Abstract
Recruitment of various stem and progenitor cells is crucial for the regeneration of an injured organ. Levels of uric acid, one of the prototypical “alarm signals,” surge after ischemia-reperfusion injury. Exogenous uric acid rapidly mobilizes endothelial progenitor cells and hematopoietic stem cells and protects the kidney from ischemia. The relatively fast responses to uric acid suggest that preformed second messengers may be released from a storage pool. Here, it is reported that monosodium urate (MSU) results in exocytosis of Weibel-Palade bodies in vitro and in vivo, leading to the release of IL-8, von Willebrand factor, and angiopoietin 2 in the culture medium or circulation. Confocal and immunoelectron microscopy confirmed depletion of von Willebrand factor in MSU-treated aortic endothelial cells. Angiopoietin 2 alone induced exocytosis of Weibel-Palade bodies, mobilized hematopoietic stem cells and depleted splenic endothelial progenitor cells, partially reproducing the actions of MSU. In addition, pretreatment with angiopoietin 2 protected the kidneys from an ischemic insult, suggesting that the previously reported renoprotection conferred by MSU likely results from exocytosis of Weibel-Palade bodies. Furthermore, experiments with toll-like receptor 4 (TLR-4)–and TLR-2–deficient mice demonstrated that uric acid–induced exocytosis of Weibel-Palade bodies is mediated by TLR-4 and that uric acid–induced release of IL-8 requires both TLR-2 and TLR-4. In summary, these results suggest that exocytosis of Weibel-Palade bodies links postischemic repair with inflammation and mobilization of stem cells.
Tissue regeneration is a finely tuned and well-orchestrated process requiring recruitment of stem and progenitor cells to the sites of injury. Mobilization of stem cells and endothelial progenitor cells (EPC) has been extensively documented in myocardial infarction, limb ischemia, vascular trauma, and acute renal ischemia.1–4 The very fact that stem and progenitor cells are mobilized and consistently found at the sites of injury, regardless of previous institution of any pharmacologic stimulation of their mobilization, alludes to the existence of intrinsic factor(s) generated during organ damage that may be responsible for their mobilization. Identification of such factors could expand the arsenal of pathogenetically relevant pharmacologic maneuvers hastening regenerative processes.
One of the default mechanistic responses to ischemia-reperfusion injury is represented by the activation of xanthine oxidoreductase and metabolism of purines.5 Potential role of metabolic products of this pathway as extracellular signaling molecules has been envisaged by Szent-Gyorgyi and pursued by Burnstock's laboratory.6 Having demonstrated renoprotective effects of ischemia-mobilized EPC in the setting of acute renal ischemia,4 we next performed a follow-up study that incriminated a product of xanthine oxidase metabolic pathway, uric acid (UA), as being a representative alarm signaling molecule discharged from the ischemic tissue and capable of downstream mobilization and recruitment of stem cells and EPS.7 Because the observed stem cells/EPC-mobilizing response to UA was relatively fast, we reasoned that putative second messenger(s) should preexist in a storage form, rather than depend on emergency on-demand synthesis. The organelle meeting this requirement is the Weibel-Palade body (WPB). These rod-shaped organelles (0.2 × 2.0 to 3.0 μm in size) contain an array of proteins, peptides, and cytokines that can be released urgently on demand. A list of compounds known to induce exocytosis of WPB is long and includes thrombin, histamine, peptido-leukotrienes, complement components, angiopoietin 2, IL-8, superoxide anion, vascular endothelial growth factor (VEGF), sphingosine-1-phosphate, ceramide, purine nucleotides, serotonin, vasopressin, and epinephrine.8 Here, we inquired whether WPB are exocytosed by the acutely and transiently elevated levels of UA, as it universally occurs after ischemia, and showed that this product of activated xanthine oxidase induces exocytosis of WPB at least in part via action on Toll-like receptor 4 (TLR-4), which was accompanied by the release of angiopoietin 2. The latter partially recapitulates the EPC-mobilizing effect of UA, raising the possibility of using UA and/or angiopoietin 2 for pharmacologic preconditioning.
RESULTS
Dynamics of Uric Acid–Induced Mobilization of Hematopoietic Stem Cells and EPC
In the previous studies, we demonstrated that renal ischemia induces transient elevation in the level of UA.7 To mimic it, here we injected monosodium urate (MSU) into male FVB mice and killed mice at various times after injection. The amount of injected MSU (50 μg) was sufficient to almost double its plasma concentration of UA, which was comparable to that detectable in the immediate postischemic period.7 As shown in Figure 1, mobilization of hematopoietic stem cells (HSC) occurred at 1 h and peaked at 3 h; mobilization and splenic sequestration of EPC was detectable at 1 h. Concurrent with the mobilization of stem/progenitor cells, the dynamics of cytokines in the peripheral blood of mice treated with MSU was examined. Notably, the plasma level of keratinocyte chemoattractant (KC), a murine analog of IL-8, which is stored in WPB and released upon exocytosis,9,10 was elevated already 1 h after injection and peaked at 3 h (from 9.5 ± 3.0 to 248.9 ± 46.8 pg/ml; Figure 2). Dynamics of other cytokines are shown in Table 1.
