional 3 h, respectively. After 2.5 h of infusion, 5-cm-long segments of duodenum (approximately 1 cm from the pylorus) or jejunum (approximately 5 cm from the ligament of Treitz) were cannulated and flushed with warm 0.9% saline, followed by air. Uptake buffer (500 μl) containing 16 mmol/L Na-HEPES, 140 mmol/L NaCl, 3.5 mmol/L KCl, 0.1 mmol/L KH2PO4, and approximately 0.5 MBq 32Pi (PerkinElmer, Bucks, UK) was instilled in the lumen, and the segment was tied off. During this period, a second 30-min urine sample was collected. Blood (0.5 ml) was taken at intervals of 5, 10, 15, and 30 min and centrifuged at high speed in a microcentrifuge for 15 min to obtain plasma. The segment of small intestine was removed and washed with 40 ml of 1 mmol/L KH2PO4 followed by 40 ml of 0.9% saline to displace any 32P bound to the intestinal mucosal surface. The segment of intestine was blotted, and length and wet weight were recorded. Scintillation counting of the plasma, wash solution, and initial uptake solution permitted calculation of phosphate transfer from the lumen into 1 ml of blood by 5 cm of small intestine and the percentage of phosphate remaining in the small intestine. At the end of the experiment, 2 ml of blood was collected for determination of plasma concentrations of phosphate, PTH, FGF-23, and 1,25(OH)2D3, and the kidneys were removed for subsequent preparation of BBM vesicles.
All procedures were carried out in accordance with the Animals (Scientific Procedures) Act 1986. Assay kits for phosphate (Universal Biologicals, Cambridge, UK), rat bioactive intact PTH and 1,25(OH)2D3 (Immunodiagnostics, Bolton, UK), and intact FGF-23 (Kainos Laboratories, Tokyo, Japan) were used according to the respective manufacturer's instructions.
Renal BBM Vesicles
After removal from the rat, kidneys were sliced into 2-mm sections and the cortex was dissected away, snap-frozen, and stored at −80°C until use. Cortical fragments were then used to prepare renal BBM vesicles, as described previously.39
Intestinal BBM Vesicles
Segments of jejunum were removed from the rat and opened longitudinally, and the mucosa was scraped off using glass slides. Mucosa samples were snap-frozen and stored at −80°C until use. Intestinal BBM vesicles were subsequently prepared as described previously.6
Western Blotting
Polyclonal antibodies for NaPi-IIa and NaPi-IIc were raised in rabbits and were a gift from Dr. Jürg Biber (Institute of Physiology, University of Zurich, Zurich, Switzerland). Rabbit polyclonal antibodies for SGLT1 were a gift from Prof. George L. Kellett (Department of Biology, The University of York, York, UK). Mouse mAb for β-actin were used as a loading control (Abcam, Cambridge, UK). For Western blotting, BBM samples (15 μg of protein) were solubilized in Laemmli sample buffer containing 5% SDS and electrophoresed on a 10% SDS polyacrylamide gel.
The proteins were transferred to nitrocellulose membranes by semidry electrophoretic blotting for 1 h at a constant current of 1 mA/cm2. Nonspecific protein-binding sites were blocked with PBS-T (0.01 mol/L PBS containing 0.1% Tween 20) and 5% fat-free milk for 1 h at room temperature. The membranes were incubated with NaPi-IIa (1:3000), NaPi-IIc (1:1000), SGLT1 (1:1000), or β-actin (1:5000) antibodies for 16 h at 4°C. The filters were then washed (2 × 15 min) with PBS-T and incubated with either anti-rabbit (1:2000; Amersham Pharmacia Biotech UK Limited, Bucks, UK) or anti-mouse (1:5000; Sigma Ltd., Poole, Dorset, UK) IgG antibody, conjugated to horseradish peroxidase for 1 h at room temperature, and finally washed again with PBS-T. Bound antibodies were detected using a Western blot detection kit (Visualizer; Upstate Cell Signaling Solutions, Dundee, UK) and visualized and quantified using a Fluor-S MultiImager System (Biorad, Hertfordshire, UK). The ratio of NaPi-IIa, NaPi-IIc, or SGLT1 to β-actin was established for each sample and expressed in arbitrary units.
