Abstract
Uropathogenic Escherichia coli (UPEC) are the most frequent causes of urinary tract infections and pyelonephritis. Renal medullary collecting duct (MCD) cells are the intrarenal site to which UPEC strains prefer to adhere and initiate an inflammatory response, but the ability of UPEC strains to translocate across impermeant MCD cells has not been demonstrated definitively. Here, several UPEC strains adhered to the apical surface and translocated across confluent murine inner MCD cells grown on filters. UPEC strains expressing cytolytic and vacuolating cytotoxins disrupted the integrity of cell layers, whereas noncytolytic UPEC strains passed through the cell layers without altering tight junctions. Apical-to-basal transcellular translocation was dramatically reduced after extinction of Toll-like receptor 4 (TLR4) and the lipid raft marker caveolin-1 by small interfering RNA. Furthermore, disruption of lipid raft integrity by filipin III and methyl-β-cyclodextrin significantly reduced both the transcellular translocation of UPEC across murine inner MCD cell layers and the stimulation of proinflammatory mediators. Bacterial translocation was also significantly reduced in primary cultures of TLR4-deficient mouse MCD cells compared with MCD cells from wild-type mice. Benzyl alcohol, an anesthetic that enhances membrane fluidity, favored the recruitment of caveolin-1 in lipid rafts and increased the translocation of UPEC across cultured TLR4-deficient MCD cells. These findings demonstrate that the transcellular translocation of UPEC strains across impermeant layers of MCD cells may occur through lipid rafts via a TLR4-facilitated process.
Urinary tract infections (UTI) and pyelonephritis, mainly caused by Escherichia coli, are among the most common infectious diseases causing significant morbidity and mortality.1,2 Bacterial attachment to mucosal epithelial cells by fimbrial adhesins represents the initial step in uropathogenic E. coli (UPEC) pathogenicity.3 The binding of adhesins to epithelial cell receptors determines tissue specificity and allows UPEC to ascend into the lower urinary tract and the kidney. Pyelonephritis-associated UPEC strains usually express type 1 fimbriae, which bind to mannosylated glycoprotein, which is abundantly expressed within the lower urinary tract,4,5 as well as P-fimbriae, which bind to the globoseries glycosphingolipids abundantly expressed on the surface of renal epithelial cells.6–8
The interaction between UPEC and bladder or renal epithelial cells induces a potent inflammatory response mainly mediated by the Toll-like receptor 4 (TLR4), which recognizes LPS, the major cell wall constituent of all Gram-negative bacteria.9 Although the process of UPEC invasion in bladder epithelial cells has been extensively studied,10–12 the mechanisms governing translocation of bacteria from the renal tubular lumen into the renal interstitium ultimately leading to systemic sepsis remain largely unknown. There is growing evidence that the detergent-resistant microdomains (DRM; or lipid rafts), highly enriched in sphingophospholipids and cholesterol,13–15 may be used by various pathogens to gain entry into cells.16 Invasion of bladder epithelial cells by type 1 fimbriated E. coli has been shown to occur through physical interaction with caveolin-1,17 which is highly expressed in caveolae, that constitute a subdomain of the biochemically defined lipid rafts.18,19 Also the integrity of lipid rafts has been shown to be required for LPS-induced cell activation.20,21
We have shown that ascending UPEC preferentially bind to and activate renal tubule medullary collecting duct (MCD) cells in an experimental model of ascending pyelonephritis.22 Using transimmortalized renal murine inner MCD cells (mpkIMCD)23 and primary cultures of MCD dissected from the kidneys of wild-type C3H/HeOuJ mice and C3H/HeJ mice exhibiting an inactive mutation of the tlr4 gene,24 we now show that type 1 and P-fimbriated UPEC strains may translocate across IMCD cell layers via distinct paracellular and transcellular routes.
RESULTS
UPEC Adhere to and Invade MCD Cells
Uropathogenic CFT073, HT7, and HT91 and nonpathogenic MG1655 E. coli isolates tested express fimH encoding the FimH adhesin subunit of type 1 fimbriae E. coli,25 whereas the commensal HT11551 isolates did not (Table 1). CFT073, HT7, and HT91 isolates express papGII encoding the PapG subunit (allelic variant II) of P fimbriae frequently expressed in pyelonephritic E. coli.26 Only CFT073 expresses sfa/foc encoding S fimbriae/F1C fimbriae.25 None of the E. coli strain tested expressed cnf1 encoding for the cytotoxic necrotizing factor 127; however, CFT073 but not HT7 and HT91 possessed the virulence factors hlyA encoding α-hemolysin25 and sat encoding a secreted vacuolating cytotoxin.28
Table 1.
