Abstract
Complement activation plays a key role in mediating apoptosis, inflammation, and transplant rejection. In this study, the role of the complement 5a receptor (C5aR) was examined in human renal allografts and in an allogenic mouse model of renal transplant rejection. In human kidney transplants with acute rejection, C5aR expression was increased in renal tissue and in cells infiltrating the tubulointerstitium. Similar findings were observed in mice. When recipient mice were treated once daily with a C5aR antagonist before transplantation, long-term renal allograft survival was markedly improved compared with vehicle-treatment (75 versus 0%), and apoptosis was reduced. Furthermore, treatment with a C5aR antagonist significantly attenuated monocyte/macrophage infiltration, perhaps a result of reduced levels of monocyte chemoattractant protein 1 and the intercellular adhesion molecule 1. In vitro, C5aR antagonism inhibited intercellular adhesion molecule 1 upregulation in primary mouse aortic endothelial cells and reduced adhesion of peripheral blood mononuclear cells. Furthermore, C5aR blockade markedly reduced alloreactive T cell priming. These results demonstrate that C5aR plays an important role in mediating acute kidney allograft rejection, suggesting that pharmaceutical targeting of C5aR may have potential in transplantation medicine.
The complement activation products C3a and C5a, known as the anaphylatoxins, are potent proinflammatory mediators that play crucial roles in the pathogenesis of infection and inflammation.1 C5a is a strong chemoattractant for neutrophils and macrophages and signals through C5a receptor (C5aR), which is a classical G protein–coupled receptor. C5aR is widely expressed on immune cells (neutrophils, macrophages, and dendritic cells [DC]),2,3 microvascular endothelial cells,4 alveolar epithelial cells,5,6 and renal glomerular mesangial cells7,8 and tubular epithelial cells.9 Different components of the complement cascade have been investigated in experimental transplant models. Strategies reducing complement activation by using soluble complement receptor 1,10 local C3 deficiency,11 or anti-C5 mAb12 resulted in partial inhibition of tissue injury and significant reduction in infiltrating leukocytes in rat renal and heart allografts. In addition, the clinical relevance of complement inhibition by soluble complement receptor 1 was shown in a multicenter trial, in which short-term blockade of complement before reperfusion led to an improved function of transplanted lungs.13
Another important aspect of complement activation is its role in ischemia-reperfusion (IR) injury. In several models of experimental IR injury of liver,14 limb,15 heart,16 and kidney,17,18 C5a or C5aR blockade successfully attenuated organ damage. Complement activation during ischemia and reperfusion contributes to the development of delayed graft function and tissue injury, which in turn negatively affects long-term outcome in transplantation19 and also activates the innate immune system of the donor and the recipient.20 C5 blockade has been shown to reduce ischemia-induced apoptosis,21 leukocyte adherence to endothelium, and decreased microvascular permeability in myocardial ischemia.16 In this study, we wanted to characterize the role of C5aR in acute kidney allograft rejection. We assessed the expression patterns of C5aR on human protocol biopsies after kidney transplantation with acute cell-mediated rejection, chronic tubulointerstitial damage (i.e., interstitial fibrosis and tubular atrophy [IF/TA]), and normal renal allograft morphology. Furthermore, we determined the efficacy of C5aR blockade in a life-supporting (no remaining host kidney) mouse model of acute renal allograft rejection. We found that short-term treatment of the recipient with a C5aR antagonist (C5aRA) significantly improved renal allograft survival when treatment was initiated before transplantation. Importantly, C5aR targeting reduced apoptosis, macrophage influx that was associated with decreased upregulation of monocyte chemoattractant protein 1 (MCP-1) and intercellular adhesion molecule 1 (ICAM-1), and alloreactive T cell priming.
RESULTS
C5aR Expression in Human and Experimental Renal Allograft Rejection
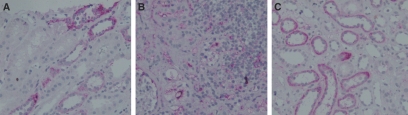
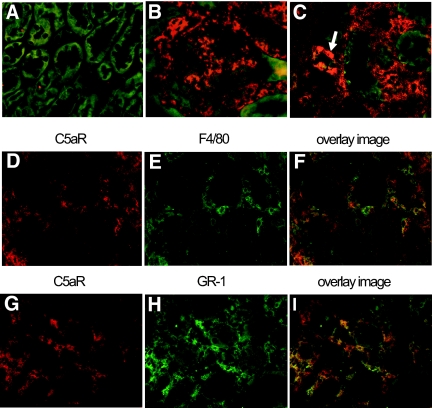
C5aR staining was performed on human protocol biopsies (Figure 1). Ten biopsies with T cell–mediated rejection (TCMR) grade Banff Ia and Ib and nine biopsies showing IF/TA grades I and II were compared with seven histologically normal protocol biopsies. Patient characteristics are shown in Table 1. The most eminent finding was that biopsies showing TCMR grade Banff Ib had high numbers of C5aR-positive infiltrating cells (Figure 1B). No specific C5aR expression was detected in the glomeruli. In the tubuli, we did not detect any significant differences between normal biopsies (Figure 1A) and biopsies with TCMR (Figure 1B) or IF/TA (Figure 1C). C5aR expression could be seen in nonatrophic and atrophic tubules of the cortex and in average was detected in <10% of proximal tubuli and 10 to 30% of distal tubuli. Next, we investigated whether transplantation-induced upregulation of C5aR occurs in rejecting kidney allografts in mice. No C5aR expression was detected in normal renal mouse tissue (Figure 2A). We detected strong upregulation of C5aR expression in the tubulointerstitial infiltrating cells and in mesangial cells of rejecting mouse kidney allografts (grade Banff Ia) at 6 d after transplantation (Figure 2, B and C). Furthermore, as observed in the human allografts, C5aR expression was detected in atrophic tubules of the cortex (Figure 2C, arrow).
Figure 1.
C5aR expression in human renal allograft rejection. (A) C5aR expression in proximal and distal tubuli of normal protocol biopsies. (B) C5aR expression in interstitial infiltrating cells of cases with TCMR Banff Ib. (C) Tubular C5aR expression in cases with IF/TA.
Table 1.
Patient characteristics of protocol biopsiesa
| Characteristic | Histology
|
|||
|---|---|---|---|---|
| Normal(n = 7) | TCMR Grade Ia(n = 5) | TCMR Grade Ib(n = 5) | IF/TA(n = 9) | |
| Recipient age | 42 ± 5 | 42 ± 7 | 51 ± 7 | 52 ± 7 |
| Donor age | 55 ± 3 | 57 ± 3 | 42 ± 12 | 51 ± 6 |
| Cold ischemia time (h) | 12 ± 4 | 14 ± 3 | 17 ± 2 | 14 ± 2 |
| No. of HLA mismatches | 3 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 |
| Serum creatinine at time of protocol biopsy (μmol/L) | 274 ± 92 | 175 ± 39 | 215 ± 66 | 228 ± 40 |
| Lowest serum creatinine within 6 wk after transplantation (μmol/L) | 142 ± 29 | 115 ± 25 | 163 ± 41 | 200 ± 25 |
Data are means ± SEM.
Figure 2.
