Abstract
The calcimimetic cinacalcet increases the sensitivity of the parathyroid calcium-sensing receptor to calcium and therefore should produce a decrease in the set point of the parathyroid hormone (PTH)-calcium curve. For investigation of this hypothesis, nine long-term hemodialysis patients with secondary hyperparathyroidism were given cinacalcet for 2 mo, the dosage was titrated per a protocol based on intact PTH and plasma calcium concentrations. Dialysis against low- and high-calcium (0.75 and 1.75 mM) dialysate was used to generate curves describing the relationship between PTH and calcium. Compared with precinacalcet levels, cinacalcet significantly reduced mean serum calcium, intact PTH and whole PTH (wPTH; all P < 0.001). The set points for PTH-calcium curves were significantly reduced, and both maximum and minimum levels of PTH (intact and whole) were significantly decreased. The calcium-mediated inhibition of PTH secretion was more marked after cinacalcet treatment. In addition, cinacalcet shifted the inverse sigmoidal curve of wPTH/non-wPTH ratio versus calcium to the left (i.e., less calcium was required to reduce the wPTH/non-wPTH ratio). In conclusion, cinacalcet increases the sensitivity of the parathyroids to calcium, causing a marked reduction in the set point of the PTH-calcium curve, in hemodialysis patients with secondary hyperparathyroidism.
Calcimimetics are widely accepted for the treatment of secondary hyperparathyroidism, commonly observed in dialysis patients. They are effective in decreasing parathyroid hormone (PTH), phosphorus, calcium, and the calcium-phosphorus product, even in patients with advanced disease.1 Decreased expression of the parathyroid calcium-sensing receptor (CaR) in severe parathyroid hyperplasia2 may reduce the sensitivity of the parathyroid cell to extracellular calcium, leading to an increase in the set point of the PTH-calcium curve. It is generally accepted that a left shift of the set point indicates an increase in the sensitivity of the parathyroid gland to calcium.3 Thus, if calcimimetics increase parathyroid CaR sensitivity to calcium, then they should produce a decrease in the set point of the PTH-calcium curve.
An immunometric assay for the measurement of intact PTH (iPTH) was, until recently, the accepted standard for the measurement of PTH; however, the iPTH assay has been shown to react not only with the bioactive form of PTH (whole PTH [wPTH]) but also with large, truncated fragments of non-wPTH,4–7 with 20 to 60% of PTH measured in normal individuals corresponding to non-wPTH.4,6–8 In dialysis patients, the percentage of non-wPTH measured in the iPTH assay is generally greater than that in healthy control subjects.4–9 A newer assay, called “whole” or “full” PTH, has been shown to be specific for wPTH.7 Plasma concentrations of non-wPTH can be determined by subtracting the wPTH value from that measured with the iPTH assay.10,11
The proportional secretion of wPTH and non-wPTH may be modified by the predialysis plasma calcium concentration in hemodialysis patients.10,11 In one study,12 we showed that although acute changes in plasma calcium produce similar secretory responses in wPTH and non-wPTH, the secretory responses are not proportional for these PTH moieties. Changes in the plasma calcium concentration modulate the ratio of wPTH/non-wPTH in a sigmoidal pattern with hypocalcemia maximizing this ratio.
The aim of this study was to evaluate the effect of cinacalcet on the dynamics of wPTH and non-wPTH secretion and also on the wPTH/non-wPTH ratio in response to the induction of hypo- and hypercalcemia during hemodialysis. The set point of the PTH-calcium curve was compared in the same hemodialysis patients before and after treatment with cinacalcet.
RESULTS
Nine patients (five men, four women) who aged 27 to 63 yr (mean 49) and had spent 18 to 240 mo on hemodialysis were included in the study. Causes of renal disease included nephroangiosclerosis (n = 1), polycystic kidney disease (n = 1), interstitial nephritis (n = 1), glomerulonephritis (n = 2), hemolytic uremic syndrome (n = 1), and unknown (n = 3).
Treatment with cinacalcet led to a significant reduction in mean plasma ionized calcium and phosphorus levels (P < 0.001 and P < 0.020, respectively; Table 1). There was no significant change in the mean serum albumin and alkaline phosphatase concentrations after cinacalcet treatment (Table 1).
Table 1.