Distribution of virulence factors in the various E. colistrains analyzeda
| E. coli Strain | fimH | papG | sfa/foc | cnf1 | hlyA | sat |
|---|---|---|---|---|---|---|
| HT11551 | − | − | − | − | − | − |
| MG1655 | + | − | − | − | − | − |
| CFT073 | + | II + | + | − | + | + |
| HT7 | + | II + | − | − | − | − |
| HT91 | + | II + | − | − | − | − |
PCR detection of virulence factors of three uropathogenic E. coli strains (CFT073, HT7, and HT91) isolated from infected urine from patients exhibiting pyelonephritis and from two nonpathogenic E. coli strains (MG1655 and HT11551). +, positive result; −, negative result; II, papG allele II.
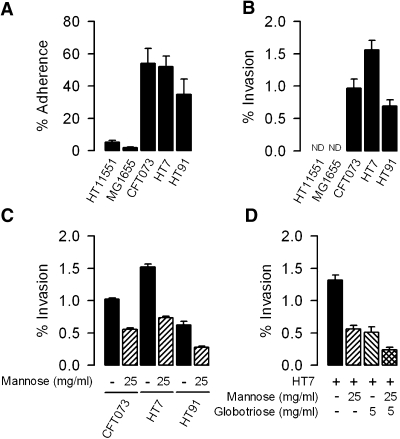
CFT073, HT7, and HT91 adhere to mpkIMCD cells more efficiently than nonpathogenic E. coli MG1655 and commensal HT11551 (Figure 1A). These UPEC strains efficiently invaded mpkIMCD cells, whereas E. coli MG1655 and the commensal HT11551 did not (Figure 1B). Preincubation of the cells with the type 1 fimbriae receptor analogue D-mannose10 induced significant inhibition of invasion by CFT073, HT7, and HT91 (45.8–55.4%; Figure 1C). Globotriose, shown to reverse binding of P fimbriated UPEC to target cells,29,30 also inhibited in an additive manner with mannose the ability of HT7, CFT073, and HT91 (data not shown) to invade cells (Figure 1D).
Figure 1.
Adherence and invasion of renal mpkIMCD cells by uropathogenic E. coli. Confluent mpkIMCD cells were incubated with various 5 × 105 E. coli isolates for 3 h and then processed for adhesion and invasion assays as described in the Concise Methods section. (A) Percentage of uropathogenic CFT073, HT7, and HT91 isolates; nonpathogenic MG1655; and commensal HT11551 adhering to the apical surface of mpkIMCD cells. (B) Gentamicin protection assays showing that CFT073, HT7, and HT91 isolates invade mpkIMCD cells, whereas MG1655 and HT11551 did not (ND, not detected). (C) Percentage of internalized CFT073, HT7, and HT91 isolates in mpkIMCD cells preincubated without or with 25 mg/ml d-mannose. (D) Percentage of internalized HT7 in mpkIMCD cells preincubated with 25 mg/ml d-mannose, 5 mg/ml globotriose, or mannose plus globotriose. Data are means ± SEM; n = 3 to 6 separate experiments for each experimental condition.
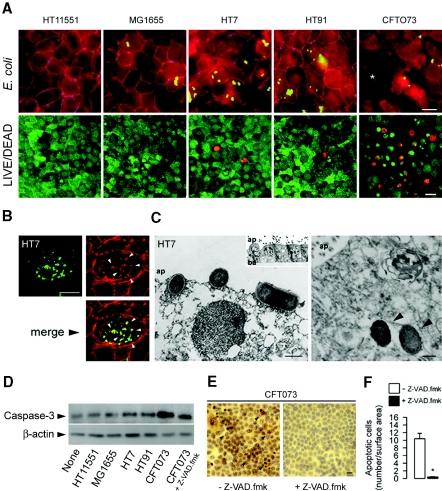
HT11551 and MG1655 did not bind to mpkIMCD cells, whereas HT7 and HT91 bound efficiently to cell membrane without altering the tight junction–associated protein ZO-1 (Figure 2A). HT7 induced remodeling of F-actin filaments in mpkIMCD cells with numerous points of bacterial attachment (Figure 2B). The binding and internalization of HT7 was confirmed by ultrastructural studies. HT7 bound to the apical membranes of mpkIMCD cells grown on permeable filters, and internalized bacteria were also detected (Figure 2C). In contrast, CFT073 induced numerous dead cells with disrupted tight junctions (Figure 2A). Western blot analysis and immunohistochemical studies using an anti–active caspase-3 antibody revealed that CFT073 but not nonpathogenic HT11551 and MG1655, and HT91, or HT7 induced cell apoptosis (Figure 2, D through F). Moreover, cell apoptosis and the rapid decrease in transepithelial resistance (RT; see Figure 3) could be prevented by preincubating cells with the pan-caspase inhibitor Z-VAD.fmk (Figure 2, D through F).
Figure 2.