Figure 1.
EPC and HSC in peripheral blood and spleen after MSU treatment. Mice received an injection of 50 μg/25 g MSU and were killed at indicated times. Blood and spleen were collected for isolation of EPC and HSC by FACS analysis, as detailed in the Concise Methods section (n = 5 in each group for each time-point). *P < 0.05.
Figure 2.
Plasma levels of KC after monosodium urate injection. Here and below, * indicates P < 0.05, ** indicates P < 0.01, and *** indicates P < 0.001 unless otherwise indicated.
Table 1.
Serum cytokine/chemokine profile after injection of MSUa
| Cytokine/Chemokine | Control
|
15 min
|
1 h
|
3 h
|
24 h
|
P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| G-CSF | <2.300 | 4.480 | 2.175 | 47.550 | 29.200 | 84.090 | 11.990 | 70.030 | 32.860 | 0.0379b | |
| GM-CSF | <4.600 | <4.600 | <4.600 | <4.600 | 20.950 | 16.350 | 0.4380 | ||||
| IFN-γ | <0.700 | <0.700 | <0.700 | <0.700 | 7.350 | 6.650 | 0.4380 | ||||
| IL-1α | <4.400 | <4.400 | 56.070 | 34.510 | 99.610 | 26.440 | 75.570 | 26.630 | 0.0327b | ||
| IL-1β | 3.283 | 1.683 | <1.600 | 1.740 | 0.140 | 3.850 | 1.160 | 4.250 | 2.910 | 0.6812 | |
| IL-10 | <10.300 | <10.300 | <10.300 | <10.300 | <10.300 | ||||||
| IL-12 | <3.700 | <3.700 | <3.700 | <3.700 | 20.510 | 16.810 | 0.4380 | ||||
| IL-13 | 36.580 | 11.980 | 25.340 | 2.310 | 17.970 | 5.700 | 9.110 | 2.550 | 34.880 | 23.530 | 0.4885 |
| IL-15 | <9.300 | <9.300 | <9.300 | <9.300 | 10.550 | 1.250 | 0.4380 | ||||
| IL-17 | <1.700 | <1.700 | <1.700 | <1.700 | 2.620 | 0.920 | 0.4380 | ||||
| IL-2 | <0.800 | <0.800 | <0.800 | <0.800 | 1.880 | 1.080 | 0.4380 | ||||
| IL-4 | <0.300 | <0.300 | <0.300 | <0.300 | <0.300 | ||||||
| IL-5 | <0.600 | <0.600 | 1.609 | 1.009 | <0.600 | <0.600 | 0.4380 | ||||
| IL-6 | <0.700 | <0.700 | 6.650 | 5.180 | 1.260 | 0.330 | 6.850 | 6.010 | 0.5308 | ||
| IL-7 | <3.600 | <3.600 | <3.600 | <3.600 | 3.970 | 0.370 | 0.4380 | ||||
| IL-9 | <10.500 | 50.720 | 30.960 | <10.500 | 293.500 | 283.600 | 39.290 | 18.590 | 0.4974 | ||
| IP-10 | <6.000 | <6.000 | <6.000 | <6.00 | 24.880 | 18.880 | 0.4380 | ||||
| KC | 9.450 | 2.990 | 6.050 | 2.910 | 101.700 | 75.820 | 248.940 | 46.830 | 162.370 | 68.030 | 0.0168b |
| MCP-1 | 7.478 | 1.178 | <6.300 | 103.600 | 76.570 | 68.600 | 24.440 | 27.400 | 8.920 | 0.2900 | |
| MIP-1α | <2.800 | <2.800 | <2.800 | <2.800 | 10.160 | 7.358 | 0.4380 | ||||
| RANTES | 2.060 | 0.820 | <0.700 | <0.700 | 4.310 | 2.120 | 4.600 | 2.490 | 0.2309 | ||
| TNF-α | <0.900 | <0.900 | 1.146 | 0.2463 | <0.900 | 1.390 | 0.490 | 0.5444 | |||
Control mice received intraperitoneal injection of the vehicle, which was treated in the same way as MSU. MCP-1, monocyte chemoattractant protein 1; MIP-1α macrophage inflammatory protein 1-α.
P < 0.05.