Levels of calcium, phosphorus, albumin, alkaline phosphatase, iPTH, and wPTH before and after cinacalcet treatmenta
| Parameter | Before Cinacalcet | After Cinacalcet | P |
|---|---|---|---|
| Basal calcium (mM) | 1.190 ± 0.110 | 1.110 ± 0.200 | <0.001 |
| Phosphorus (mM) | 1.740 ± 0.160 | 1.410 ± 0.090 | <0.020 |
| Albumin (g/dl) | 3.960 ± 0.400 | 4.030 ± 0.500 | NS |
| Alkaline phosphatase (IU/L) | 117.000 ± 20.000 | 134.000 ± 28.000 | NS |
| Basal iPTH (pg/ml) | 645.000 ± 86.000 | 336.000 ± 55.000 | <0.001 |
| Maximal iPTH (pg/ml) | 997.000 ± 116.000 | 762.000 ± 107.000 | <0.001 |
| Minimal iPTH (pg/ml) | 148.000 ± 29.000 | 53.000 ± 10.000 | <0.001 |
| iPTH basal/maximal ratio | 0.640 ± 0.060 | 0.460 ± 0.070 | <0.050 |
| iPTH set point | 1.210 ± 0.020 | 1.100 ± 0.010 | <0.001 |
| iPTH set point (midrange) | 1.190 ± 0.020 | 1.090 ± 0.010 | 0.001 |
| Basal wPTH (pg/ml) | 358.000 ± 46.000 | 181.000 ± 11.000 | <0.010 |
| Maximal wPTH (pg/ml) | 624.000 ± 93.000 | 467.000 ± 65.000 | <0.020 |
| Minimal wPTH (pg/ml) | 56.000 ± 14.000 | 23.000 ± 5.300 | <0.020 |
| wPTH basal/maximal ratio | 0.530 ± 0.050 | 0.430 ± 0.050 | <0.050 |
| wPTH set point | 1.190 ± 0.020 | 1.075 ± 0.010 | <0.001 |
| wPTH set point (midrange) | 1.180 ± 0.010 | 1.060 ± 0.010 | <0.001 |
Data are means ± SE.
There was a significant decrease in basal iPTH and basal wPTH levels after the 2-mo treatment with cinacalcet (both P < 0.001; Table 1). Before cinacalcet treatment, the mean wPTH level was 56 ± 12% of the iPTH value; after cinacalcet treatment, wPTH accounted for 62 ± 10% of the iPTH value (NS versus precinacalcet treatment).
Set Point of the PTH-Calcium Curve
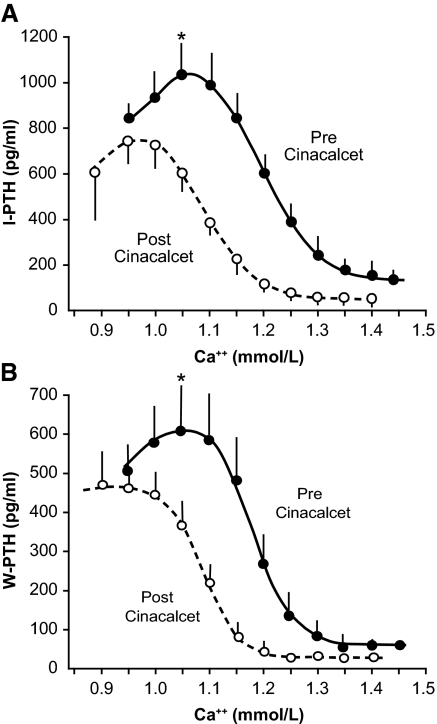
Changes in plasma ionized calcium concentration induced by hemodialysis with low and high concentrations of calcium in the dialysate produced a sigmoid-shaped response of iPTH and wPTH (Figure 1). Set points measured from iPTH-calcium and wPTH-calcium curves were not statistically different. Before treatment with cinacalcet, the set point of the iPTH-calcium curve was 1.21 ± 0.02; after treatment with cinacalcet, the set point decreased significantly to 1.10 ± 0.01 (P < 0.001). Similar results were obtained from the wPTH-calcium curve (Table 1). Results obtained when the set point was calculated as the plasma calcium midrange between the minimal and maximal PTH led to similar results (Table 1).
Figure 1.
(A) PTH-calcium curve for iPTH before and after treatment with cinacalcet. •, before cinacalcet; ○, after cinacalcet. (B) PTH-calcium curve for wPTH before and after treatment with cinacalcet (*P < 0.05 for both iPTH and wPTH before and after cinacalcet treatment for ionized calcium concentrations >1.05 mM). •, before cinacalcet; ○, after cinacalcet.