Effects of UPEC on the integrity of confluent renal mpkIMCD cell layers. (A) E. coli isolates (5 × 105 bacteria/well) were added (3 h) to the apical side of confluent mpkIMCD cells grown on 1-μm-pore-size permeable filters, then fixed and processed for indirect immunofluorescence using an anti–ZO-1 antibody (in purple), and then stained with phalloidin (in red) to visualize F-actin. Cells were also stained with LIVE/DEAD fluorescent dye staining (red, dead cells; green, viable cells). CFT073 but not HT7 and HT91 altered the integrity of the cell layer with numerous dead cells (*). Bars = 10 μm. (B) Indirect immunofluorescence of cells grown on glass slide showing actin rearrangements (in red) forming close contacts (arrowhead) with an adherent HT7 bacteria (in green, and merge image in yellow). (C) Transmission electron micrographs of adherent HT7 bacteria to the apical membrane of mpkIMCD cells (left) and internalized bacteria (arrowheads; right). Bars = 1 μm. (D) Western blot analysis of active caspase-3 expression in nonpathogenic and uropathogenic E. coli strains. (E and F) Illustrations (E) and number of caspase-3–positive stained cells (F) incubated with CFT073 and without or with the pan-caspase inhibitor Z-VAD.fmk (20 μM, 30 min). Bars are means ± SEM; n = 3 independent experiments. *P < 0.05 between groups. Bar = 10 μm.
Figure 3.
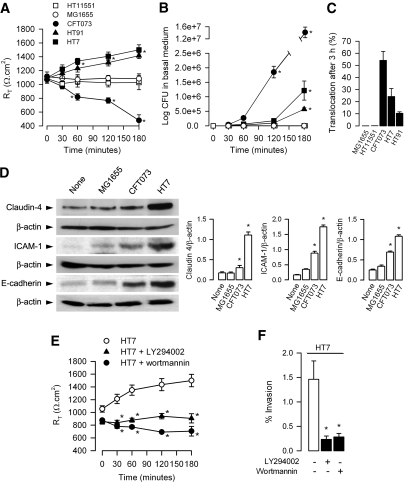
Differential effects of UPEC on paracellular permeability, bacterial translocation, and expression of tight junction and adhesion proteins in mpkIMCD cells. (A through C) E. coli (5 ± 105 bacteria/well) were added to the apical side of confluent layers of mpkIMCD cells grown on permeable filters. RT (A), number of bacteria recovered in the basal medium (B), and percentage of bacterial translocation determined as described in the Concise Methods section (C) were measured during a 3-h period. Data are means ± SEM; n = 6 to 9 experiments for each condition tested. *P < 0.05 versus time 0 values. (D) Western blot analyses of expression of claudin-4, intercellular adhesion molecule 1 (ICAM-1), E-cadherin, and the corresponding β-actin in mpkIMCD cells incubated without (None) or with MG1655, CFT073, or HT7 (5 ± 105 bacteria/well) for 3 h. Bars are mean ratio values ± SEM of densitometric analyses of claudin-4, ICAM-1, or E-cadherin over β-actin–labeled bands from three to four separate cultures of mpkIMCD in each group tested. *P < 0.05 versus None values. (E and F) RT values (E) and bacterial invasion (F) in mpkIMCD cells preincubated (30 min) without or with LY294002 (50 μM) or wortmannin (1 μM) before addition of HT7. The percentage of bacterial invasion was determined using the gentamicin protection assay as described in the Concise Methods section. Data are means ± SEM; n = 4 separate filters for each condition tested. *P < 0.05 versus None values.
Apical-to-Basal Translocation of UPEC across MCD Cells
We then next analyzed the capacity of UPEC to translocate across confluent layers of mpkIMCD cells grown on permeable filters when added (5 × 105 bacteria/filter) to the apical side of the cells. Nonpathogenic MG1655 and HT11551 did not alter RT and did not translocate across cell layers (Figure 3, A through C). CFT073 caused a significant time-dependent fall in RT (Figure 3A), and more than 50% of the bacteria added to the apical side of the cell layers were recovered in the basal medium (Figure 3, B and C). In sharp contrast, HT7 and HT91 caused a significant increase in RT, and the number of HT7 and HT91 recovered in basal medium significantly increased as a function of time (Figure 3, B and C). Incubating mpkIMCD cells with HT7 induced a marked increase in the tight junction protein claudin-4, which is highly expressed in collecting duct cells,31 as compared with uninfected cells or cells incubated with MG1655 (Figure 3D). HT7, HT91 (not shown), and, to a lesser extent, CFT073 also increased the expression of the adhesion molecule E-cadherin and intercellular adhesion molecule 1 involved in cell-to-cell contact–mediated host responses32 (Figure 3D). The increase in RT caused by HT7 could result from remodeling of the host cell actin cytoskeletal network secondary to the activation of phosphoinositide 3-kinase (PI3-K)10 and downstream proteins, such as α-actinin and vinculin, involved in the reorganization and stabilization of host actin.33 Indeed, the PI3-K inhibitors LY294002 (50 μM) and wortmannin (1 μM) both inhibited bacterial invasion of mpkIMCD and blocked the rise in RT caused by HT7 (Figure 3, E and F).