C5aR expression in experimental renal allograft rejection in mice. (A) No C5aR expression was detected in a normal renal mouse tissue. (B and C) Upregulation of C5aR expression in rejecting mouse renal allografts 6 d after transplantation in mesangial cells of the glomeruli (B), tubulointerstitial infiltrates (C), and atrophic tubuli (C, arrow). Immunohistochemistry for C5aR and infiltrating cells was performed on renal allografts at day 6 after transplantation. (D, E, G, and H) Cryosections were immunostained with goat anti-mouse C5aR antibody visualized by Cy3-conjugated secondary antibodies (D and G), rat anti-mouse monocytes/macrophages F4/80 antibody (E), or monoclonal rat anti-mouse neutrophils Gr-1 antibody (H) visualized by Cy2-conjugated secondary antibodies. (F and I) The overlay Cy3/Cy2 color fluorescence cell images demonstrate the expression of C5aR on infiltrating monocytes/macrophage (F) and on infiltrating neutrophils (I).
Double-staining experiments of saline-treated allografts at 6 d after transplantation revealed C5aR expression only on F4/80-positive monocytes/macrophages (Figure 2, D through F) and Gr-1–positive neutrophils (Figure 2, G through I), but not on CD4+ or CD8+ infiltrating T lymphocytes (data not shown). These data point toward an activation of the C5a/C5aR system in both human and mouse allograft rejection.
C5aR Antagonism Resulted in Long-Term Survival after Experimental Allogeneic Kidney Transplantation
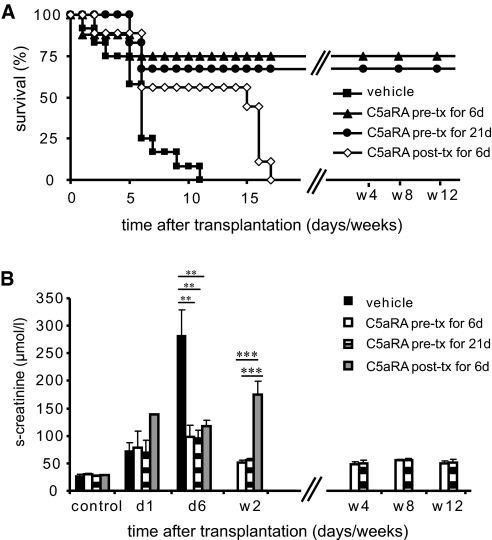
To study the effect of C5aR inhibition on acute kidney allograft rejection, we used a mouse renal transplant model. Recipients were treated once daily with either vehicle or C5aRA for 6 d or 3 wk. Treatment was initiated either before transplantation or immediately after transplantation once reperfusion had already occurred. All vehicle-treated recipients died within 11 d after surgery. In contrast, initiation of treatment with the C5aRA JPE-1375 before transplantation caused long-term survival (12 wk) in 75% of the recipients receiving C5aRA treatment for 6 d and in 67% of the recipients receiving C5aRA treatment for 21 d. When C5aRA treatment was initiated immediately after transplantation, allograft rejection was delayed but not prevented. Long-term survival was not obtained (Figure 3A). These results suggest that C5aR is a critical effector in acute renal allograft rejection and may indicate that C5aR contributes more to the initial ischemia-induced events of allograft injury.
Figure 3.
C5aR inhibition caused long-term survival and attenuated loss of renal function after allogeneic kidney transplantation. (A) Survival after allogeneic kidney transplantation in a mouse model. Vehicle treatment (▪) caused 100% mortality within 11 d after transplantation. In contrast, C5aR inhibitor treatment initiated before transplantation for 6 (▴) and 21 d (•) caused long-term allograft survival over 12 wk. When C5aRA was initiated after transplantation, no long-term survival was obtained (⋄). (n = 10 mice were investigated for each group.) (B) Renal function by serum creatinine measurement. Recipients receiving vehicle treatment (▪) and both C5aR inhibitor–pretreated groups receiving treatment over 6 d (□) or over 3 wk (  ) had a similar rise of serum creatinine at 24 h after transplantation, correlating with the early ischemic injury. In contrast, at 6 d after transplantation, significant differences were detected between groups. Vehicle treatment caused a further rise in serum creatinine, whereas C5aR inhibitor–treated recipients remained stable at slightly elevated serum creatinine levels. C5aRA treatment initiated after transplantation (
) had a similar rise of serum creatinine at 24 h after transplantation, correlating with the early ischemic injury. In contrast, at 6 d after transplantation, significant differences were detected between groups. Vehicle treatment caused a further rise in serum creatinine, whereas C5aR inhibitor–treated recipients remained stable at slightly elevated serum creatinine levels. C5aRA treatment initiated after transplantation ( ) and given over 6 d caused a delayed rise in serum creatinine at day 14, indicating delayed rejection with loss of renal function. Data are means ± SEM; **P < 0.01; ***P < 0.001.
) and given over 6 d caused a delayed rise in serum creatinine at day 14, indicating delayed rejection with loss of renal function. Data are means ± SEM; **P < 0.01; ***P < 0.001.
C5aR Inhibition Preserved Renal Function after Experimental Allogeneic Kidney Transplantation
Renal function was studied by measurement of serum creatinine levels (Figure 3B). An early increase in serum creatinine was detected in all groups 24 h after transplantation as a result of transplant-associated IR injury. Six days after transplantation, vehicle-treated recipients developed severe loss of renal function as reflected in profound serum creatinine elevation (281 ± 48 μmol/L). In contrast, the renal function of recipients receiving C5aRA treatment (98 ± 21, 95 ± 15, and 116 ± 10.9 μmol/L for C5aRA-treated recipients) remained stable without any further significant increase in serum creatinine. At 14 d, allograft recipients that received C5aRA treatment immediately after transplantation for 6 d showed delayed loss of renal function with a marked increase of serum creatinine (175 ± 25 μmol/L). In contrast, recipients that received C5aRA treatment before transplantation showed only moderate serum creatinine elevation (55 ± 6 and 55 ± 3 μmol/L) at 14 d. These results suggest that C5aR activation contributes significantly to the loss of renal function in this model of allogenic kidney transplantation.
C5aRA Treatment Markedly Reduced Apoptosis
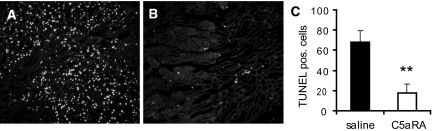
Organ transplantation is invariably associated with ischemia-induced apoptosis and might contribute to delayed graft function. To detect cell death, we performed a terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) assay (Figure 4) 1 d after transplantation. Allografts from vehicle-treated recipients showed high numbers of TUNEL-positive cells (Figure 4A). In contrast, initiation of C5aRA treatment before transplantation significantly reduced (P < 0.01) the frequency of TUNEL-positive cells (Figure 4B), indicating the crucial role of C5aR in IR-induced apoptosis. Because the proinflammatory cytokine TNF-α is an established inducer of apoptosis22,23 and upregulation of TNF-α has been demonstrated in IR injury,24,25 we investigated the expression of TNF-α mRNA in allografts from vehicle-treated recipients compared with those from C5aRA-treated recipients. As expected, we observed a strong transplant-induced upregulation of TNF-α mRNA expression in allografts from vehicle-treated recipients at 1 d after transplantation (P < 0.01 versus control). A similar upregulation of TNF-α mRNA expression in allografts from C5aRA-treated recipients was observed (data not shown), suggesting that TNF-α is not responsible for the diminished apoptosis in response to C5aRA treatment.