Uric Acid Triggers Exocytosis of WPB In Vitro and In Vivo
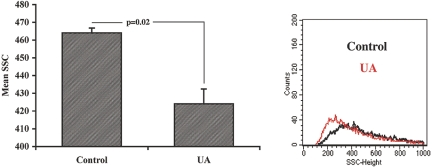
To gain insights into the cellular mechanism of the observed EPC-mobilizing effect of MSU, we considered the urgency of this reaction as an indicator of a release of an already synthesized messenger compound(s), intracellularly stored and awaiting activation or exocytosis. The previous demonstration of the surge in KC level after administration of MSU prompted us to examine other constituents of WPB. Application of MSU to cultured human umbilical vein endothelial cells (HUVEC) resulted in the rapid depletion of von Willebrand factor (vWF) and angiopoietin 2, markers of WPB, from endothelial cells (Figure 3). Immunocytochemical detection of vWF and angiopoietin 2 in HUVEC revealed that this effect occurred already at 5 min of exposure (Figure 3B). This effect was detectable even with the lowest concentration of MSU used (10 μg/ml), which resulted in the depletion of angiopoietin 2 from the HUVEC (Figure 3, C and D). Exocytosis of WPB was independently confirmed by FACS analysis. As shown in Figure 4, side scatter of HUVEC was reduced after MSU treatment, indicative of the reduction in cell volume and consistent with the degranulation reaction.
Figure 3.
Exocytosis of WPB induced by UA. (A and B) Treatment of HUVEC with MSU (100 μg/ml) resulted in a rapid exocytosis of WPB and the loss of cellular vWF and angiopoietin 2. Images were obtained and processed as detailed in the Concise Methods section. Quantitative analysis of fluorescence intensity for A. Approximately 100 individual cells were analyzed in each of three independent experiments. (C and D) Dosage dependence (10, 50, and 100 μg/ml) of MSU effect on the expression of angiopoietin 2 and vWF. Quantitative analysis of fluorescence intensity. *Significant decrease in the expression of vWF and angiopoietin 2.
Figure 4.
Decreased granularity (side scatter) of HUVEC after MSU treatment. (Right) Representative side-scatter tracings from control and MSU-treated HUVEC. Left) Summary of side-scatter experiments (P < 0.05, n = 3; Kolmogorov-Smirnov statistical analysis).
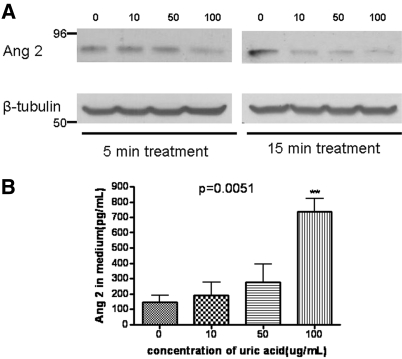
Western blot analysis of cell lysates (Figure 5A) and immunocytochemical staining (data not shown) revealed that the most prominent decrease of angiopoietin 2 abundance occurred at 15 min after application of MSU. Reciprocal changes in angiopoietin 2 were observed in the culture medium (Figure 5B). The concentration of angiopoietin 2 in the culture medium exhibited a mild elevation in response to 10 and 50 μg/ml UA and showed a five-fold increase to 100 μg/ml already after 15 min of incubation.
Figure 5.
Time course and dosage-response of angiopoietin 2 expression. (A) Western blot analysis of HUVEC lysates. (B) ELISA detection of angiopoietin 2 in the conditioned medium. Note that the most prominent effect was detectable after 15 min of treatment with the reciprocal changes in the culture medium.
In the in vivo study of a highly vascularized organ, the kidney, the decrease of angiopoietin 2 was also observed 15 min after the injection of MSU (Figure 6A). In addition, the serum level of angiopoietin 2 in MSU-treated mice (50 μg intraperitoneally) was elevated already after 20 min with a significant two-fold increase documented after 40 min (Figure 6B).
Figure 6.
Decreased expression of angiopoietin 2 in the kidneys and a reciprocal increase in the serum level after MSU administration. (A, top) Typical results of Western blot analysis of angiopoietin 2 abundance in the mouse kidney 15 min after intraperitoneal injection of MSU (50 μg). (A, bottom) Summary of the results presented in A. (B) Serum angiopoietin 2 in mice after MSU treatment. *Significant difference from control (P = 0.046; n = 3).
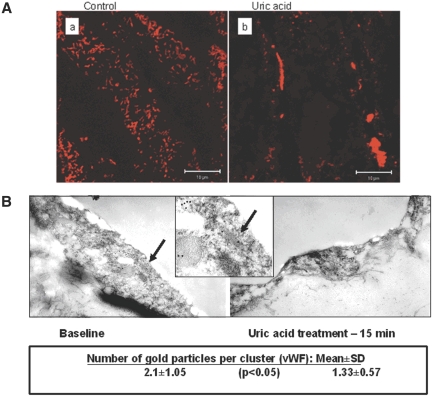
Immunohistochemical staining of en face aortic preparations obtained from mice pretreated with MSU (Figure 7A) showed a rich pattern of immunodetectable vWF in control but a scarce expression of this main component of WPB after administration of MSU. Immunoelectron microscopy of vWF and angiopoietin 2 in aortic endothelium was next performed. Mice administered an injection of MSU or a vehicle and studied 15 min later. As shown in Figure 7B, 10 nm of gold nanoparticles labeling vWF were abundant in control endothelia but decreased significantly after in vivo administration of MSU. Immunodetectable angiopoietin 2 was expressed at a much lower level, not always co-clustering with vWF, and could not be quantified. Collectively, these data amply complement the results of in vitro study in establishing that MSU acutely induces exocytosis of WPB and release of vWF and angiopoietin 2 into the circulation.