Maximal and Minimal PTH
Hemodialysis with a low concentration of calcium in the dialysate produced an increase in iPTH and wPTH to their maximal levels. Cinacalcet produced a significant decrease in the maximal iPTH and wPTH levels (P < 0.001 and P < 0.020, respectively; Table 1). The degree of decrease in maximal PTH was similar for iPTH and wPTH (24 and 26%, respectively). Similarly, hemodialysis-induced hypercalcemia produced a decrease in iPTH and wPTH to minimal levels (Table 1). Cinacalcet treatment led a significant decrease in the minimal iPTH and wPTH levels (P < 0.001 and P < 0.020, respectively). Again, the degree of decrease in minimal PTH was similar for iPTH and wPTH (64 and 59%, respectively).
Mean plasma calcium concentrations required to stimulate iPTH and wPTH maximally were higher before (1.075 ± 0.020 and 1.070 ± 0.002) than after (0.98 ± 0.04 and 0.95 ± 0.03) treatment with cinacalcet (both P < 0.01). Plasma calcium concentrations required to achieve minimal iPTH and wPTH were lower after (1.25 ± 0.04 and 1.22 ± 0.03) than before (1.39 ± 0.05 and 1.33 ± 0.03) cinacalcet therapy (both P < 0.001).
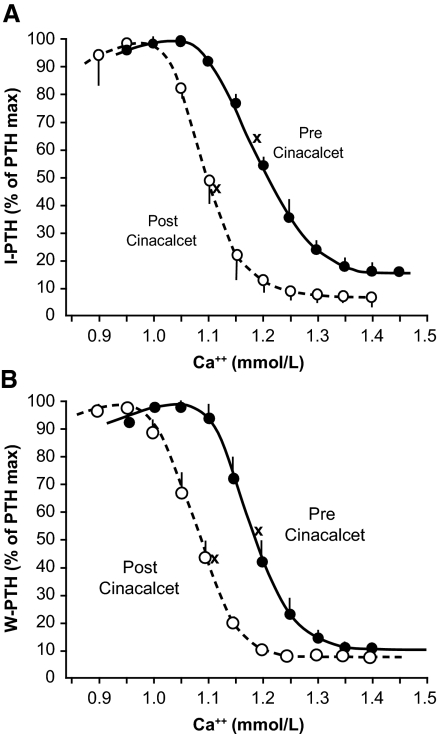
The relationships between iPTH and wPTH secretion, measured as a percentage of maximal PTH stimulation, and ionized calcium concentrations are shown in Figure 2. The reduction of PTH secretion (iPTH and wPTH) by calcium was more marked after treatment with cinacalcet than before. Before cinacalcet, for a plasma ionized calcium concentration of 1.15 mM, both iPTH and wPTH were stimulated at 70 to 80% of the maximal PTH; however, after cinacalcet, the same calcium concentration produced almost total inhibition of PTH secretion. For ionized calcium concentrations >1.05 mM, the values for iPTH and wPTH were significantly lower (P < 0.05) after than before treatment with cinacalcet (Figure 1).
Figure 2.
(A) Relationship between the percentage of maximal PTH stimulation for iPTH and the ionized calcium concentration. •, before cinacalcet; ○, after cinacalcet; x, basal values. (B) Relationship between the percentage of maximal PTH stimulation for wPTH and the ionized calcium concentration. •, before cinacalcet; ○, after cinacalcet; x, basal values.
Ratio of Basal To Maximal PTH
One important observation is a greater decrease in set point (0.11 mmol/L) than in basal serum calcium (0.08 mmol/L) levels, resulting in a lower basal/maximal PTH ratio. The ratio of basal to maximal PTH for iPTH and wPTH decreased after treatment with cinacalcet (both P < 0.05; Figure 2, Table 1). Thus, the relative degree of PTH stimulation at the basal plasma calcium concentration was reduced after treatment with cinacalcet.
The wPTH to Non-wPTH Ratio
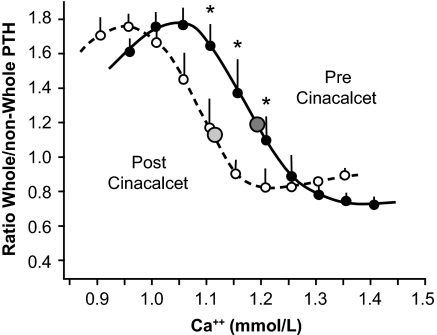
The relationship between the ratio wPTH/non-wPTH and the plasma ionized calcium concentration before and after treatment with cinacalcet are shown in Figure 3. The wPTH/non-wPTH ratio decreased as the plasma calcium concentration increased. After treatment with cinacalcet, the curve shifted to the left; thus, less calcium was required to reduce the wPTH/non-wPTH ratio. Before treatment, the basal calcium was 1.19 ± 0.11 mM and the corresponding ratio wPTH/non-wPTH was 1.18 ± 0.14. After treatment with cinacalcet, the plasma calcium decreased to 1.11 ± 0.20 mM; however, the ratio wPTH/non-wPTH remained almost the same (1.12 ± 0.17).