Figure 4.
C5aRA treatment significantly reduced posttransplantation apoptosis. Representative specimens after TUNEL assay 24 h after transplantation are shown. (A) Allografts from vehicle-treated recipients had increased numbers of TUNEL-positive cells mainly in the tubular epithelium of the outer strip of the outer medulla. (B) Allografts from C5aRA-treated recipients showed markedly decreased numbers of TUNEL-positive cells. (C) Quantification of TUNEL-positive cells is presented as mean ± SEM; **P < 0.01; six mice were investigated in each group. Magnification, ×200.
C5aRA Treatment Attenuated Inflammatory Cell Infiltration
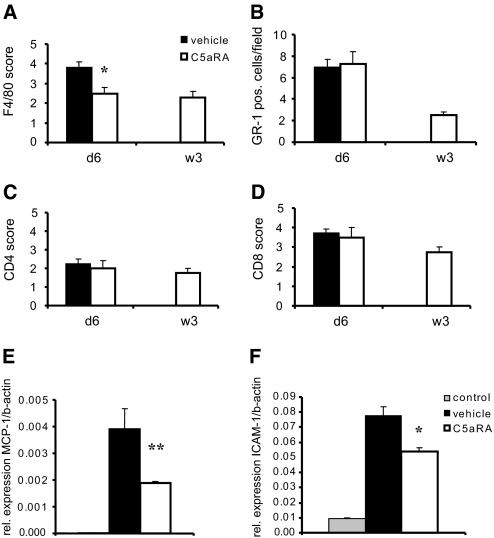
Inflammatory cell infiltration of allografts with host leukocytes is a hallmark of acute allograft rejection; therefore, we performed immunohistochemistry for different leukocyte subsets to elucidate the composition of the cell infiltrates at 6 d (Figure 5, A through D). We found significantly reduced amounts of monocytes/macrophage infiltration in allografts from C5aRA-pretreated recipients (P < 0.05) compared with those from vehicle-treated recipients. No significant differences in the amount of infiltrating neutrophils or CD4+ or CD8+ cells between the groups were observed at 6 d.
Figure 5.
Effect of C5aRA treatment on inflammatory cell infiltration. For analysis of infiltrating cells, subsets of leukocytes were stained in renal allografts at 6 d and 3 wk after transplantation. (A) C5aRA treatment caused reduced numbers of infiltrating F4/80-positive monocytes/macrophages that were still detectable at 3 wk after transplantation. (B through D) No significant differences in numbers of Gr-1–positive neutrophils (B) or CD4+ (C) and CD8+ (D) T lymphocytes were detected. *P < 0.05; six mice were investigated for each group. (E and F) MCP-1 (E) and ICAM-1 (F) mRNA expression of normal control kidneys versus allografts from vehicle- and C5aRA-pretreated recipients was investigated at 24 h after transplantation. Allogeneic kidney transplantation caused a massive upregulation of MCP-1 and ICAM-1 expression in allografts from vehicle-treated recipients. C5aRA treatment reduced both MCP-1 and ICAM-1 upregulation significantly compared with vehicle treatment. Data are means ± SD. **P < 0.01; six mice were investigated for each group.
C5aRA Treatment Attenuated Upregulation of Chemokines and Adhesion Molecules
To explore the contribution of chemokines to the attenuated monocyte/macrophage infiltration by C5aRA treatment, we investigated MCP-1 expression. Members of the chemokine family play a central role in inflammatory cell infiltration into extravascular sites by attracting and stimulating specific subsets of leukocytes.26 MCP-1 is an important mediator for monocyte/macrophage recruitment27; therefore, we compared the transplantation-induced changes of MCP-1 mRNA expression in allografts of vehicle-treated recipients with those obtained from C5aRA-pretreated recipients (Figure 5E). As expected, low basal expression of MCP-1 mRNA seen in normal mice kidney was strongly upregulated at 24 h after allogeneic kidney transplantation; however, we observed a significant reduction of transplant-induced upregulation of MCP-1 in allografts from C5aRA- compared with vehicle-treated recipients (P < 0.01).
Similarly, we examined expression of ICAM-1, which plays a crucial role in adhesive interaction between activated endothelium and blood leukocytes. Twenty-four hours after transplantation, strong ICAM-1 upregulation occurred in allografts from vehicle-treated recipients (Figure 5F). C5aRA treatment of the recipient significantly reduced ICAM-1 upregulation (P < 0.05).
Inhibition of C5aR Blocked ICAM-1 Upregulation and Reduced Leukocyte Adhesion In Vitro
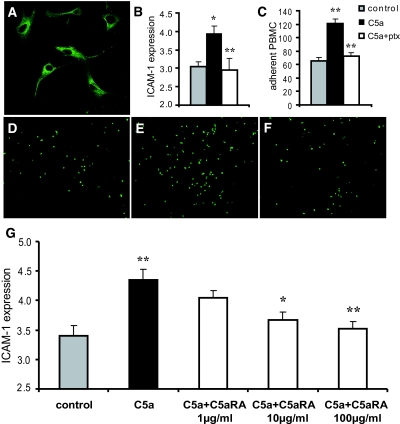
The interaction of C5a with C5aR was shown to induce a strong increase in gene expression for cell adhesion molecules in human umbilical vein endothelial cells.28 To confirm the role of the C5aR pathway for ICAM-1 expression and adhesion of leukocytes, we performed additional in vitro experiments with primary cultures of mouse aortic endothelial cells (MAEC). First, we demonstrated cell surface expression of C5aR on MAEC by immunocytochemistry (Figure 6A). When primary antibody was omitted for negative control, no signal was detected (data not shown). Next, we investigated the expression of ICAM-1 after stimulation of cells with murine C5a (mrC5a) for 16 h by cell ELISA. C5a-stimulation significantly induced (P < 0.05) upregulation of ICAM-1 on MAEC (Figure 6B). C5aR belongs to the family of G protein–coupled seven-span transmembrane receptors that can be blocked by pertussis toxin.29 Pretreatment of MAEC with pertussis toxin (P < 0.01 versus cells without pertussis toxin incubation) completely prevented C5a-induced upregulation of ICAM-1, suggesting a role for C5aR in this effect. These results were further corroborated by in vitro experiments that examined PBMC adhesion to the endothelial surface of mouse aortas. Stimulation of mouse aorta with mrC5a induced a significant increase (P < 0.01 versus control) in adhesion of PBMC. This effect could be completely abolished when aortas were pretreated with pertussis toxin (P < 0.01 versus aortas without pertussis toxin incubation; Figure 6C). Images of adherent cells are shown in Figure 6, D through F. Next, we examined the effect of C5aRA on ICAM-1 expression in C5a-stimulated MAEC. Indeed, the pretreatment of MAEC for 3 h with the C5aRA prevented the C5a-induced upregulation of ICAM-1 in MAEC in a dosage-dependent manner, directly verifying the role of C5aR (Figure 6G). These results clearly demonstrate that C5aR blockade on endothelial cells impairs upregulation of the ICAM-1 mediating leukocyte adhesion and confirm our in vivo findings.
Figure 6.