Immunohistochemistry of NaPi-IIa for Light Microscopy
The aorta of anesthetized rats infused with HEPES buffer or 3 mg/kg MEPE was cannulated and the left kidney perfused with 0.9% sodium chloride to remove blood, followed by perfusion with 40 ml of fixative solution containing 3% paraformaldehyde dissolved in 0.1 mol/L cacodylate buffer (pH 7.4, adjusted to 300 mOsmol/L with sucrose). The left kidney was removed, decapsulated, and embedded in OCT compound (BDH, Poole, UK), then frozen in isopentane precooled in liquid nitrogen. Samples were stored at −80°C until use.
Ten-micrometer cryostat sections were mounted onto polylysine-coated slides (BDH) and air-dried for 1 h. Sections were then washed for 3 × 5 min in 0.1 mol/L PBS (pH 7.4), followed by treatment with 0.3% methanolic H2O2 for 30 min to eliminate endogenous peroxidase activity. Sections were washed (1 × 5 min) with 0.1 mol/L PBS and then 2 × 5 min in 0.1 mol/L PBS containing 0.1% Triton X-100 (PBST) and then blocked for 30 min with 10% normal donkey serum (Sigma) in PBST. Sections were then incubated overnight at 4°C with NaPi-IIa antibody at 1:500 dilution in 1% normal donkey serum in PBST.
After overnight incubation, sections were washed 1 × 5 min in 0.1 M PBST, then 2 × 5 min in 0.1 mol/L PBS, and then incubated with biotin-conjugated goat anti-rabbit antibody (Vector Laboratory, Burlingame, CA) at 1:500 dilution in PBS for 1 h at room temperature. Subsequently, sections were washed 1 × 5 min with 0.1 mol/L PBS and then 2 × 5 min in 0.5 mol/L Tris-HCl buffer (pH 7.4) before incubation with ExtrAvidin (Sigma) at 1:500 dilution in 0.5 mol/L Tris-HCl buffer (pH 7.4) for 1 h at room temperature. Sections were washed (3 × 5 min) using Tris-HCl and then incubated in a filtered mixture of 0.1 mg/ml 3,3′-diaminobenzidine tetrahydrochloride, 0.01 g/ml nickel ammonium sulfate, 0.5 mol/L Tris-HCl, and 0.1% H2O2. The reaction was stopped by three brief washes with cold Tris-HCl followed by 2 × 10 min in Tris-HCl.
Slides were dehydrated, cleared, and coverslipped using DPX (BDH). Results were photographed using a Nikon Coolpix 4500 digital camera (Tokyo, Japan) attached to a Vanox-T microscope. Composite images were prepared using Adobe PhotoshopElements (San Jose, CA).
Immunofluorescence for Confocal Imaging of NaPi-IIa
Sections were treated as for light microscopy up to the addition of ExtrAvidin; instead, sections were incubated with Texas Red Avidin D (Vector Laboratories) for 1 h at room temperature. After washing, 3 × 5 min in PBS, slides were coverslipped using Citiflour (Agar Scientific, Essex, UK).
The distribution of NaPi-IIa was acquired using a Leica LSM 510 laser scanning confocal microscope equipped with an Argon and helium-neon laser using a ×40/1.3 oil immersion objective. Green autofluorescence was imaged using the 488-nm excitation line of the Argon laser with emission at 505 to 530 nm. The specific, red fluorescence of the NaPi-IIa signal was acquired using the 543-nm excitation line of the HeNe laser and a 560-nm long-pass filter. Single optical sections of 3.2 μm were collected using an averaging filter of four iterations to reduce electronic noise. Composite images were prepared using Adobe PhotoshopElements.
Chemicals
Recombinant full-length human MEPE produced in E. coli was a gift from Acologix (Hayward, CA). Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (Poole, Dorset, UK) or VWR International Ltd. (Lutterworth, Leicestershire, UK) and were of analytical grade.