Figure 3.
Relationship between wPTH/non-wPTH and serum ionized calcium concentration before and after treatment with cinacalcet. Values of wPTH/non-wPTH were significantly decreased (*P < 0.05) after cinacalcet at ionized calcium concentrations of 1.10, 1.15, and 1.20 mmol/L. •, before cinacalcet; ○, after cinacalcet;  , basal serum calcium values.
, basal serum calcium values.
DISCUSSION
Cinacalcet lowers PTH, phosphorus, calcium, and the calcium-phosphorus product1 and is licensed for the treatment of secondary hyperparathyroidism in hemodialysis patients with ESRD. Because cinacalcet targets the parathyroid CaR, increasing sensitivity to extracellular calcium, it was hypothesized that cinacalcet should produce a decrease in the set point of the PTH-calcium curve in patients with secondary hyperparathyroidism.
The dynamics of PTH secretion were evaluated using low and high concentrations of calcium dialysate before and after treatment with cinacalcet. After 2 mo of treatment with cinacalcet, there was a left shift of the PTH-calcium curves for both iPTH and wPTH. Thus, cinacalcet reduces the set point of the PTH-calcium curves, and, therefore, the sensitivity of the parathyroid cells to calcium is increased. Previous studies showed there was a greater reduction in PTH levels 4 h after administration of cinacalcet than at the 24-h time point, which was used to monitor the efficacy of treatment13,14; therefore, 24 h after treatment, any acute effect of cinacalcet is absent. In this study, patients received cinacalcet the day before blood sampling. The half-life of the cinacalcet molecule is 34 ± 9 h15; therefore, the PTH-calcium curve was obtained when serum cinacalcet levels were minimal, and the change in the set point was not the result of an acute calcimimetic effect.
Several reports16–18 have shown that there is an “adaptation” of the PTH-calcium curve set point to the prevailing plasma calcium concentration. A decrease in plasma calcium was associated with a reduction in the set point, with an increase in basal minimum and maximum PTH secretion and even in the basal-to-maximal PTH ratio. These changes in PTH secretion are increased responses of parathyroid cells to the low calcium levels. In our study, the decrease in set point was due to increased sensitivity of the receptor to the inhibitory effect of calcium. The decrease in set point after cinacalcet was associated with a decrease in basal minimal and maximal PTH secretion and a decrease in the basal to maximal PTH secretion, which is a clear sign of inhibition of parathyroid gland activity. Possible explanations for an alteration in the set point of the PTH-calcium curve include changes in the extracellular ionized calcium concentration,16,17 which is not supported by this study, or a calcium-independent effect.19
After cinacalcet treatment, minimal wPTH and iPTH were accomplished with a normal calcium concentration (1.22 and 1.25 mM, respectively). A small increase in plasma calcium concentration within the normal range may produce a marked decrease in PTH and explains the effectiveness of cinacalcet in the treatment of secondary hyperparathyroidism. In addition, basal PTH, maximal PTH, and minimal PTH for wPTH and iPTH were reduced after treatment with cinacalcet.
The patients included in this study had high PTH levels; therefore, they benefited from the treatment with cinacalcet. These patients were not on vitamin D. Because vitamin D can modify basal PTH levels,20 treatment with vitamin D analogs in the 6 mo before and during the study was not permitted, to avoid any possible confounding effect produced by the action of vitamin D receptor activation. The dynamic of PTH secretion was evaluated in the same patients using low- and high-calcium hemodialysis (which effectively decreases and increases plasma calcium, respectively), resulting in a concomitant stimulation and inhibition of PTH secretion. This method has been used by different investigators to assess the dynamics of PTH secretion in hemodialysis patients with secondary hyperparathyroidism.16–18,21–23
The relative degree of PTH stimulation was measured using the ratio of basal to maximal PTH. In normal volunteers, this ratio is 20 to 25%24 and is higher in hemodialysis patients.12,16 In this study, the ratio of basal to maximal PTH for wPTH and iPTH was 53 and 64%, respectively, before cinacalcet treatment. After treatment with cinacalcet, the ratio was reduced (43 and 46%), indicating a reduction in PTH stimulation relative to the maximal capacity of the gland.