Figure 7.
Laser-scanning confocal microscopy of en face aorta and transmission immunoelectron microscopy of aortic endothelium. (A) Representative views of laser-scanning confocal microscopy of the endothelial layer of en face aorta from control and MSU-treated mice. (B) Immunoelectron microscopy of vWF in aortic endothelial cells. Note the labeling of vWF within WPB (insert) and reduction of immunodetectable vWF after MSU. vWF (10 nm gold) and angiopoietin 2 (6 nm gold particles) labeling of aortic endothelia at baseline (left) and 15 min after administration of MSU (right). Central panel provides a zoomed view (arrows) of WPB with vWF labeling. Angiopoietin 2 labeling is too sparse to be quantified. Quantification of vWF labeling showed a significant decline after UA. Magnification, ×29,000.
Angiopoietin 2–Induced Mobilization of HSC and EPC and Protection of Renal Function after Ischemia
Administration of angiopoietin 2 (1 μg per mouse, intravenously) to intact mice resulted in the surge of the number of HSC in the blood and spleen detected after 3 h (Figure 8A). EPC levels decreased in the splenic niche (Figure 8B). A similar phenomenon was previously observed under conditions of ischemic preconditioning, when the mobilized EPC evaded splenic sequestration and were shunted directly to the ischemic site.4
Figure 8.
Effects of angiopoietin 2 on HSC and EPC. (A) Rapid elevation of HSC (CD150+/c-Kit+ cells) in peripheral blood and spleen 3 h after angiopoietin 2 injection. *P = 0.02. (B) Mobilization of the splenic pool of CD34+/Flk-1+ EPC 3 h after angiopoietin 2 injection. *P = 0.03 (n = 3 in each group for each time point).
Because our previous studies showed that MSU pretreatment afforded renoprotection against ischemia-reperfusion injury,7 we next inquired whether this phenomenon could occur as a result of exocytosis of WPB; therefore, we tested the ability of angiopoietin 2–induced HSC/EPC mobilization to affect the severity of ischemic renal injury. Angiopoietin 2 (1 μg/25 g, intravenously) was administered 3 h before the induction of 30 min of bilateral renal ischemia in mice. Angiopoietin 2 pretreatment afforded renoprotection against ischemic insult, as judged from the stable plasma creatinine level in the treated group compared with the elevation in plasma creatinine in untreated ischemic mice (Figure 9). Thus, administration of a component of WPB, angiopoietin 2, released by a surge in UA level mimicked the effect of MSU administration. Similar results were obtained with intravenous injection of vWF (D.P. et al., manuscript in preparation).
Figure 9.
Angiopoietin 2 pretreatment prevents renal functional deterioration after renal ischemia (n = 3 to 6; *P = 0.0005 control versus ischemia; P = 0.02 control versus ischemia+angiopoietin 2; P = 0.005 ischemia versus ischemia+angiopoietin 2).
TLR-4 Mediates the Action of Uric Acid on WPB
The question of cellular target(s) of UA that is (are) involved in the observed action of MSU was addressed next. There are data implicating both TLR-2 and -4 in mediating some cellular actions of UA.11 Moreover, a recent report suggested that TLR-2 activation triggers exocytosis of WPB.12 Consequently, we attempted to dissect relations between UA, TLR, and exocytosis of WPB in vivo using TLR-2 and TLR-4 knockout mice. As shown in Figure 10, administration of UA to TLR-2–deficient mice resulted within 30 min in an increase in plasma levels of vWF and angiopoietin 2. In contrast, this effect was curtailed in TLR-4–deficient mice, indicating that TLR-4 was necessary for the execution of MSU effect on the release of vWF and angiopoietin 2. This was not the case in the wild-type mice: C57Bl/6J (background for TLR-2 knockout mice) and Balb/CJ (background for TLR-4 knockout mice) both showed significant increases in vWF and angiopoietin 2 levels 30 and 180 min after MSU administration.
Figure 10.
Release of vWF and angiopoietin 2 in TLR-2 but not in TLR-4 knockout mice after administration of MSU. After administration of UA (50 μg/mouse), TLR-2–deficient mice (n = 8 at baseline, n = 5 at 30 min, and n = 3 at 3 h) within 30 min had increased plasma levels of vWF and angiopoietin 2. This effect was abrogated in TLR-4–deficient mice (n = 8 at baseline, n = 5 at 30 min, and n = 3 at 3 h). In contrast, plasma KC was not increased in both TLR-2–and TLR-4–deficient mice.
Interestingly, MSU-induced elevation in circulating KC levels was absent in either TLR-2 or TLR-4 knockout mice, suggesting that the presence of both receptors is necessary for release of KC and that separate mechanism(s) of regulation of release of different constituents of WPB may exist. Three hours after MSU administration, levels of vWF and angiopoietin 2 returned to baseline and were indistinguishable between TLR-4–and TLR-2–deficient mice.