Caveolin-1 Is Required for the Internalization of UPEC in MCD Cells
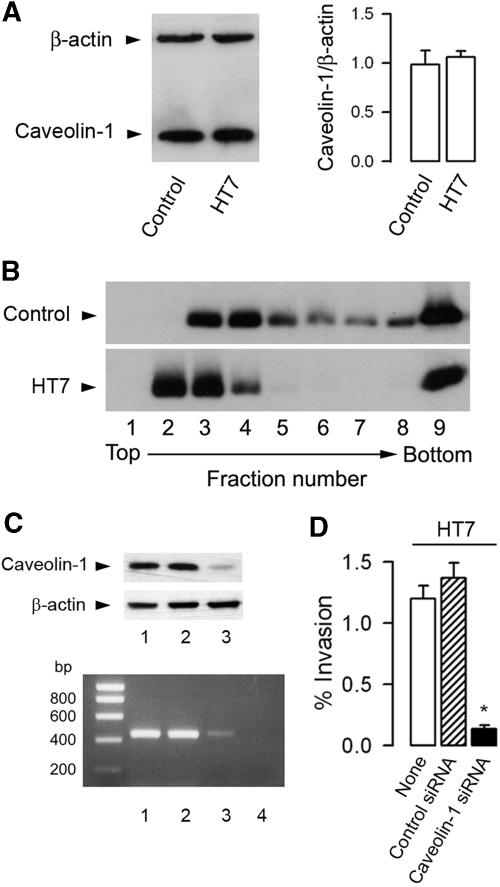
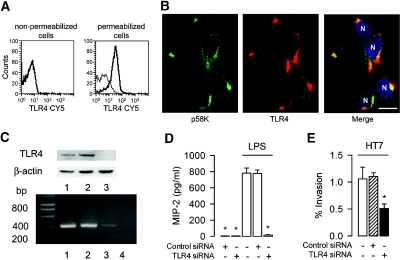
The integrity of lipid rafts has been shown to play a key role in the invasion of UPEC in bladder epithelial cells.17 All subsequent experiments were therefore performed using invasive HT7 to analyze the role of lipid rafts in the apical internalization of HT7 isolates by mpkIMCD cells. DRM were extracted from confluent mpkIMCD cells incubated with or without HT7 isolates for 3 h. The amount of the lipid raft marker caveolin-118 was nearly identical in uninfected and HT7-infected mpkIMCD cells (Figure 4A). Flotation-centrifugation gradient of DRM revealed that caveolin-1–labeled bands were detected mainly in fractions 3 through 6 from uninfected cells, whereas more intensely labeled bands were detected in fractions 2 through 4 from HT7-infected cells (Figure 4B). This could suggest that the adhesion and/or internalization of UPEC provokes the recruitment of caveolin-1 in DRM. To confirm these results, we conducted experiments on cells after caveolin-1 mRNA gene silencing. The level of caveolin-1 mRNA and the amount of caveolin-1 protein were significantly lower 2 d after the cells had been transfected with a caveolin-1 siRNA than in untreated cells or cells transfected with a negative control siRNA (Figure 4C). The decreased expression of caveolin-1 was associated with a significant decrease in the percentage of internalized HT7 isolates as compared with that of untreated cells (−88%) or cells transfected with a control siRNA (−90%; Figure 4D).
Figure 4.
Caveolin-1 requirement for internalization of UPEC in mpkIMCD cells. (A) Western blot analysis of caveolin-1 and β-actin in mpkIMCD cells incubated without (control) or with HT7 (5 ± 105 bacteria/well, 3 h). Bars are the mean ratio values ± SEM of densitometric analyses of caveolin-1 over β-actin–labeled bands from three to four separate cultures. (B) Western blot analysis of caveolin-1 in DRM fractions fractionated by flotation centrifugation gradient in uninfected and HT7-infected mpkIMCD cells. (C) Caveolin-1 mRNA (bottom) and protein (top) expressions in uninfected mpkIMCD cells (lane 1) or cells transfected with a negative control siRNA (lane 2), a caveolin-1 siRNA (lane 3), or with non–reverse-transcribed caveolin-1 siRNA (lane 4). (D) Nontransfected cells (None) and transfected cells with caveolin-1 siRNA or negative control siRNA (control siRNA) were incubated with HT7 (5 ± 105 bacteria/well, 3 h) before invasion assay. Bars (means ± SEM) represent the percentage of internalized bacteria. *P < 0.05 versus None values.