To our knowledge, no published written report has shown the effect of calcimimetics on set point in dialysis patients. In this study, there was a decrease in the wPTH/non-wPTH ratio as the plasma calcium concentration increased. After treatment with cinacalcet, the wPTH/non-wPTH ratio versus calcium curve shifted to the left. Thus, less calcium was required to reduce the wPTH/non-wPTH ratio. Despite the hypocalcemic effect of cinacalcet, the wPTH/non-wPTH ratio was unchanged. The left shift of the wPTH/non-wPTH ratio versus calcium curve is an interesting finding. The plasma calcium response to PTH is nearly linear between 0.625 and 1.250 mM; however, above a plasma calcium concentration of 1.25 mM, the response decreases.20 This reduced response is due, in part, to an increase in carboxy-terminal and large truncated amino-terminal PTH fragments.25,26 These fragments antagonize the calcemic effect of PTH.10,27,28 Some authors proposed that a decrease in wPTH/non-wPTH ratio was associated with decreased turnover.11 Because cinacalcet inhibits parathyroid gland function,12 a decrease in the wPTH/non-wPTH ratio would have been expected, with a consequent increase in the risk for low bone turnover as a result of a decrease in wPTH relative to non-wPTH; however, cinacalcet treatment shifted the inverse sigmoidal curve of wPTH/non-wPTH–calcium leftward, and hypocalcemia, induced by cinacalcet, could offset the decreased wPTH/non-wPTH ratio. A previous study by Martin et al.29 compared the effect of long-term treatment with cinacalcet or placebo on the ratio of biointact PTH (biPTH) to iPTH-biPTH in patients with secondary hyperparathyroidism. They found significant variations in this ratio with respect to plasma calcium. Higher plasma calcium levels were associated with a lower ratio of biPTH to iPTH-biPTH in both treatment groups. For any given plasma calcium concentration, the ratio was lower in patients receiving cinacalcet compared with those receiving placebo. This is an interesting observation that does not contradict our results. As depicted in Figure 3, hypercalcemia was associated with a lower ratio of wPTH to non-wPTH, and for serum calcium levels from 1.00 to 1.25 mM, the ratio was lower after cinacalcet treatment than before treatment. In this study, phosphorus levels were reduced after cinacalcet therapy; however, this decrease in serum phosphorus levels is not expected to have a large impact on the set point of the PTH-calcium curve.30,31
The maximal secretion of PTH obtained during hemodialysis with low calcium is a reflection of the size of the parathyroid gland or the number of parathyroid cells that are actively secreting PTH. The parathyroid gland size was not measured in this study; however, it is unlikely that, in such a short period of time, cinacalcet was able to reduce the size of the parathyroid glands. Although it could be that the number of cells secreting PTH has decreased, the minimal PTH may also reflect the total mass of parathyroid cells. In this study, the minimal PTH decreased by as much as 65% after treatment with cinacalcet. We propose that cinacalcet may decrease maximal and minimal PTH secretion by restraining PTH secretion in a significant number of parathyroid cells.
In conclusion, as cinacalcet increases the sensitivity of the CaR to the inhibitory effect of calcium in patients with secondary hyperparathyroidism, a high calcium level is not required to inhibit PTH secretion.
CONCISE METHODS
Five male and four female patients who were younger than 65 yr and met the following criteria were eligible for inclusion in the study: On long-term hemodialysis for >6 mo and clinically stable with no recent illnesses, iPTH 300 to 1000 pg/ml, normal plasma calcium concentration (9.0 to 10.6 mg/dl), serum phosphorus concentration <6 mg/dl, no treatment with vitamin D analogs during the previous 6 mo and not added to treatment during the study period, no diabetes, and serum aluminum levels <35 μg/L. The protocol adhered to the Declaration of Helsinki and was approved by the institutional review board at the Hospital Universitario Reina Sofia (Cordoba, Spain). Informed written consent was obtained before inclusion in the study.
PTH-calcium curves were measured before and after 2 mo of treatment with cinacalcet. Patients were started on cinacalcet 30 mg/d, and iPTH concentration was measured at weekly intervals. The dosage of cinacalcet was increased by 30 mg/d when the PTH was >300 ng/ml and the plasma calcium concentration was >8.5 mg/dl, whereas the cinacalcet dosage was decreased (withheld) when PTH was <150 ng/ml or plasma calcium was <8.5 mg/dl.
Maximal secretion and suppression of iPTH and wPTH as well as the entire PTH-calcium curve were determined by performing hemodialysis with low (0.75 mM) and high (1.75 mM) concentrations of calcium dialysate separated by 1 wk.16,17,21,22 Blood was drawn at regular intervals (basal and 10, 20, 30, 45, 60, 90, 120, 150, 180, and 210 min from the beginning of the hemodialysis session) for measurement of ionized calcium, iPTH, and wPTH. Patients received cinacalcet the day before blood sampling.