DISCUSSION
Data presented herein provide the first demonstration of the effect of UA, a universal companion of ischemia-reperfusion tissue injury, on TLR-mediated exocytosis of WPB, resulting in increased circulating levels of angiopoietin 2, which seemed to be sufficient to induce mobilization of HSC and EPC. This latter action has been linked to induction of pharmacologic preconditioning in various cardiovascular and renal diseases.4,13 As such, the findings presented herein may serve as a blueprint toward design of novel strategies for pharmacologic preconditioning.
Distinct mechanistic pathways may be implicated in accomplishing local and systemic reactions to ischemia or other stressors. It has been shown that hypoxia per se activates exocytosis of WPB14 (although UA levels were not determined), the event that may take place within the ischemic organ. Distant regulation of exocytosis of WPB can be accomplished, according to the data presented here, via release of the product of xanthine oxidase activation in the ischemic organ—UA. The same mediator was previously implicated in the cell injury–induced activation of dendritic cells.15 This type of regulation may represent a novel paradigm of alarm signaling in response to injury. Data demonstrated (Figure 10) that this action of urate can be mediated via both TLR-4 and TLR-2: TLR-4 is necessary for the release of vWF and angiopoietin 2, whereas both receptors are required for release of KC. Intriguingly, vWF and angiopoietin 2 responses to urate in TLR-2 knockout mice seem to be somewhat exaggerated compared with the wild-type mice. It is not clear whether it reflects some antagonistic relations between TLR-2 and TLR-4 or other mechanisms contribute to this enhanced response. Recent demonstration of bacterial lipotheicoic acid recognition by TLR-2 resulting in exocytosis of WPB, although TLR-4 actions were not investigated, is in concert with our findings (Figure 11).12
Figure 11.
A schematic view of the mechanistic cascade triggered by renal ischemia-reperfusion acute and transient surge in UA, which acting on TLR-4 and TLR-2 leads to mobilization of different components of WPB and eventually determines the balance between the pro-repair and proinflammatory sequelae. Antagonistic relations between TLR-2 and TLR-4 may exist, but clarification of this will require further studies.
In addition to UA, other mediators may use a similar mechanism of exocytosis of WPB to induce mobilization of EPC. It was previously found that VEGF is a potent EPC-mobilizing agonist16; however, VEGF has been shown to induce exocytosis of WPB.17 On the basis of the present demonstration that angiopoietin 2 is capable of mobilizing HSC and EPC, such a mechanism may explain at least in part the described effect of VEGF.
Angiopoietin 2 has been localized to WPB from which it can be rapidly released.18 Competing with angiopoietin 1 for Tie-2 receptor, angiopoietin 2 has been implicated in pleiotropic actions depending on the biologic context, from proinflammatory with increased vascular permeability to cytoprotective in stressed endothelial cells19–24; therefore, a delicate balance could be envisaged between the proinflammatory actions of angiopoietin 2 (among other released constituents of WPB) and its HSC- and EPC-mobilizing action serving as a pharmacologic preconditioner. It is quite possible that a more severe tissue injury and/or higher levels of UA result in an intense angiopoietin 2 signaling with proinflammatory actions prevailing over its HSC- and EPC-mobilizing effect. In fact, angiopoietin 2 release has been shown to be responsible for the sepsis-induced leakage of pulmonary blood vessels.25 It was also suggested that angiopoietin 2 participates in the recruitment of bone marrow–derived endothelial precursors, as well as stimulates EPC migration.26,27 It is quite possible that the observed recruitment of stem cells in our studies represents another facet of the same phenomenon—the stress-induced response.
On a broader scale, our findings provide further experimental support to the “danger model”—a conceptual link between immune- and non–immune-mediated tissue injury.28 The essence of this theory is that many signaling elements initiating inflammation in response to invading organisms and alarm signals released from injured/necrotic tissues are shared and elicit similar responses at the tissue level. A host of alarm signals, “alarmins,” has been described in immunologic literature, UA being one of them.15,29 Thus, our studies enrich the spectrum of physiologic actions of the acute and reversible surge in UA levels by demonstrating the ability of UA to effect exocytosis of WPB and mobilize stem cells in response to ischemic signaling.
CONCISE METHODS
Endothelial Cell Culture and Treatments
HUVEC were purchased from Clonetics (Walkersville, MD) and used between passages 5 and 7. Cells were cultured in EBM-2 medium (Cambrex Bio Science, Walkersville, MD) with EGM-2 SingleQuots supplements (Cambrex Bio Science) and maintained at 37°C incubator with 5% CO2. For cell culture experiments, UA was prepared as previously reported.30 Briefly, UA sodium salt (MSU), 10 to 100 μg/ml (Sigma-Aldrich, St. Louis, MO) was added to prewarmed EBM-2 medium (37°C). The mixture was agitated at 37°C for 30 min and then passed through a sterile 0.22-μm filter. Control media were treated similarly but with the omission of UA. For animal experiments, MSU was dissolved in sterile water, heated in a microwave, and filtered through a 0.22-μm filter.