C5aR inhibition reduced C5a-induced upregulation of ICAM-1 in endothelial cells and completely abrogated C5a-induced increase of leukocyte adhesion in vitro. (A) C5aR was expressed on MAEC. Stimulation of MAEC with 50 ng/ml C5a resulted in a significant upregulation of ICAM-1 (*P < 0.05). (B) Receptor inhibition by pertussis toxin (ptx) prevented the C5a-induced ICAM-1 upregulation significantly (**P < 0.01). (C) Adhesion of PBMC to the endothelial surface of aortas was studied in vitro. Stimulation of mouse aorta with C5a induced a significant increase in adhesion of PBMC (**P < 0.01 versus control). Pretreatment with ptx inhibited C5a-induced PBMC adhesion significantly (**P < 0.01 versus C5a stimulation). (D through F) Images of adherent cells are shown in control (D), stimulation with C5a (E), and stimulation with C5a and ptx (F). Data are presented as means ± SEM for five individual experiments performed in triplicate (**P < 0.01). (G) C5a-induced upregulation of ICAM-1 in MAEC (**P < 0.01 versus control) was blocked by C5aRA pretreatment in a dosage-dependent manner. Data are means ± SEM from three independent experiments, performed in four parallels for each condition. *P < 0.05; **P < 0.01 versus C5a stimulation.
C5aRA Treatment Attenuates Priming of Alloreactive T Cells
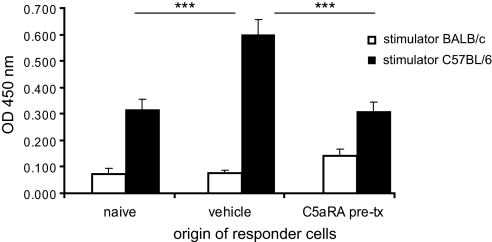
To elucidate further the possible functional effect of C5aR blockade on T lymphocyte priming, we investigated the proliferative response of naive and primed T cells after stimulation with allogenic C57BL/6 splenocytes by performing a mixed lymphocyte reaction (MLR) assay. The responder splenocytes were isolated from naive BALB/c mice, untreated allograft recipients, and C5aRA-treated recipients (treatment was initiated before transplantation) at day 6 after transplantation. Responder splenocytes from all groups were stimulated with allogenic C57BL/6 splenocytes. After co-culture for 48 h, bromodeoxyuridine (BrdU) was added for 16 h; thereafter, BrdU incorporation was measured. Syngenic controls with BALB/c splenocytes as stimulators revealed very low BrdU incorporation. A moderate proliferative response was observed for splenocytes obtained from naive BALB/c mice after stimulation with allogenic C57BL/6 splenocytes, and this response was strongly increased when primed responder cells were obtained from untreated allograft recipients (Figure 7). In contrast, the splenocytes from C5aRA-treated recipients had significantly reduced proliferative response compared with those of untreated recipients (P < 0.001). This result suggests that C5aR-mediated signaling contributes to alloreactive T cell activation in transplantation.
Figure 7.
Effect of C5aRA on T cell priming. In vitro MLR assay was performed with splenocytes isolated from naive BALB/c mice, untreated recipients, and C5aRA-treated (initiated before transplantation [pre-tx]) recipients of kidney allografts at 6 d after transplantation. Splenocytes of untreated recipients co-cultured with C57BL/6 stimulator cells showed significantly (***P < 0.001) more proliferation (BrdU incorporation) compared with splenocytes of naive BALB/c mice. Splenocytes of C5aRA-treated recipients showed significantly reduced (***P < 0.001) proliferative response compared with those of untreated recipients. Experiments were performed in triplicate, and five mice for each group were investigated; data are means ± SEM.
DISCUSSION
Acute rejection and the initial injury caused by ischemia reperfusion are major determinants of long-term allograft function and survival. Current therapeutic strategies predominantly concentrate on prevention of T cell–mediated cellular rejection. Direct targeting of genes involved in IR injury and inflammation might prove useful to inhibit processes of tissue rejection and irreversible damage.
Complement activation occurs early after IR injury, and the C5a anaphylatoxin generated during complement activation is an important mediator of IR injury and transplant rejection.18,30,31 Furthermore, elevated urinary excretion of C5a has been correlated to renal allograft rejection.32 C5a is not only a potent neutrophil and macrophage chemoattractant but also recognized as a pleiotropic molecule modulating the activity of many cell types with a broad range of biologic functions via its receptor.
In human protocol biopsies of renal allografts with cellular rejection (Banff IB), we detected C5aR expression on infiltrating cells. A similar C5aR expression pattern was found in a mouse model of acute cell- and antibody-mediated rejection. To explore further the functional role of C5aR in acute kidney allograft rejection, we used a specific C5aRA in the mouse transplant model. C5aRA administration for 6 or 21 d caused similar improvement of long-term survival and renal function, yet this effect was achieved only when C5aRA treatment was started before reperfusion. We speculated that initiation of C5a blockade before reperfusion could reduce transplantation-associated IR injury. In line with this hypothesis, we found significantly reduced numbers of TUNEL-positive cells at posttransplantation day 1 in the C5aRA-pretreated group. This effect was independent of TNF-α expression, because we found no differences in TNF-α mRNA expression between the C5aRA- and vehicle-treated groups. This result is in line with a study demonstrating that C5 blockade during experimental renal IR injury reduced late apoptosis.21 The same group also showed that C5aR targeting abrogated upregulation of CXC chemokines but not of TNF-α.18 C5-mediated regulation of apoptosis seems to depend on cell types and stress models. In experimental sepsis, C5a inhibited neutrophil apoptosis via phosphatidylinositol-3-kinase signaling and extracellular signal–regulated kinase–signaling pathway, resulting in phosphorylation of Bad and blockade of proteolytic cleavage of caspases.33–35 Direct interaction of C5a with members of the caspase family was shown previously in other models. Reduced thymocyte apoptosis by C5a blockade was shown to depend on inhibition of caspase 3, 6, and 9 activation in experimental sepsis.36 Caspase inhibition by C5aRA treatment might be relevant in our model as well; however, the exact molecular mechanisms underlying the attenuated apoptosis in our model of renal allograft rejection by C5aRA treatment remain to be investigated.
Macrophages constitute 40 to 60% of infiltrating cells during acute allograft rejection.37 In a human kidney transplant biopsy study, macrophage infiltration 3 mo after transplantation correlated inversely with graft survival.38 Furthermore, macrophage depletion reduced acute rejection in a rat model of allogeneic kidney transplant rejection.39 Our detailed analysis of the composition of infiltrating cells revealed a significantly reduced monocyte/macrophage influx into allografts of C5aRA-pretreated recipients, which might have contributed to superior survival after C5aRA treatment in our model.