The following terms were defined for wPTH and iPTH, using the data obtained during dialysis-induced hypo- and hypercalcemia: (1) Basal PTH was the mean of the predialysis PTH level with low- or high-calcium dialysate; (2) maximal PTH was the highest PTH level observed in response to hypocalcemia; an additional reduction of the plasma calcium concentration did not further increase the PTH; (3) minimal PTH was the lowest PTH level during suppression by hypercalcemia; a further increase in the plasma calcium concentration did not result in any additional decrease in PTH; (4) ratio of basal to maximal PTH (%) was (basal PTH/maximal PTH) × 100 (this ratio is 20 to 25% in normal volunteers24) by correcting the actual PTH for the overall capacity to produce PTH (maximal PTH), a measure of the relative degree of PTH stimulation is obtained; (5) the set point of calcium was defined as the plasma calcium concentration at which maximal PTH secretion was reduced by 50%.16,17,21,22 The method of Brown and colleagues32–34 was also used, where the set point of calcium is the plasma calcium at the midrange between the minimal and maximal PTH (curve fitting was achieved using spectral analysis Running-Means software.35); the dependent variable (PTH) was in the form of means of three consecutive serum calcium values, limiting analytical variability; (6) basal plasma calcium was the mean of plasma calcium concentrations at basal (predialysis) PTH with low- or high-calcium dialysate; (7) plasma calcium at maximal PTH secretion was the plasma calcium level at which maximal PTH secretion was achieved; and (8) plasma calcium at minimal PTH secretion was the plasma calcium level at which minimal PTH secretion was achieved.
For assessment of the calcium-dependent changes in iPTH and wPTH, graphs were constructed interpolating the PTH value from the PTH-calcium curve for each patient at the following calcium concentrations: 0.90, 0.95, 1.00, 1.05, 1.10, 1.15, 1.20, 1.25, 1.30, 1.35, 1.40, and 1.45 mM. After the calculation of individual PTH values, curves were constructed with mean values of interpolated PTH values. The ratio of wPTH to non-wPTH was calculated at baseline and during changes in the ionized calcium concentration.8,11
Laboratory Measurements
During the low- and high-calcium dialysis phases, ionized calcium was measured using a calcium-selective electrode (Bayer Diagnostics, Barcelona, Spain) immediately after the blood sample was obtained. Plasma calcium, phosphorus, alkaline phosphatase, albumin, and aluminum were measured using standard laboratory techniques. PTH was measured using the Duo PTH Kit (Scantibodies Laboratory, Santee, CA). The kit contains two immunoradiometric assays. Both assays share a polyclonal antibody (anti-PTH 39-84) coated onto the surface of polystyrene beads as a solid phase. The immunoradiometric assay for wPTH uses a tracer antibody directed against the 1-4 amino-terminal region of PTH. The use of this antibody is designed to be specific for 1-84 PTH.7 The immunoradiometric assay for iPTH uses a specific polyclonal antibody directed against 7-34 PTH. With this antibody, both wPTH and non-wPTH are detected. For iPTH and wPTH assays, the normal reference ranges are 14 to 66 and 7 to 36 pg/ml, respectively. The intra- and interassay coefficients of variation were <5 and <7%, respectively, for both assays. The plasma concentration of non-wPTH was determined by subtracting the wPTH assay value from the iPTH assay value.
Statistical Analysis
Results are presented as means ± SE. When the data were normally distributed, as assessed by the Shapiro-Wilks test, means were compared using paired t test or general linear model for repeated measures, followed by the Bonferroni post hoc test. Differences were considered significant at P < 0.05.
DISCLOSURES
None.