Immunocytochemical Staining
HUVEC (approximately 104 cells) were seeded on chamber slides (Lab-TekII Chamber Slide System; Nalge Nunc Int., Rochester, NY) and grown in EGM-2 medium until at least 80% confluent. The cells were treated with various concentrations of UA (0, 10, 50, and 100 μg/ml) for various periods of time (5, 15, and 60 min). After treatment, cells were fixed with 4% paraformaldehyde, permeabilized with 0.25% Triton-X 100 in PBS (pH 7.4), and stained with antibodies against vWF (1:200; F3520, rabbit anti-human vWF; Sigma) and angiopoietin 2 (1:100; Sc-7017; Santa-Cruz Biotechnology, Santa Cruz, CA). Nonspecific protein binding was blocked by 1 h of incubation with 1% BSA (Sigma) in PBS for vWF staining and 5% rabbit serum (Sigma) in PBS for angiopoietin 2 staining. Incubations with primary and secondary antibodies (FITC-conjugated rabbit anti-goat and Texas Red dye–conjugated donkey anti-rabbit; Jackson Immunoresearch, West Grove, PA) were performed for 1 h at room temperature. For visualization of the nuclei, cells were co-stained with Hoechst 33342 (Invitrogen, Carlsbad, CA). Slides were mounted using an antifade reagent (Slowfade; Invitrogen). Each experiment was performed at least three times with fluorescence intensity of approximately 100 cells digitally quantified using MetaMorph image analysis routines.
Western Blot Analysis
Passages 5 through 7 HUVEC were cultured in 100-mm tissue culture dishes (BD Falcon, San Jose, CA) until confluent. After washing twice with PBS, cells were cultured in serum-free EBM-2 medium with various concentrations of MSU (0, 10, 50, and 100 μg/ml) for various times (5, 15, and 60 min). The harvested cells were lysed in RIPA buffer (1% Triton-X 100, 0.1% SDS, and 0.5% sodium deoxycholate in PBS) with proteinase inhibitors (Complete tablet; Roche, Basel, Switzerland). Cell lysates were dissolved in Laemmli buffer, boiled at 95°C for 5 min, and separated on 4 to 12% SDS-PAGE (Invitrogen). The proteins were electrotransferred to a polyvinylidene difluoride membrane (Millipore, Medford, MA). After blocking with 5% wt/vol nonfat dry milk, membranes were incubated at 4°C overnight with primary antibody (goat anti–angiopoietin 2, Sc-7017, 1:50; Santa-Cruz Biotechnology), followed by incubation with horseradish peroxidase–conjugated anti-goat IgG (Sc- 2020, 1:10,000; Santa-Cruz Biotechnology) for angiopoietin 2. Detection was performed using enhanced chemiluminescence (Pierce, Rockford, IL) and exposure to x-ray film. The same membrane was reprobed with β-tubulin–specific antibody (T 5293; Sigma) to ensure equal protein loading. Relative protein levels were calculated as densitometric ratios to β-tubulin.
ELISA for Angiopoietin 2 and vWF
Confluent HUVEC were washed with PBS, and medium was exchanged to serum-free EBM-2 with additions of UA and collected at various times for measurements of secreted angiopoietin 2 by ELISA Quantikine (R&D Systems, Minneapolis, MN) kit. For determination of angiopoietin 2 in the serum of mice, indirect ELISA was performed by coating standard antigen (Recombinant Ang2; R&D Systems) or serum at 4°C overnight on a microplate (Maxisorp; Nunc [Fisher Scientific, Rochester, NY]) and incubating at room temperature for 1 h with goat anti–angiopoietin 2 (Sc-7017, 1:40; Santa-Cruz Biotechnology), followed by incubation with horseradish peroxidase–conjugated anti-goat IgG (Sc-2020, 1:500; Santa-Cruz Biotechnology). Detection was performed by adding peroxidase substrate (SureBlue TMB; KPL, Gaithersburg, MD) and TMB stop solution (KPL). For detection of vWF, capture antibodies were used at 1:200 dilution and reaction with 100 μl of the plasma sample (1:32 dilution), followed by secondary antibodies at 1:750 dilution with extensive washings between these procedures. Plates were read at a wavelength of 450 nm.
FACS Analysis
For quantification of peripheral circulating and splenic EPC and HSC by FACS, mononuclear cells were isolated either from 500 μl of peripheral blood or from tissue homogenates by density gradient centrifugation using Histopaque-1077 (Sigma-Aldrich) solution. For preparing splenic tissue homogenates, the whole organ was placed in 2 ml of RPMI 1640 (Invitrogen) at 4°C. The tissue was minced and immediately homogenized, according to a previously published technique with minor modifications.31 Cells were incubated for 30 min on ice with FITC-conjugated anti-mouse CD34 (RAM34; eBioscience, San Diego, CA) and PE-conjugated anti-mouse Flk-1 (Avas12α1; BD Biosciences, Rockville, MD) or with FITC-conjugated anti-mouse CD117 (c-Kit; BD Biosciences) and PE-conjugated anti-mouse CD150 (eBioscience). After incubation, cells were washed with PBS and fixed in 4% paraformaldehyde. Data were acquired using a FACScan cytometer equipped with a 488-nm argon laser and a 635-nm red diode laser and analyzed using CellQuest software (Becton Dickinson, San Jose, CA). The setup of FACScan was performed using unstained cells. For quantification of EPC, the number of CD34/Flk-1 double-positive cells within the monocytic cell population was counted. Side-scatter parameter was used to assess exocytosis of WPB in HUVEC after UA treatment (100 μg/ml) and compared with nontreated cells.