The migration of inflammatory cells into extravascular sites requires a series of coordinated signals, including the generation of a chemotactic gradient by the cells of the extravascular compartment and upregulation of adhesion molecules on activated endothelium. Strong upregulation of MCP-1 has been demonstrated in animal models during renal ischemia as well as in renal biopsies from patients with acute and chronic renal allograft rejection.40 In line with this report, we observed a strong upregulation of the proinflammatory mediator MCP-1 in allografts of vehicle-treated recipients, whereas allografts from C5aRA-treated recipients showed significantly decreased renal MCP-1 mRNA expression. This coincides with previous observations in experimental lupus nephritis, in which C5aR blockade reduced proinflammatory cytokine expression.41 C5a activation is also involved in upregulation of a variety of adhesion molecules on endothelial cells28; therefore, we analyzed expression of ICAM-1 in renal allografts at 1 d after transplantation. Indeed, we could demonstrate that the transplantation-induced upregulation of ICAM-1 mRNA expression observed in allografts from vehicle-treated recipients was strongly attenuated by C5aRA pretreatment. It should be noted that we were not able to detect C5aR expression on the endothelium of renal allografts by immunohistochemistry; however, by performing in vitro experiments with primary MAEC, we could demonstrate C5aR expression on these cells by immunocytochemistry. The divergence of these data might be explained by different antibody-binding specificity. In vitro, we could completely abrogate C5a-induced upregulation of ICAM-1 by pretreating of the cells with either pertussis toxin or C5aRA. In additional experiments, PBMC adhesion to the endothelium of isolated mouse aortas stimulated with C5a was significantly reduced as a result of C5aR inactivation by pertussis toxin. This result supports our in vivo observation that C5aR has a critical role in the transplant-associated upregulation of ICAM-1 and subsequent macrophage infiltration.
New data are emerging on C5a/C5aR-induced DC activation, affecting the diverse range of DC functions relevant to the allospecific T cell response.42–46 We were not able to demonstrate C5aR expression on infiltrating CD4+ and CD8+ T lymphocytes in allografts by immunohistochemistry; however, C5aRA treatment (initiated before transplantation) significantly reduced priming of alloreactive T cells in allograft recipients. Such reduced T cell priming by C5aRA may be mediated via the ability of C5a/C5aR to induce tolerogenic DC subsets.47,48 Another mechanism linking complement to T cell function is the decay accelerating factor. Decay accelerating factor–regulated IL-12 production and subsequent T cell differentiation into IFN-γ–producing effectors was prevented by the deficiency of either C3aR or C5aR in antigen-presenting cells.49,50 Collectively, our data highlight C5aR signaling as an important mediator not only of IR injury but also of the subsequent alloreactive T cell priming leading to allograft rejection.
In summary, we demonstrated a crucial role of activated C5a/C5aR in mediating transplant-induced IR injury and acute allograft rejection. Improved allograft function and survival by pharmacologic inhibition of C5aR includes three important pathways: Ischemia-induced apoptosis, the infiltration of host monocytes/macrophages, and priming of alloreactive T cells. Reduced monocyte/macrophage infiltration by C5aRA treatment is mediated by inhibition of renal MCP-1 and ICAM-1 expression, leading to decreased PBMC adhesion to blood vessel endothelium. Targeting the C5aR in patients who receive cadaveric allografts might be considered as novel therapeutic option to prevent delayed graft function and to improve allograft outcome.
CONCISE METHODS
Human Protocol Biopsies
C5aR staining was performed on human protocol biopsies taken as standard of care after kidney transplantation at 6 wk, 12 wk, and 6 mo, respectively. The Hannover protocol biopsy program has been approved by the ethics board of the Hannover Medical School. Protocol biopsies have been classified according to the updated Banff classification51 by a nephropathologist. For further immunohistologic staining, seven biopsies without any sign of rejection were chosen; 10 biopsies showed acute TCMR (grades Ia and Ib; no borderline cases were included), and nine biopsies showed IF/TA (Banff grades I and II) without active rejection. Cases with TCMR had no simultaneous rise in serum creatinine and could hence be considered as subclinical rejection. All human biopsies were C4d negative. Patient characteristics are shown in Table 1. Immunohistochemistry for C5aR expression was evaluated on paraffin sections. Anti-human C5aR antibody (CD88 clone S5/1; HyCult Biotechnology, Uden, Netherlands) was used. Semiquantitative classification of C5aR expression was performed as follows: 0, no expression; 1, <10% of glomeruli or tubuli; 2, 10 to 20% of glomeruli or tubuli; 3, 30 to 50% of glomeruli or tubuli; 4, >50% of glomeruli or tubuli. The absolute amount of C5aR-positive interstitial infiltrating cells was counted in a high-power field with the densest infiltrate (×40 magnification).
C5aR Antagonist
The C5aRA JPE-1375 was provided by Jerini AG (Berlin, Germany) and has been characterized previously.52 JPE-1375 is a hexameric linear peptidomimetic molecule (molecular weight 955) that has been used previously in an experimental model of renal fibrosis in mice.53 Potent inhibition of human C5aR and high activity on murine C5aR makes JPE-1375 particularly suitable for testing in murine models.52
Animals
Male inbred C57BL/6 (H2b) and female BALB/c (H2d) mice were supplied by Charles River (Sulzfeld, Germany) and were housed at the animal facilities of Phenos GmbH (Hannover, Germany) under conventional conditions. Ten- to 12-wk-old mice weighing between 20 and 30 g were used for all experiments. Mice were cared for in accordance with the institution's guidelines for experimental animals. The animal protection committee of the local authorities approved all experiments.
Allogeneic Kidney Transplantation
Vascularized kidney transplantation from C57BL/6 to BALB/c mice was performed as described previously.54 Briefly, mice were anesthetized with isoflurane, the left donor kidney was attached to a cuff of the aorta and the renal vein with a small caval cuff, and the ureters were removed en block. After left nephrectomy of the recipient, the vascular cuffs were anastomosed to the recipient abdominal aorta and vena cava, respectively, below the level of the native renal vessels. The ureter was directly anastomosed into the bladder. Cold ischemia time was 60 min, and warm ischemia time was 30 min. The right native kidney was removed at the time of allograft transplantation or in the long-term survival studies at posttransplantation day 4. General physical condition of the mice was monitored for evidence of rejection.
Treatment of the recipient with C5aRA was started before surgery or immediately after transplantation by intraperitoneal injection once daily. No other immunosuppressive treatment was administered. Four experimental groups were evaluated. The first group (control) received vehicle (saline) treatment over 6 d; the second group received short-term treatment with JPE-1375 1 m/kg body wt initiated before transplantation over 6 d; the third group was treated for 3 wk with JPE-1375 1 mg/kg body wt starting before transplantation; and the fourth group received JPE-1375 1 m/kg body wt over 6 d starting immediately after transplantation, once reperfusion already had occurred. For studying long-term survival, 10 mice per group were followed for 12 wk; six additional mice per group and time point were killed at posttransplantation day 1 and day 6 for histologic and molecular analysis. Six additional mice from the group receiving C5aRA treatment for 21 d were killed after 3 wk. Mice that underwent transplantation were studied for renal function and survival. Serum creatinine levels were measured by an automated method (Beckman Analyzer, Krefeld, Germany).