Intracellular TLR4 Activation Caused by LPS and Uropathogenic E. coli Occurs through a Lipid Raft–and Clathrin-Dependent Internalization Processes
The interaction between adherent UPEC and apical membranes of MCD cells causes an intense inflammatory response mainly mediated via LPS-mediated TLR4 activation.22 mpkIMCD cells expressed intracellular TLR4 (Figure 5A), which co-localized with the microtubule-binding Golgi membrane protein p58K34 (Figure 5B), suggesting that TLR4 is mainly located in the Golgi apparatus. Initiation of cellular activation by intracellular TLR4 must therefore require internalization and trafficking of bacterial virulent factors.21,34,35 The consequences of inhibiting cell traffic and membrane internalization pathways were analyzed on the LPS-mediated activation of the transcription factor NF-κB and on the LPS-mediated secretion of the chemokine macrophage inflammatory protein 2 (MIP-2) in mpkIMCD cells. Cytochalasin D, which disrupts actin filaments and inhibits actin polymerization, methyl-β-cyclodextrin (MβCD), and filipin III, which alter DRM by complexing cholesterol or removing cholesterol from membranes, respectively,36,37 and monodansylcadaverin, which inhibits clathrin-dependent endocytosis, significantly decreased LPS-mediated cell activation and the secretion of MIP-2 (Supplemental Figure 1). As a control, we showed that monodansylcadaverin had no effect on IL-1β–mediated cellular activation. These agents also reduced the production of MIP-2 by mpkIMCD cells incubated with HT7 (Supplemental Figure 1).
Figure 5.
Intracellular TLR4 requires intact cell trafficking endocytosis pathways and mediates the internalization of UPEC in mpkIMCD cells. (A) FACS analysis for TLR4 in nonpermeabilized and permeabilized cells. Nonbold lines correspond to the isotype control. (B) Double immunofluorescence using antibodies raised against the Golgi apparatus marker p58K (in green) and TLR4 (in red). Nuclei (N) were counterstained with Hoechst 33258 (in blue). (C) tlr4 mRNA and protein expressions in uninfected mpkIMCD cells (lane 1) and cells transfected with a negative control siRNA (lane 2), tlr4 siRNA (lane 3), or non–reverse-transcribed tlr4 siRNA (lane 4). (D) MIP-2 secretion in nontransfected cells and cells transfected with a negative control siRNA or tlr4 siRNA incubated with LPS (10 ng/ml, 6 h). Data are means ± SEM; n = 5 separate cultures in each condition tested. (E) Nontransfected cells and cells transfected with tlr4 siRNA or negative control siRNA were incubated with HT7 before invasion assay as described already. Bars (means ± SEM) represent the percentage of internalized bacteria. *P < 0.05 between groups.
TLR4 Favors the Adhesion to and Internalization of UPEC in MCD Cells
We next analyzed the consequence of tlr4 gene silencing on the capacity of HT7 to invade mpkIMCD cells. Transient transfection of the cells with a tlr4 siRNA resulted in a significant decrease in tlr4 mRNA and in protein expressions as compared with untreated cells and cells transfected with a negative control siRNA (Figure 5C). The decreased expression of TLR4 was associated with an almost complete inhibition of LPS-mediated secretion of MIP-2 (Figure 5D) and also significantly inhibited the cellular internalization of HT7 (Figure 5E). These findings suggest that the internalization of UPEC is linked to TLR4-mediated cell activation. That the secretion of MIP-2 elicited by HT7 was significantly reduced in cells preincubated with the PI3-K inhibitors LY294002 and wortmannin and in cells transfected with the caveolin-1 siRNA also suggests that blockade of bacterial invasion impairs TRL4-mediated cell activation (Supplemental Figure 2).
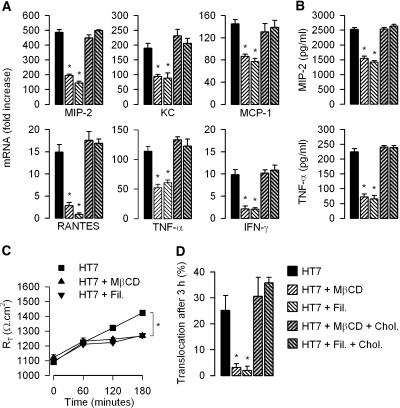
Cholesterol-Affecting Drugs Impair the TLR4-Mediated Inflammatory Response and the Translocation of UPEC across MCD Cells
Because lipid rafts seem to be critical in both cell activation and bacterial invasion, we tested whether the disruption of lipid rafts concomitantly impaired both the stimulation of proinflammatory mediators and the translocation of bacteria across mpkIMCD cells. The cholesterol-affecting drugs MβCD (10 mM) and filipin III (5 μM) both significantly reduced the stimulated mRNA expression levels of MIP-2, keratinocyte-derived chemokine (KC), monocyte chemoattractant protein 1 (MCP-1), RANTES, TNF-α, and IFN-γ and secretion of MIP-2 and TNF-α resulting from the incubation of mpkIMCD with HT7 isolates (Figure 6, A and B). MβCD and filipin III also impaired the increase in RT induced by apical addition of HT7 isolates on confluent mpkIMCD cells grown on filters (Figure 6C) and significantly reduced the apical-to-basal translocation of bacteria (Figure 6D). As a control, HT7-infected cells treated with MβCD or filipin III were rinsed and incubated in the presence of 100 μM cholesterol together with the HT7 isolates for an additional 3 h. Cholesterol repletion fully restored the downregulated expression of proinflammatory mediators (Figure 6, A and B) and fully antagonized the inhibition of HT7 translocation across mpkIMCD cells (Figure 6D) caused by the cholesterol-affecting drugs. These results suggest that the activation and secretion of proinflammatory chemokines/cytokines and translocation of UPEC are functionally coupled.