Surgical Procedures
Male FVB/NJ mice were obtained from Jackson Laboratory and used at the age of 11 to 12 wk. UA (50 μg/100 μl in distilled water) was injected intraperitoneally. Mice (n = 4 in each group) were killed at 5 and 15 min and 1, 3, and 24 h after injection.
Male TLR-2 knockout (B6.129-Tlr2tm1Kir/J) and TLR-4 knock out (C.C3-Tlr4Lps-d/J) mice were purchased from Jackson Laboratory. UA (50 μg/100 μl in distilled water) was injected intraperitoneally. Mice (n = 5 in each group) were killed at 30 min and 3 h after injection. Background strains Balb/CJ (for TLR-4 knockout) and C57Bl/6J (for TLR-2 knockout) were treated and examined similarly.
For renal ischemia induction, mice were anesthetized with intraperitoneal injection of ketamine hydrochloride (6 mg/100 g) and xylazine hydrochloride (0.77 mg/100 g) and placed on a heated surgical pad. Rectal temperature was maintained at 37°C. After a 1.5-cm mid-laparotomy, the kidneys were exposed and clamping of the renal pedicles performed with microserrefines (Fine Science Tools, Foster City, CA). After 25 min, the clamps were released. Mice were killed 48 h after surgery, and blood, kidneys, and spleen were collected for further analysis. For sham operation, mid-laparotomy without vascular clamping was performed.
Chemokine and Cytokine Measurements
Multiplex assay kit (Linco Research, MCYTO-70K-PMX, Millipore Corp.) was used for the simultaneous quantification of the following mouse cytokines and chemokines: IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12(p70), IL-13, IL-15, IL-17, IFN-γ, IP-10, G-CSF, GM-CSF, TNF-α, KC, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and RANTES. All analytes were tested individually and in combination to ensure that there were no cross-reactions. Briefly, the cytokine standards were resuspended in assay buffer and serially diluted. Twenty-five microliters of standard, quality controls, or sample was added to each well of a 96-well plate with 25 μl of the bead solution. The plate was sealed, covered with aluminum foil, and incubated overnight (16 to 18 h) with agitation on a plate shaker at 4°C. Then the plate was washed twice with 200 μl/well wash buffer, with removal of buffer by vacuum filtration between each wash. This was followed by addition of 25 μl of a detection antibody cocktail into each well and incubation at room temperature for 1.5 h. Streptavidin-phycoerythrin solution (25 μl) was added to each well and incubated at room temperature for 30 min. The plate was then analyzed on the Luminex IS100 analyzer (Luminex, Austin, TX). The data were saved and evaluated as median fluorescence intensity using appropriate curve-fitting software (Luminex 100IS 2.3). A five-parameter logistic method with weighting was used. All measurements were performed in duplicate.
Immunoelectron Microscopy of vWF.
Anesthetized mice received intraperitoneally injection of MSU (50 μg/mouse) or the equal amount of vehicle, killed after 15 min, and perfused with PBS followed by 4% PFA. Thoracic aortas were gently removed, sectioned, and postfixed. Tissue wedges were dissected and stored in PBS overnight at 4°C. Tissue wedges were further processed as follows: Dehydration in 50, 70, 85, 95, 100, 100, and 100% cold (4°C) ethanol, 15 min at each step; infiltration with LRWhite resin (resin:ethanol 1:1) for 90 min then pure resin for 24 h, both steps at 4°C; and embedding with fresh LRWhite resin and polymerization at 55°C overnight. Ultrathin sections were collected on Nickel grids. For immunolabeling, sections were incubated in the blocking buffer (BB) for donkey secondary antibodies (Aurion; Electron Microscopy Sciences, Hatfield, PA) for 15 min; anti-vWF 1:50 in BB or angiopoietin 2 1:50 in BB for 1 h; washed three times for 5 min each in BB, followed by the secondary antibodies donkey anti-rabbit IgG/10 nm gold or donkey anti-goat IgG/6 nm gold both at 1:100 in BB (Aurion) for 1 h. Grids were washed three times for 3 min in PBS then two times for 1 min in water and counterstained with uranyl acetate (aqueous) for 15 min, followed by lead citrate for 5 min. Preparations were examined at 80 Kv in a JEOL (Peabody, MA) 100-cx II. Images were captured on Kodak (Rochester, NY) 4489 film, negatives were scanned into digital format on an Epson Expression 1600 professional flatbed scanner with transillumination, and the number of nano-gold particles per cluster was counted.