Mouse Allograft Pathology
Kidney allografts from animal experiments were harvested 24 h or 6 d after transplantation, and one half of each allograft was immediately fixed in buffered formalin and embedded in paraffin. Three-micrometer sections were stained with periodic acid-Schiff stain and evaluated according to the updated Banff classification55 by a nephropathologist, who was masked to the experimental groups. Immunohistochemistry was performed using the following primary antibodies: Rat anti-mouse monocytes/macrophages (F4/80; Serotec, Oxford, UK), monoclonal rat anti-mouse T lymphocytes (CD4 and CD8; BD Pharmingen, Heidelberg, Germany), monoclonal rat anti-mouse neutrophils (Gr-1; BD Pharmingen), and polyclonal goat anti-mouse C5aR (Santa Cruz Biotechnology, Heidelberg, Germany). Stainings were performed on cryosections with Cy2- or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Lab, West Grove, PA) for fluorescence visualization. Specimens were analyzed without knowledge of the animal assignment. Analysis of infiltrating neutrophils was done by semiquantitative counting of cells in 10 randomly chosen, nonoverlapping fields of cortex and outer medulla, in Gr-1 stained sections (×200 magnification). For monocytes/macrophages (F4/80-positive cells) and subsets of T lymphocytes (CD4+ and CD8+ cells), semiquantitative scoring was done as follows: 0, no; 1, weak (<10% of the tubulointerstitial infiltrates); 2, moderate (10 to 30%); 3, high (30 to 50%); and 4, very high (>50%) infiltrating cells. Six mice were analyzed from each group.
TUNEL-Diaminobenzidine Assay
For TUNEL assay, 2 μm sections of 4% paraformaldehyde (PFA)-fixed paraffin-embedded tissues were deparaffinized, treated with the terminal deoxynucleotidyl transferase enzyme, and incubated in a humidified chamber at 37°C for 1 h. After washing, the tissue was treated with FITC-labeled anti-digoxygenin, incubated for 60 min, and washed. Negative controls were prepared under the same conditions, with the omission of the terminal deoxynucleotidyl transferase enzyme. TUNEL-positive cell numbers were counted in 20 nonoverlapping view fields per specimen without knowledge of the animal assignment.
RNA Extraction and Real-Time Quantitative PCR
Frozen kidneys were ground in liquid nitrogen, and total RNA was extracted using Trizol reagent (Invitrogen, Karlsruhe, Germany). For real-time quantitative PCR, 1 μg of DNase-treated total RNA was reverse-transcribed using Superscript II Reverse transcriptase (Invitrogen), and quantitative PCR was performed on an SDS 7700 system (Applied Biosystems, Darmstadt, Germany) using Rox dye (Invitrogen), FastStart taq Polymerase (Roche Diagnostics, Mannheim, Germany), and gene-specific primers and Fam-Tamra–labeled probes (BioTez, Berlin, Germany). PCR amplification was carried out for 10 min 96°C, and 40 cycles for 10 s at 95°C, and 1 min at 60°C. Mouse-specific primer sequences for MCP-1, TNF-α, and ICAM-1 were used. β-Actin served as the reference gene for normalization. Primer sequences are available on request. Quantification was carried out using qgene software.56 Five mice were analyzed in each group.
Mixed Lymphocyte Reactivity
Priming of alloantigen-specific T cells from kidney allograft recipients was investigated by performing a MLR assay based on the measurement of BrdU incorporation during DNA synthesis. The responder spleen cells were prepared from naive BALB/c mice or at day 6 after transplantation from untreated or C5aRA-pretreated allograft recipients. Cells were treated with ammonium chloride solution (CellSystems GmbH, St. Katharinen, Germany) to lyse erythrocytes, washed three times, and resuspended at 3 × 106 cells/ml in complete RPMI medium (Life Technologies, Gaithersburg, MD) supplemented with 10% FCS (Sigma-Aldrich, Seelze, Germany), 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. Aliquots (100 μl per well) were delivered into a 96-well, flat-bottom tissue culture plate in triplicate. Stimulator cells were prepared from the spleens of syngeneic mice (i.e., BALB/c) and allograft donors (i.e., C57BL/6). After lysis of erythrocytes, stimulator cells were treated with 50 μg/ml mitomycin C for 30 min at 37°C, washed, and resuspended in culture medium at 3 × 106 cells/ml. Aliquots (100 μl) were co-cultured with responder cells for 48 h. Afterward, BrdU was added, and, 18 h later, responder cell proliferation was quantified using colorimetric Cell Proliferation ELISA kit (Roche Diagnostics) in accordance with the manufacturer's instructions.
MAEC Culture
Isolation of MAEC from 6- to 8-wk-old wild-type mice was performed as described previously.57 The cells were cultivated in medium consisting of Endothelial Cell Growth Medium 2 (Clonetics/Cambrex, Baltimore, MD) and DMEM (1:1) supplemented with 20% FCS, 100 μg/ml endothelial cell growth supplements (Sigma Aldrich), 100 U/ml penicillin, 100 mg/ml streptomycin, 2 mM l-glutamine, 0.5% nonessential amino acids, and 0.1 mg/ml heparin. MAEC at passage 3 were used for endothelial cell characterization and for all experiments. The endothelial nature of cells was confirmed by the typical cobblestone morphology of confluent monolayers, by Dil-Ac-LDL uptake, and by surface expression of CD31 and CD106 analyzed by immunocytochemistry (data not shown).
MAEC Immunocytochemistry
MAEC were seeded and cultured on glass coverslips. Serum-starved cells were fixed with 4% PFA in PBS for 20 min at room temperature. Nonspecific binding was blocked by 2 h of incubation at room temperature with 3% BSA in PBS, and the preparations were washed three times with PBS. Incubations with primary antibody (rat anti-mouse C5aR clone 20/70; HyCult Biotechnology) were performed for 2 h at room temperature. Incubations with Alexa 488–conjugated secondary antibodies (Invitrogen, Karlsruhe, Germany) were performed for 1 h. After staining, cells were embedded in Poly-Mount mounting media (Polysciences, Eppelheim, Germany). The fluorescence cell images were captured using a Leica TCS-SP2 AOBS confocal microscope (Leica Microsystems, Wetzlar, Germany). All of the images were taken with oil-immersed ×63 objective (NA = 1.4).
ICAM-1 Cell ELISA
Cell surface expression of ICAM-1 on MAEC was measured by cell ELISA as described previously.58 Briefly, MAEC were grown in 96-well plates until 80% confluent, starved for 4 h, and then stimulated for 16 h with 50 ng/ml recombinant mrC5a (Sigma). The mrC5a concentration was chosen in preliminary experiment ranging from 0.5 to 200.0 ng/ml. For inhibition experiments, MAEC were preincubated for 2 h with 100 ng/ml pertussis toxin (Sigma Aldrich) or with various concentrations of the C5aRA ranging from 1 to 100 μg/ml. MAEC incubated in medium without stimuli served as a control. After fixation with 3% PFA and blocking with 3% BSA to prevent nonspecific binding, the cells were incubated for 2 h with polyclonal rabbit-anti mouse ICAM-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA).The specific binding of antibodies was then evaluated by incubation of cells for 1 h with secondary peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) followed by addition of tetramethylbenzidine substrate solution (R&D, Minneapolis, MN), stopping the reaction with 0.5 M H2SO4, and measuring the OD at 450 nm. The substrate was then washed away with deionized water, the plate was allowed to dry, and 0.5% trypan blue was added to stain for the number of cells per well. Excess of trypan blue was washed away, and 1% SDS was added to solubilize the trypan blue–stained cells. Each well was then read at 595 nm. The OD of ICAM-1 staining was divided by the OD of trypan blue staining to yield ICAM-1 expression (OD 450/OD 595) for each well.