Figure 6.
Effects of cholesterol-affecting drugs and cholesterol repletion on the expression of proinflammatory mediators and translocation of UPEC across renal mpkIMCD cells. The relative mRNA expression levels of proinflammatory mediators quantified by real-time PCR (A), secretion of MIP-2 and TNF-α in cell supernatants (B), RT (C), and apical-to-basal translocation of HT7 (D) were measured in confluent cultures of mpkIMCD cells grown on filters and preincubated (30 min) without (black bars, ▪) or with 10 mM MβCD (hatched bars, ▴) or 5 μM filipin III (hatched bars, ▾) and then incubated with HT7 (5 × 105 bacteria, 3 h). Sets of MβCD- or filipin III–treated cells incubated with HT7 were rinsed and then further incubated with HT7 in the presence of 100 μM cholesterol for an additional 3 h (hatched gray bars). Data are means ± SEM; n = 5 to 7 separate cultures for each experimental condition. *P < 0.05 versus HT7 values.
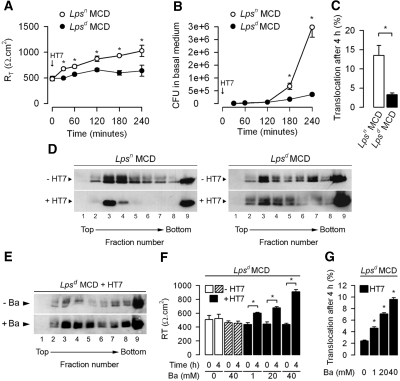
We next addressed the question of whether TLR4, in addition to its key role in the induction of proinflammatory mediators, contributes to the transcellular passage of bacteria across tight confluent epithelial MCD cells. The capacity of translocation of UPEC was analyzed in primary cultures of MCD dissected from the kidneys of TLR4-expressing Lpsn and TLR4-defective Lpsd mice. Like in mpkIMCD cells, apical addition of HT7 to confluent layers of Lpsn MCD grown on filters caused a significant time-dependent increase in RT (Figure 7A). In contrast, RT values from Lpsd MCD were not affected after apical exposure to bacteria (Figure 7A). The number of colony-forming units (CFU) recovered from the basal medium and the percentage of bacteria translocated after 4 h was significantly greater in Lpsn MCD than in Lpsd MCD (Figure 7, B and C). As in mpkCCDcl4 cells, marked redistribution of caveolin-1 into fractions 3 and 4 was observed in DRM fractions of Lpsn MCD incubated with HT7 (Figure 7D). In contrast, no marked redistribution of caveolin-1 was observed between the DRM fractions in uninfected and HT7-infected Lpsd MCD (Figure 7D). These findings suggest that TLR4-mediated cellular activation facilitates the transcellular translocation of bacteria. They also suggest that the recruitment of cholesterol-enriched lipid rafts into plasma membrane resulting from the TLR4-mediated activation of proinflammatory mediators should increase membrane fluidity and thereby facilitate bacterial translocation. To test this hypothesis, we undertook analysis of the caveolin-1 distribution in DRM and bacterial translocation assays in TLR4-defective Lpsd MCD preincubated without or with benzyl alcohol, a local anesthetic shown to increase membrane fluidity,38,39 before adding HT7 to the apical side of the cell layers. Flotation-centrifugation gradient analyses revealed that the intensity of caveolin-1–labeled bands was much more marked in DRM fractions from HT7-infected Lpsd MCD treated with benzyl alcohol (20 mM, 4 h) than those of HT7-infected Lpsd MCD that were not incubated with benzyl alcohol (Figure 7E). Benzyl alcohol, which had no effect on RT in uninfected Lpsd MCD, also significantly increased RT and bacterial translocation in a dosage-dependent manner when Lpsd MCD cell layers were incubated in the presence of HT7 (Figure 7, F and G).
Figure 7.
Translocation of UPEC across confluent cultures of Lpsn and Lpsd MCD cells. (A through C) RT (A), number of HT7 recovered in the basal medium (B), and percentage of apical-to-basal translocation of bacteria (C) measured in confluent cultures of Lpsn and Lpsd MCD grown on permeable filters and incubated on the apical side with HT7 (5 × 105 bacteria/well). (D) Western blot analysis of caveolin-1 in DRM fractions fractionated by flotation centrifugation gradient in uninfected (−HT7) and HT7-infected (+HT7) Lpsn MCD and Lpsd MCD. (E) Western blot analysis of caveolin-1 in flotation centrifugation fractionated DRM fractions of confluent cultures of Lpsd MCD incubated with HT7 and with (+Ba) or without (−Ba) 20 mM benzyl alcohol (Ba) for 4 h. (F and G) Effects of increasing concentrations of Ba on RT (F) and percentage of apical-to-basal translocation of HT7 (G) in confluent cultures of Lpsd MCD. Data are means ± SEM; n = 6 separate cultures of MCD dissected from the kidneys of two to three mice for each group tested. *P < 0.05 between groups.