Statistical Analysis
Data are presented as means ± SEM. Normal distribution was determined by Kolmogrov-Smirnov test of normality; all statistical comparisons were made using a one-way ANOVA with post hoc comparisons performed using the Bonferroni multiple comparisons test. Analyses were performed using Graphpad Instat software with statistical significance taken as P < 0.05; each group consisted of n = 6.
DISCLOSURES
R.J.U. has previously acted as a medical advisor to Acologix.
Acknowledgments
We gratefully acknowledge Acologix (Hayward, CA) for providing the recombinant Hu-MEPE and also financial support of this project and the St. Peter's Trust for Kidney, Bladder and Prostate Research for its support.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Rowe PS: The wrickkened pathways of FGF23, MEPE and PHEX. Crit Rev Oral Biol Med 15: 264–281, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sommer S, Berndt T, Craig T, Kumar R: The phosphatonins and the regulation of phosphate transport and vitamin D metabolism. J Steroid Biochem Mol Biol 103: 497–503, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Berndt TJ, Schiavi S, Kumar R: “Phosphatonins” and the regulation of phosphorus homeostasis. Am J Physiol Renal Physiol 289: F1170–F1182, 2005 [DOI] [PubMed] [Google Scholar]
- 4.White KE, Larsson TE, Econs MJ: The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: Frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocr Rev 27: 221–241, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Marks J, Churchill LJ, Srai SK, Biber J, Murer H, Jaeger P, Debnam ES, Unwin RJ: Intestinal phosphate absorption in a model of chronic renal failure. Kidney Int 72: 166–173, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Marks J, Srai SK, Biber J, Murer H, Unwin RJ, Debnam ES: Intestinal phosphate absorption and the effect of vitamin D: A comparison of rats with mice. Exp Physiol 91: 531–537, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Radanovic T, Wagner CA, Murer H, Biber J: Regulation of intestinal phosphate transport: I. Segmental expression and adaptation to low-P (i) diet of the type IIb Na(+)-P (i) cotransporter in mouse small intestine. Am J Physiol Gastrointest Liver Physiol 288: G496–G500, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J: Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc Natl Acad Sci U S A 95: 14564–14569, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murer H, Hernando N, Forster L, Biber J: Molecular mechanisms in proximal tubular and small intestinal phosphate reabsorption (plenary lecture). Mol Membr Biol 18: 3–11, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Murer H, Forster I, Biber J: The sodium phosphate cotransporter family SLC34. Pflugers Arch 447: 763–767, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Eto N, Tomita M, Hayashi M: NaPi-mediated transcellular permeation is the dominant route in intestinal inorganic phosphate absorption in rats. Drug Metab Pharmacokinet 21: 217–221, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Berndt T, Thomas LF, Craig TA, Sommer S, Li X, Bergstralh EJ, Kumar R: Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci U S A 104: 11085–11090, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamoto K, Ito M, Kuwahata M, Kato S, Segawa H: Inhibition of intestinal sodium-dependent inorganic phosphate transport by fibroblast growth factor 23. Ther Apher Dial 9: 331–335, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK: 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289: G1036–G1042, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Fisher LW, Fedarko NS: Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res 44[Suppl 1]: 33–40, 2003 [PubMed] [Google Scholar]
- 16.Rowe PS, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, Oudet CL: MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics 67: 54–68, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Petersen DN, Tkalcevic GT, Mansolf AL, Rivera-Gonzalez R, Brown TA: Identification of osteoblast/osteocyte factor 45 (OF45), a bone-specific cDNA encoding an RGD-containing protein that is highly expressed in osteoblasts and osteocytes. J Biol Chem 275: 36172–36180, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Jain A, Fedarko NS, Collins MT, Gelman R, Ankrom MA, Tayback M, Fisher LW: Serum levels of matrix extracellular phosphoglycoprotein (MEPE) in normal humans correlate with serum phosphorus, parathyroid hormone and bone mineral density. J Clin Endocrinol Metab 89: 4158–4161, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Argiro L, Desbarats M, Glorieux FH, Ecarot B: Mepe, the gene encoding a tumor-secreted