PBMC Adhesion Assay In Vitro
The adhesion of mouse PBMC isolated by Ficoll-Paque separation to the endothelial surface of mouse aortas was determined by counting adherent cells fluorescently labeled with the acetyloxymethyl ester of calcein (Calbiochem, Bad Soden, Germany). One- to 2-mm pieces of aortas cleaned carefully of periadventitial fat and connective tissue and opened longitudinally were placed adventitia side down on collagen I–coated 96-well plates containing 10 μl of endothelial cell basal medium 2 (Clonetics/Cambrex) supplemented with 5% FCS to allow adherence of the aortic pieces to the substratum. When the pieces were well attached (after 4 h), 200 μl of endothelial cell basal medium 2 containing 50 ng/ml mrC5a was added. These concentrations were chosen in preliminary experiment ranging from 0.5 to 200.0 ng/ml. For inhibitory experiments, the pieces were preincubated for 2 h with 100 ng/ml pertussis toxin. Aorta pieces incubated in medium alone served as a control. After 16 h, the aorta pieces were washed twice and 100 × 103 fluorescently labeled PBMC in 200 μl medium were added. The cells were allowed to adhere for 45 min at 37°C. Unbound cells were removed by washing three times. Photographs of aorta pieces were then made using the Leica imaging microscope with the digital image–processing program. The bound leukocytes were counted without knowledge of the group assignment in four different view fields per aorta piece.
Statistical Analysis
Data are shown as means ± SEM. Normal distribution was analyzed by Klomogorov-Smirnov test, and statistical significance was calculated by t test for independent groups. SPSS 12.01 software was used (SPSS, Chicago, IL).
DISCLOSURES
None.
Acknowledgments
This work was supported by Jerini AG (Berlin, Germany).
We thank Yvonne Nikolai, Herle Chlebusch, and Kerstin Bankes for excellent technical assistance and Annette Fiebeler for critically reading the manuscript.
Published online ahead of print. Publication date available at www.jasn.org.
F.G. and S.R. contributed equally to this work.
See related editorial, “Should Complement Activation Be a Target for Therapy in Renal Tranplantation?” on pages 2250–2251.
REFERENCES
- 1.Haas PJ, van Strijp J: Anaphylatoxins: Their role in bacterial infection and inflammation. Immunol Res 37: 161–175, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Gutzmer R, Kother B, Zwirner J, Dijkstra D, Purwar R, Wittmann M, Werfel T: Human plasmacytoid dendritic cells express receptors for anaphylatoxins C3a and C5a and are chemoattracted to C3a and C5a. J Invest Dermatol 126: 2422–2429, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Connelly MA, Moulton RA, Smith AK, Lindsey DR, Sinha M, Wetsel RA, Jagannath C: Mycobacteria-primed macrophages and dendritic cells induce an up-regulation of complement C5a anaphylatoxin receptor (CD88) in CD3+ murine T cells. J Leukoc Biol 81: 212–220, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Laudes IJ, Chu JC, Huber-Lang M, Guo RF, Riedemann NC, Sarma JV, Mahdi F, Murphy HS, Speyer C, Lu KT, Lambris JD, Zetoune FS, Ward PA: Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol 169: 5962–5970, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Riedemann NC, Guo RF, Sarma VJ, Laudes IJ, Huber-Lang M, Warner RL, Albrecht EA, Speyer CL, Ward PA: Expression and function of the C5a receptor in rat alveolar epithelial cells. J Immunol 168: 1919–1925, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Drouin SM, Kildsgaard J, Haviland J, Zabner J, Jia HP, McCray PB Jr, Tack BF, Wetsel RA: Expression of the complement anaphylatoxin C3a and C5a receptors on bronchial epithelial and smooth muscle cells in models of sepsis and asthma. J Immunol 166: 2025–2032, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Braun M, Davis AE 3rd: Cultured human glomerular mesangial cells express the C5a receptor. Kidney Int 54: 1542–1549, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Shushakova N, Tkachuk N, Dangers M, Tkachuk S, Park JK, Hashimoto K, Haller H, Dumler I: Urokinase-induced activation of the gp130/Tyk2/Stat3 pathway mediates a pro-inflammatory effect in human mesangial cells via expression of the anaphylatoxin C5a receptor. J Cell Sci 118: 2743–2753, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Fayyazi A, Scheel O, Werfel T, Schweyer S, Oppermann M, Gotze O, Radzun HJ, Zwirner J: The C5a receptor is expressed in normal renal proximal tubular but not in normal pulmonary or hepatic epithelial cells. Immunology 99: 38–45, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratt JR, Hibbs MJ, Laver AJ, Smith RA, Sacks SH: Effects of complement inhibition with soluble complement receptor-1 on vascular injury and inflammation during renal allograft rejection in the rat. Am J Pathol 149: 2055–2066, 1996 [PMC free article] [PubMed] [Google Scholar]
- 11.Pratt JR, Basheer SA, Sacks SH: Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med 8: 582–587, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Jiang J, Liu W, Kubelik D, Chen G, Gies D, Garcia B, Zhong R, Rother RP: Prevention of acute vascular rejection by a functionally blocking anti-C5 monoclonal antibody combined with cyclosporine. Transplantation 79: 1121–1127, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Keshavjee S, Davis RD, Zamora MR, de Perrot M, Patterson GA: A randomized, placebo-controlled trial of complement inhibition in ischemia-reperfusion injury after lung transplantation in human beings. J Thorac Cardiovasc Surg 129: 423–428, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM: The role of the complement system in ischemia-reperfusion injury. Shock 21: 401–409, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Woodruff TM, Arumugam TV, Shiels IA, Reid RC, Fairlie DP, Taylor SM: Protective effects of a potent C5a receptor antagonist on experimental acute limb ischemia-reperfusion in rats. J Surg Res 116: 81–90, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Qin G, Liang G, Li J, Barrington RA, Liu DX: C5aR-mediated myocardial ischemia/reperfusion injury. Biochem Biophys Res Commun 357: 446–452, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Arumugam TV, Shiels IA, Strachan AJ, Abbenante G, Fairlie DP, Taylor SM: A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int 63: 134–142, 2003 [DOI] [PubMed] [Google Scholar]
- 18.de Vries B, Kohl J, Leclercq WK, Wolfs TG, van Bijnen AA, Heeringa P, Buurman WA: Complement factor C5a mediates renal ischemia-reperfusion injury independent from neutrophils. J Immunol 170: 3883–3889, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Mengel M, Bogers J, Bosmans JL, Seron D, Moreso F, Carrera M, Gwinner W, Schwarz A, De Broe M, Kreipe H, Haller H: Incidence of C4d stain in protocol biopsies from renal allografts: Results from a multicenter trial. Am J Transplant 5: 1050–1056, 2005 [DOI] [PubMed] [Google Scholar]
- 20.El-Sawy T, Belperio JA, Strieter RM, Remick DG, Fairchild RL: Inhibition of polymorphonuclear leukocyte-mediated graft damage synergizes with short-term costimulatory blockade to prevent cardiac allograft rejection. Circulation 112: 320–331, 2005 [DOI] [PubMed] [Google Scholar]