Acknowledgments
Funding was provided by government grants: PI 070315 from instituto Carlos III and 195/04 and 202/05 from Consejeria de Salud de la Junta de Andalucia and Fundación Nefrologica and Red de Investigación Renal (RD 06/0016//0007). M.R., C.V., and R.S. are supported by instituto Carlos III within specific programs for research personnel (intensification and postresidency research fellowship). Y.A. is supported by Consejeria de Salud de la Junta de Andalucia. Jane Blackburn assisted in the writing of this manuscript. Funding for the writing was provided by Amgen Europe.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drüeke TB, Goodman WG: Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Gogusev J, Duchambon P, Hory B, Giovannini M, Goureau Y, Sarfati E, Drüeke TB: Depressed expression of calcium receptor in parathyroid gland tissue of patients with primary or secondary uremic hyperparathyroidism. Kidney Int 51: 328–336, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Felsenfeld AJ, Llach F: Parathyroid gland function in chronic renal failure. Kidney Int 43: 771–789, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Brossard JH, Cloutier M, Roy L, Lepage R, Gascon-Barré M, D'Amour P: Accumulation of a non-(1-84) molecular form of parathyroid hormone (PTH) detected by intact PTH assay in renal failure: importance in the interpretation of PTH values. J Clin Endocrinol Metab 81: 3923–3929, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Lepage R, Roy L, Brossard JH, Rousseau L, Dorais C, Lazure C, D'Amour P: A non-(1-84) circulating parathyroid hormone (PTH) fragment interferes significantly with intact PTH commercial assay measurements in uremic samples. Clin Chem 44: 805–809, 1998 [PubMed] [Google Scholar]
- 6.Brossard JH, Lepage R, Cardinal H, Roy L, Rousseau L, Dorais C, D'Amour P: Influence of glomerular filtration rate on non-(1-84) parathyroid hormone (PTH) detected by intact PTH assays. Clin Chem 46: 697–703, 2000 [PubMed] [Google Scholar]
- 7.Gao P, Scheibel S, D'Amour P, John MR, Rao SD, Schmidt-Gayk H, Cantor TL: Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1-84: Implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res 16: 605–614, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Coen G, Bonucci E, Ballanti P, Balducci A, Calabria S, Nicolai GA, Fischer MS, Lifrieri F, Manni M, Morosetti M, Moscaritolo E, Sardella D: PTH 1-84 and PTH “7-84” in the noninvasive diagnosis of renal bone disease. Am J Kidney Dis 40: 348–354, 2002 [DOI] [PubMed] [Google Scholar]
- 9.John MR, Goodman WG, Gao P, Cantor TL, Salusky IB, Jüppner H: A novel immunoradiometric assay detects full-length human PTH but not amino-terminally truncated fragments: Implications for PTH measurements in renal failure. J Clin Endocrinol Metab 84: 4287–4290, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Slatopolsky E, Finch J, Clay P, Martin D, Sicard G, Singer G, Gao P, Cantor T, Dusso A: A novel mechanism for skeletal resistance in uremia. Kidney Int 58: 753–761, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Monier-Faugere MC, Geng Z, Mawad H, Friedler RM, Gao P, Cantor TL, Malluche HH: Improved assessment of bone turnover by the PTH-(1-84)/large C-PTH fragments ratio in ESRD patients. Kidney Int 60: 1460–1468, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Santamaria R, Almaden Y, Felsenfeld A, Martin-Malo A, Gao P, Cantor T, Aljama P, Rodriguez M: Dynamics of PTH secretion in hemodialysis patients as determined by the intact and whole PTH assays. Kidney Int 64: 1867–1873, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Quarles LD, Sherrard DJ, Adler S, Rosansky SJ, Mccary LC, Liu W, Turner SA, Bushinsky DA: The calcimimetic AMG073 as a potential treatment for secondary hyperparathyroidism of end-stage renal disease. J Am Soc Nephrol 14: 575–583, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Goodman WG, Hladik GA, Turner SA, Blaisdell PW, Goodkin DA, Liu W, Barri YM, Cohen RM, Coburn JW: The calcimimetic agent AMG073 lowers plasma parathyroid hormone levels in haemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol 13: 1017–1024, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Kumar GN, Sproul C, Poppe L, Turner S, Gohdes M, Ghoborah H, Padhi D, Roskos L: Metabolism and disposition of calcimimetic agent cinacalcet HCl in humans and animal models. Drug Metab Dispos 32: 1491–1500, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Pahl M, Jara A, Bover J, Rodriguez M, Felsenfeld AJ: The set point of calcium and the reduction of parathyroid hormone in hemodialysis patients. Kidney Int 49: 226–231, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez M, Caravaca F, Fernandez E, Borrego MJ, Lorenzo V, Cubero J, Martin-Malo A, Betriu A, Rodriguez AP, Felsenfeld AJ: Evidence for both abnormal set point of PTH stimulation by calcium and adaptation to serum calcium in hemodialysis patients with hyperparathyroidism. J Bone Miner Res 12: 347–355, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Borrego MJ, Felsenfeld AJ, Martin Malo A, Almaden Y, Concepción MT, Aljama P, Rodriguez M: Evidence for adaptation of the entire PTH-calcium curve to sustained changes in the serum calcium in haemodialysis patients. Nephrol Dial Transplant 12: 505–513, 1997 [DOI] [PubMed] [Google Scholar]