DISCUSSION
Attachment to and translocation of bacteria across the normally impermeant epithelial cell barriers are important features of pathogen infection. This is of particular importance during pyelonephritis, because the translocation of bacteria across the renal tubule epithelium facilitates their dissemination into the renal interstitium. We show that UPEC adhere to the apical surface of renal IMCD cells and may traverse cell layers either via paracellular route by disrupting cell layers and tight junctional complexes or via a transcellular route without altering cell–cell contacts.
Adherence and invasive assays indicate that UPEC bind to the apical surface of MCD mpkIMCD via a mannose- and globotriose-inhibitable mechanism. Purified P-fimbriae bind to the apical surface of renal tubule cells and are specifically inhibited by globotriose, a trisaccharide analog of the Gal1α,4Gal motif present in the globoseries glycosphingolipids abundantly expressed on the surface of renal epithelial cells.7,29 The interaction of UPEC with mpkIMCD cells is of particular importance given our study showing that UPEC preferentially, if not exclusively, bind to collecting duct cells using an experimental in vivo model of ascending pyelonephritis.22 Many UPEC strains have the capacity to induce cell death. The cytolytic E. coli strain CFT073 causes severe alterations of mpkIMCD cell layers. α-Hemolysin could be responsible for the apoptosis of host cells caused by CFT073. This pore-forming toxin induces intracellular Ca2+ oscillation process and sustained entry of Ca2+, which can activate the caspase-mediated apoptotic pathway.40 According to this view, the pan-caspase inhibitor Z-VAD.fmk protects MCD cells against the cytotoxic action of CFT073. These findings contrast with those of Wu et al.41 using Madin-Darby canine kidney (MDCK) cells, which remained impermeant to the apical addition of UPEC, unless the cell polarity was disrupted by pretreating cells with the hepatocyte growth factor. These apparently divergent results may be due to the inability of PapG adhesins to bind to the apical surface of MDCK cells.26
Adhesion of the noncytolytic HT7 isolates to collecting duct cells induces a marked inflammatory response, mainly via the activation of intracellular TLR4 signaling.42 These findings are very similar to those seen after interaction between type 1 piliated and P-fimbriated E. coli and bladder epithelial cells or human renal epithelial A498 cells, respectively.10,11,43 TLR4-mediated signaling has also been shown to be involved in the phagocytosis and internalization of E. coli across intestinal cells.44 Interestingly, TLR4 was found to reside mainly in the Golgi apparatus of renal MCD cells. A similar intracellular localization of TLR4 was previously reported in a variety of epithelial and nonepithelial cell types.21,34,35,45 We show that bacterial LPS, the main virulent factor of UPEC, is internalized via a lipid raft–and a clathrin-mediated process to activate TLR4 signaling.21 We also show that inhibiting caveolin-1 mRNA expression and disrupting lipid raft integrity inhibited (1) the adhesion and internalization of the HT7 strain, (2) the stimulation of proinflammatory mediators, and (3) the transcytosis of UPEC across mpkIMCD cell layers grown on permeable filters.
Lipid rafts may be used by various pathogens to gain entry into cells.16 For example, type 1 piliated E. coli invade bladder epithelial cells through lipid raft recruitment of caveolin-1 protein in DRM from UPEC-infected bladder epithelial cells.17 Similarly, this study suggests that the adherence of UPEC at the apical surface of mpkIMCD cells increases the density of lipid raft structures to facilitate transcytosis of bacteria. Interestingly, IFN-γ was found to increase the levels of GM1, a ganglioside used as a marker of raft formation,46 and to induce the translocation of commensal E. coli across impermeant intestinal cells via lipid rafts.47 These findings, together with this study, suggest that the induction of a proinflammatory response may directly affect lipid rafts. Cholesterol-affecting drugs, which inhibit the TLR4-mediated inflammatory response resulting from the interaction of UPEC with lipid rafts, also impaired the translocation of UPEC across mpkIMCD cell layers. Thus, lipid rafts are critical structures for both TLR4-mediated cellular activation and for the internalization and translocation of UPEC.