Measurement of Serum Creatinine and UA.
Serum creatinine concentration was measured using a commercially available kit (Raichem, San Diego, CA). Serum concentration of UA was measured using the Amplex Red UA/uricase assay kit (Invitrogen).
Statistical Analysis
All of the values were expressed as means ± SE. Significant of difference between two groups were tested by two-tailed t test. One-way ANOVA and post test with Tukey multiple comparison test was used for three or more groups. P < 0.05 was considered as a significant difference.
DISCLOSURES
None.
Acknowledgments
These studies were supported in part by National Institutes of Health grants DK 52783, DK45462, and DK54602 (M.S.G.) and Westchester Artificial Kidney Foundation. M.-C.K. was on sabbatical from Kaohsiung Medical University Hospital (Kaohsiung, Taiwan).
We express our gratitude to Carl Nathan (Weill Medical College, Cornell University, New York, NY) for valuable insights.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T: Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5: 434–438, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T: Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 103: 2776–2779, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S: Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res 88: 167–174, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS: Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: Modulation by ischemic preconditioning. Am J Physiol Renal Physiol 291: F176–F185, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Zimmet JM, Hare JM: Nitroso-redox interactions in the cardiovascular system. Circulation 114: 1531–1544, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Ralevic V, Burnstock G: Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 7.Patschan D, Patschan S, Gobe GG, Chintala S, Goligorsky MS: Uric acid heralds ischemic tissue injury to mobilize endothelial progenitor cells. J Am Soc Nephrol 18: 1516–1524, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Lowenstein CJ, Morrell CN, Yamakuchi M: Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med 15: 302–308, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G: Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J Exp Med 188: 1751–1756, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolff B, Burns AR, Middleton J, Rot A: Endothelial cell “memory” of inflammatory stimulation: Human venular endothelial cells store interleukin 8 in Weibel-Palade bodies. J Exp Med 188: 1757–1762, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R: Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum 52: 2936–2946, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Into T, Kanno Y, Dohkan J, Nakashima M, Inomata M, Shibata K, Lowenstein CJ, Matsushita K: Pathogen recognition by Toll-like receptor 2 activates Weibel-Palade body exocytosis in human aortic endothelial cells. J Biol Chem 282: 8134–8141, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Losordo DW, Dimmeler S: Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: Angiogenic cytokines. Circulation 109: 2487–2491, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Pinsky DJ, Naka Y, Liao H, Oz MC Wagner DD, Mayadas TN, Johnson RC, Hynes RO, Heath M, Lawson CA, Stern DM: Hypoxia-induced exocytosis of endothelial cell Weibel-Palade bodies: A mechanism for rapid neutrophil recruitment after cardiac preservation. J Clin Invest 97: 493–500, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Evans JE, Rock KL: Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425: 516–521, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM: VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18: 3964–3972, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita K, Yamakuchi M, Morrell CN, Ozaki M, O’Rourke B, Irani K, Lowenstein CJ: Vascular endothelial growth factor regulation of Weibel-Palade-body exocytosis. Blood 105: 207–214, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG: The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103: 4150–4156, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55–60, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, Papapetropoulos A: Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther 314: 738–744, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Lemieux C, Maliba R, Favier J, Theoret JF, Merhi Y, Sirois MG: Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood 105: 1523–1530, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Daly C, Pasnikowski E, Burova E, Wong V, Aldrich TH, Griffiths J, Ioffe E, Daly TJ, Fandl JP, Papadopoulos N, McDonald DM, Thurston G, Yancopoulos GD, Rudge JS: Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci U S A 103: 15491–15496, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harfouche R, Hussain SN: Signaling and regulation of endothelial cell survival by angiopoietin-2. Am J Physiol Heart Circ Physiol 291: H1635–H1645, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, Lee PJ, Geick A, de Fougerolles AR, Elias JA: Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med 12: 1286–1293, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP: Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 3: e46, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udani V, Santarelli J, Yung Y, Cheshier S, Andrews A, Kasad Z, Tse V: Differential expression of angiopoietin-1 and angiopoietin-2 may enhance recruitment of bone-marrow-derived endothelial precursor cells into brain tumors. Neurol Res 27: 801–806, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Gill KA, Brindle NP: Angiopoietin-2 stimulates migration of endothelial progenitors and their interaction with endothelium. Biochem Biophys Res Commun 336: 392–396, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Matzinger P: The danger model: A renewed sense of self. Science 296: 301–305, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Bianchi ME: DAMPs PAM: Ps and alarmins—All we need to know about danger. J Leukoc Biol 81: 1–5, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ: Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 41: 1287–1293, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Church LD, Hessler G, Goodall JE, Rider DA, Workman CJ, Vignali DA, Bacon PA, Gulbins E, Young SP: TNFR1-induced sphingomyelinase activation modulates TCR signaling by impairing store-operated Ca2+ influx. J Leukoc Biol 78: 266–278, 2005 [DOI] [PubMed] [Google Scholar]