- 19.de Francisco AL: Cinacalcet HCl: A novel therapeutic for hyperparathyroidism. Expert Opin Pharmacother 6: 441–452, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Felsenfeld AJ, Rodríguez M, Aguilera-Tejero E: Dynamics of parathyroid hormone secretion in health and secondary hyperparathyroidism. Clin J Am Soc Nephrol 2: 1283–1305, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Felsenfeld AJ, Rodriguez M, Dunlay R, Llach F: A comparison of parathyroid-gland function in haemodialysis patients with different forms of renal osteodystrophy. Nephrol Dial Transplant 6: 244–251, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez M, Felsenfeld AJ, Williams C, Pederson JA, Llach F: The effect of long-term intravenous calcitriol administration on parathyroid function in hemodialysis patients. J Am Soc Nephrol 2: 1014–1020, 1991 [DOI] [PubMed] [Google Scholar]
- 23.De Cristofaro V, Colturi C, Masa A, Comelli M, Pedrini LA: Rate dependence of acute PTH release and association between basal plasma calcium and set point of calcium-PTH curve in dialysis patients. Nephrol Dial Transplant 16: 1214–1221, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Brent GA, LeBoff MS, Seely EW, Conlin PR, Brown EM: Relationship between the concentration and rate of change of calcium and serum intact parathyroid hormone levels in normal humans. J Clin Endocrinol Metab 67: 944–950, 1988 [DOI] [PubMed] [Google Scholar]
- 25.D'Amour P: Effects of acute and chronic hypercalcemia on parathyroid function and circulating parathyroid hormone molecular forms. Eur J Endocrinol 146: 407–410, 2002 [DOI] [PubMed] [Google Scholar]
- 26.D'Amour P, Räkel A, Brossard JH, Rousseau L, Albert C, Cantor T: Acute regulation of circulating parathyroid hormone (PTH) molecular forms by calcium: utility of PTH fragments/PTH(1-84) ratios derived from three generations of PTH assays. J Clin Endocrinol Metab 91: 283–289, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Nguyen-Yamamoto L, Rousseau L, Brossard JH, Lepage R, D'Amour P: Synthetic carboxyl-terminal fragments of parathyroid hormone (PTH) decrease ionized calcium concentration in rats by acting on a receptor different from the PTH/PTH-related peptide receptor. Endocrinology 142: 1386–1392, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Divieti P, John MR, Jüppner H, Bringhurst FR: Human PTH-(7-84) inhibits bone resorption in vitro via actions independent of the type 1 PTH/PTHrP receptor. Endocrinology 143: 171–176, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Martin KJ, Jüppner H, Sherrard DJ, Goodman WG, Kaplan MR, Nassar G, Campbell P, Curzi M, Charytan C, McCary LC, Guo MD, Turner SA, Bushinsky DA: First- and second-generation immunometric PTH assays during treatment of hyperparathyroidism with cinacalcet HCl. Kidney Int 68: 1236–1243, 2005 [DOI] [PubMed] [Google Scholar]
- 30.De Francisco AL, Cobo M, Setien M, Rodrigo E, Fresnedo GF, Unzueta MT, Amado JA, Ruiz JC, Arias M, Rodriguez M: Effect of serum phosphate on parathyroid hormone secretion during hemodialysis. Kidney Int 54: 2140–2145, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Estepa JC, Aguilera-Tejero E, Lopez I, Almaden Y, Rodriguez M, Felsenfeld AJ: Effect of phosphate on parathyroid hormone secretion in vivo. J Bone Miner Res 14: 1848–1854, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Brown EM: Four-parameter model of the sigmoidal relationship between parathyroid hormone release and extracellular calcium concentration in normal and abnormal parathyroid tissue. J Clin Endocrinol Metab 56: 572–581, 1983 [DOI] [PubMed] [Google Scholar]
- 33.Ramirez JA, Goodman WG, Belin TR, Gales B, Segre GV, Salusky IB: Calcitriol therapy and calcium-regulated PTH secretion in patients with secondary hyperparathyroidism. Am J Physiol 267: E961–E967, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Goodman WG, Belin T, Gales B, Juppner H, Segre GV, Salusky IB: Calcium-regulated parathyroid hormone release in patients with mild or advanced secondary hyperparathyroidism. Kidney Int 48: 1553–1558, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Helwig JT, Council KA, eds: Statistical Analysis System User's Guide, Cary, NC, SAS Institute, 1979