- 21.De Vries B, Matthijsen RA, Wolfs TG, Van Bijnen AA, Heeringa P, Buurman WA: Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: Inhibition of late apoptosis and inflammation. Transplantation 75: 375–382, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Meldrum KK, Metcalfe P, Leslie JA, Misseri R, Hile KL, Meldrum DR: TNF-alpha neutralization decreases nuclear factor-kappaB activation and apoptosis during renal obstruction. J Surg Res 131: 182–188, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Wong M, Ziring D, Korin Y, Desai S, Kim S, Lin J, Gjertson D, Braun J, Reed E, Singh RR: TNFalpha blockade in human diseases: Mechanisms and future directions. Clin Immunol 126: 121–136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnahoo KK, Shames BD, Harken AH, Meldrum DR: Review article: The role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol 162: 196–203, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Gueler F, Rong S, Park JK, Fiebeler A, Menne J, Elger M, Mueller DN, Hampich F, Dechend R, Kunter U, Luft FC, Haller H: Postischemic acute renal failure is reduced by short-term statin treatment in a rat model. J Am Soc Nephrol 13: 2288–2298, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Perez de Lema G, Maier H, Nieto E, Vielhauer V, Luckow B, Mampaso F, Schlondorff D: Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol 12: 1369–1382, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Wenzel U, Schneider A, Valente AJ, Abboud HE, Thaiss F, Helmchen UM, Stahl RA: Monocyte chemoattractant protein-1 mediates monocyte/macrophage influx in anti-thymocyte antibody-induced glomerulonephritis. Kidney Int 51: 770–776, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, Ward PA: C5a-induced gene expression in human umbilical vein endothelial cells. Am J Pathol 164: 849–859, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skokowa J, Ali SR, Felda O, Kumar V, Konrad S, Shushakova N, Schmidt RE, Piekorz RP, Nurnberg B, Spicher K, Birnbaumer L, Zwirner J, Claassens JW, Verbeek JS, van Rooijen N, Kohl J, Gessner JE: Macrophages induce the inflammatory response in the pulmonary arthus reaction through Galphai2 activation that controls C5aR and Fc receptor cooperation. J Immunol 174: 3041–3050, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Nakashima S, Qian Z, Rahimi S, Wasowska BA, Baldwin WM 3rd: Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J Immunol 169: 4620–4627, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Nicholas SB, Aguiniga E, Ren Y, Kim J, Wong J, Govindarajan N, Noda M, Wang W, Kawano Y, Collins A, Hsueh WA: Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int 67: 1297–1307, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Muller TF, Kraus M, Neumann C, Lange H: Detection of renal allograft rejection by complement components C5A and TCC in plasma and urine. J Lab Clin Med 129: 62–71, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Perianayagam MC, Balakrishnan VS, Pereira BJ, Jaber BL: C5a delays apoptosis of human neutrophils via an extracellular signal-regulated kinase and Bad-mediated signalling pathway. Eur J Clin Invest 34: 50–56, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Perianayagam MC, Balakrishnan VS, King AJ, Pereira BJ, Jaber BL: C5a delays apoptosis of human neutrophils by a phosphatidylinositol 3-kinase-signaling pathway. Kidney Int 61: 456–463, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Guo RF, Sun L, Gao H, Shi KX, Rittirsch D, Sarma VJ, Zetoune FS, Ward PA: In vivo regulation of neutrophil apoptosis by C5a during sepsis. J Leukoc Biol 80: 1575–1583, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Guo RF, Huber-Lang M, Wang X, Sarma V, Padgaonkar VA, Craig RA, Riedemann NC, McClintock SD, Hlaing T, Shi MM, Ward PA: Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis. J Clin Invest 106: 1271–1280, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sund S, Reisaeter AV, Scott H, Mollnes TE, Hovig T: Glomerular monocyte/macrophage influx correlates strongly with complement activation in 1-week protocol kidney allograft biopsies. Clin Nephrol 62: 121–130, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Srinivas TR, Kubilis PS, Croker BP: Macrophage index predicts short-term renal allograft function and graft survival. Transpl Int 17: 195–201, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Jose MD, Ikezumi Y, van Rooijen N, Atkins RC, Chadban SJ: Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation 76: 1015–1022, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Segerer S, Nelson PJ, Schlondorff D: Chemokines, chemokine receptors, and renal disease: From basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol 11: 152–176, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Bao L, Osawe I, Puri T, Lambris JD, Haas M, Quigg RJ: C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur J Immunol 35: 2496–2506, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Li K, Sacks SH, Zhou W: The relative importance of local and systemic complement production in ischaemia, transplantation and other pathologies. Mol Immunol 44: 3866–3874, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Zhou W, Peng Q, Li K, Sacks SH: Role of dendritic cell synthesis of complement in the allospecific T cell response. Mol Immunol 44: 57–63, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Wenderfer SE, Ke B, Hollmann TJ, Wetsel RA, Lan HY, Braun MC: C5a receptor deficiency attenuates T cell function and renal disease in MRLlpr mice. J Am Soc Nephrol 16: 3572–3582, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, Best J, Herman NS, Sproles AA, Zwirner J, Whitsett JA, Gerard C, Sfyroera G, Lambris JD, Wills-Karp M: A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest 116: 783–796, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohl J, Wills-Karp M: Complement regulates inhalation tolerance at the dendritic cell/T cell interface. Mol Immunol 44: 44–56, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Wills-Karp M: Complement activation pathways: A bridge between innate and adaptive immune responses in asthma. Proc Am Thorac Soc 4: 247–251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wills-Karp M, Koehl J: New insights into the role of the complement pathway in allergy and asthma. Curr Allergy Asthma Rep 5: 362–369, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Lalli PN, Strainic MG, Lin F, Medof ME, Heeger PS: Decay accelerating factor can control T cell differentiation into IFN-gamma-producing effector cells via regulating local C5a-induced IL-12 production. J Immunol 179: 5793–5802, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME: Decay-accelerating factor modulates induction of T cell immunity. J Exp Med 201: 1523–1530, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K: Antibody-mediated rejection criteria: An addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 3: 708–714, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Schnatbaum K, Locardi E, Scharn D, Richter U, Hawlisch H, Knolle J, Polakowski T: Peptidomimetic C5a receptor antagonists with hydrophobic substitutions at the C-terminus: Increased receptor specificity and in vivo activity. Bioorg Med Chem Lett 16: 5088–5092, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Boor P, Konieczny A, Villa L, Schult AL, Bucher E, Rong S, Kunter U, van Roeyen CR, Polakowski T, Hawlisch H, Hillebrandt S, Lammert F, Eitner F, Floege J, Ostendorf T: Complement C5 mediates experimental tubulointerstitial fibrosis. J Am Soc Nephrol 18: 1508–1515, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Ziegler E, Gueler F, Rong S, Mengel M, Witzke O, Kribben A, Haller H, Kunzendorf U, Krautwald S: CCL19-IgG prevents allograft rejection by impairment of immune cell trafficking. J Am Soc Nephrol 17: 2521–2532, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Racusen LC: The Banff schema and differential diagnosis of allograft dysfunction. Transplant Proc 36: 753–754, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Muller PY, Janovjak H, Miserez AR, Dobbie Z: Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32: 1372–1374, 1376, 1378–1379, 2002 [PubMed] [Google Scholar]
- 57.Kreisel D, Krupnick AS, Szeto WY, Popma SH, Sankaran D, Krasinskas AM, Amin KM, Rosengard BR: A simple method for culturing mouse vascular endothelium. J Immunol Methods 254: 31–45, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Hubbard AK, Giardina C: Regulation of ICAM-1 expression in mouse macrophages. Inflammation 24: 115–125, 2000 [DOI] [PubMed] [Google Scholar]