protein in oncogenic hypophosphatemic osteomalacia, is expressed in bone. Genomics 74: 342–351, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Rowe PS, Vierthaler L, Zhou J, Quarles LD: Phosphorylated acidic serine-aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J Endocrinol 192: 261–267, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowe PS, Garrett IR, Schwarz PM, Carnes DL, Lafer EM, Mundy GR, Gutierrez GE: Surface plasmon resonance (SPR) confirms that MEPE binds to PHEX via the MEPE-ASARM motif: A model for impaired mineralization in X-linked rickets (HYP). Bone 36: 33–46, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bresler D, Bruder J, Mohnike K, Fraser WD, Rowe PS: Serum MEPE-ASARM-peptides are elevated in X-linked rickets (HYP): Implications for phosphaturia and rickets. J Endocrinol 183: R1–R9, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Brown TA, Zhou J, Xiao ZS, Awad H, Guilak F, Quarles LD: Role of matrix extracellular phosphoglycoprotein in the pathogenesis of X-linked hypophosphatemia. J Am Soc Nephrol 16: 1645–1653, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobbie H, Unwin RJ, Faria NJ, Shirley DG: Matrix extracellular phosphoglycoprotein causes phosphaturia in rats by inhibiting tubular phosphate reabsorption. Nephrol Dial Transplant 23: 730–733, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Rowe PS, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, Cundy J, Navvab S, Chen D, Drezner MK, Quarles LD, Mundy GR: MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone 34: 303–319, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hattenhauer O, Traebert M, Murer H, Biber J: Regulation of small intestinal Na-P(i) type IIb cotransporter by dietary phosphate intake. Am J Physiol 277: G756–G762, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Brown AJ, Dusso A, Slatopolsky E: Vitamin D. Am J Physiol 277: F157–F175, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Sriussadaporn S, Wong MS, Pike JW, Favus MJ: Tissue specificity and mechanism of vitamin D receptor up-regulation during dietary phosphorus restriction in the rat. J Bone Miner Res 10: 271–280, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Zineb R, Zhor B, Odile W, Marthe RR: Distinct, tissue-specific regulation of vitamin D receptor in the intestine, kidney, and skin by dietary calcium and vitamin D. Endocrinology 139: 1844–1852, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Armbrecht HJ, Boltz MA, Hodam TL, Kumar VB: Differential responsiveness of intestinal epithelial cells to 1,25-dihydroxyvitamin D3: Role of protein kinase C. J Endocrinol 169: 145–151, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Hebert SC, Cheng S, Geibel J: Functions and roles of the extracellular Ca2+-sensing receptor in the gastrointestinal tract. Cell Calcium 35: 239–247, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Brown EM, Pollak M, Hebert SC: Sensing of extracellular Ca2+ by parathyroid and kidney cells: Cloning and characterization of an extracellular Ca(2+)-sensing receptor. Am J Kidney Dis 25: 506–513, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Gama L, Baxendale-Cox LM, Breitwieser GE: Ca2+-sensing receptors in intestinal epithelium. Am J Physiol 273: C1168–C1175, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, Hebert SC, Soybel DI, Brown EM: Identification and localization of extracellular Ca(2+)-sensing receptor in rat intestine. Am J Physiol 274: G122–G130, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Riccardi D, Traebert M, Ward DT, Kaissling B, Biber J, Hebert SC, Murer H: Dietary phosphate and parathyroid hormone alter the expression of the calcium-sensing receptor (CaR) and the Na+-dependent Pi transporter (NaPi-2) in the rat proximal tubule. Pflugers Arch 441: 379–387, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Brown AJ, Zhong M, Finch J, Ritter C, McCracken R, Morrissey J, Slatopolsky E: Rat calcium-sensing receptor is regulated by vitamin D but not by calcium. Am J Physiol 270: F454–F460, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Ba J, Brown D, Friedman PA: Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. Am J Physiol Renal Physiol 285: F1233–F1243, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Ogbureke KU, Fisher LW: Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs). Kidney Int 68: 155–166, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Goestemeyer AK, Marks J, Srai SK, Debnam ES, Unwin RJ: GLUT2 protein at the rat proximal tubule brush border membrane correlates with protein kinase C (PKC)-betal and plasma glucose concentration. Diabetologia 50: 2209–2217, 2007 [DOI] [PubMed] [Google Scholar]