That the apical-to-basal translocation of HT7 is significantly greater in primary cultures of Lpsn MCD expressing TLR4 than in Lpsd MCD suggests that the TLR4-mediated inflammatory response facilitates the translocation of UPEC across MCD. The increase in membrane fluidity caused by benzyl alcohol (the increase in membrane fluidity caused by 40 mM benzyl alcohol being equivalent to that induced by an increase in temperature of approximately 6°C)38 significantly increased the translocation of UPEC across Lpsd MCD, which develop only limited TLR4-independent cellular activation as compared with Lpsn MCD.22,42 Collectively, these findings suggest that TLR4-mediated cell activation resulting from the attachment of UPEC to lipid raft structures would then promote their recruitment into the plasma membrane, induce an increase in membrane fluidity, and thereby facilitate the internalization and translocation of UPEC across renal collecting duct cells; however, this in vitro study only partially reflects the complex consequences of the inflammatory response caused by UPEC during ascending pyelonephritis in vivo. In particular, the in vitro experiments do not take into account the recruitment and activation of bone marrow–derived cells at the site of inflammation, which in fact play a critical role in clearing bacteria from the kidneys during ascending UTI. Using experimental models of ascending UTI, we and others previously showed that the number of polymorphonuclear neutrophils infiltrating kidneys is much greater in TLR4-expressing Lpsn mice than in TLR4-defective Lpsd mice, whereas the amount of UPEC invading kidneys is significantly greater in Lpsd mice than in Lpsn mice.22,42,48,49 Further in vivo studies will be needed to determine and quantify precisely the presence of UPEC infiltrating the renal interstitium of infected TLR4-expressing Lpsn mice.
In conclusion, this study provides the first demonstration that pyelonephritic UPEC can traverse the impermeant MCD barrier to invade the renal interstitium either through a paracellular pathway or via a TLR4-facilitated, lipid raft–mediated, transcytosis pathway.
CONCISE METHODS
Bacteria
Two E. coli isolates (HT7 and HT91) were isolated from the urine of patients hospitalized for pyelonephritis at the Tenon Hospital (Paris, France). Uropathogenic E. coli strain CFT073,50 nonpathogenic E. coli K-12 strain MG1655,51 and a commensal E. coli strain (HT11551) isolated from the feces of a hospitalized patient were also used. Bacteria were grown in Luria-Bertani broth at 37°C for 24 h. The E. coli strains were screened for virulence factors chosen as extraintestinal pathogenic E. coli markers. Total DNA was extracted using the QIAamp DNA Minikit according to the manufacturer's instructions (Qiagen, GmbH, Courtaboeuf, France). PCR was performed using specific primers to detect fimH, papG (allelic variants I, II, and III), sfa/foc, cnf1, hlyA, and sat as described previously.52,53 Gentamicin susceptibility was determined using a disk diffusion assay according to the guidelines of the French Society of Microbiology (http://www.sfm.asso.fr).
Cultured Cells
Experiments were carried out on transimmortalized mpkIMCD cells that had been derived from isolated MCD microdissected from the kidneys of a transgenic mouse expressing the large T antigen under the control of a 1.1-kb sequence of L-type pyruvate kinase promoter fused to a SV40 enhancer.23 Cells were seeded on glass coverslips, Petri dishes, or Transwell permeable filters (1 μm pore size, 0.33 cm2 insert growth area; Corning Costar Corp., Cambridge, MA) and grown until confluent in a modified, hormonally defined, antibiotic-free medium as described previously.23 Experiments were also carried out on primary cultures of isolated MCD dissected from the kidneys of 9- to 13-wk-old C3H/HeJ Lpsd mice and C3H/HeOuJ Lpsn mice as described previously.22 Pools of isolated MCD (eight to 12 fragments) were seeded onto collagen-precoated, 1-μm-pore-size Transwell filters and grown to confluence for 15 d at 37°C in a 5% CO2/95% air atmosphere in the same modified defined medium (DM) as described already. Experiments were carried out 2 wk after seeding, using confluent cells with a transepithelial electrical resistance ≥400 Ω.cm2. All experiments were carried out in accordance with the French legislation governing animal studies.
Transient Transfection and Luciferase Reporter Assay
mpkCCDcl4 cells (15 × 106 cells/ml) were transfected by electroporation with the p(κB)3 IFN-Luc plasmid (a Luciferase cis-reporter system containing 7 × AP-1 and 3 × NF-κB enhancer elements) as described previously.54 Luciferase activity was measured with a luminometer using the Luciferase Assay System (Promega, Charbonnières, France) according to the manufacturer's instructions. All experiments were performed at least in triplicate.
Adherence and Invasion Assays
Adherence and invasion assays were performed as described previously10 on confluent mpkIMCD cells grown in 24-well plates. After replacing fresh medium 2 h before infection, host cells were infected with bacteria (5 × 105 bacteria per well) for 3 h at 37°C. Sets of infected cells (three wells per bacterium tested) were then lysed in 0.1% Triton X-100 in PBS and then plated on LB-agar plates to count the total (extracellular and intracellular) number of CFU present in total cell lysates to take into account bacterial growth over the time course of the experiments. For the adherence assay, the same sets of infected cells (three wells per bacterium tested) were rinsed five times in PBS supplemented with 1 mM Ca2+ then lysed in 0.1% Triton X-100 in PBS and plated on LB-agar plates. The percentage of adherent bacteria was calculated as the number of CFU recovered after PBS rinses divided by the total number of CFU. Cellular invasion by the bacteria was determined using the gentamicin protection assay.55 Sets of infected cells (three wells per bacterium tested) were rinsed three times in PBS and then incubated for an additional 2 h in medium containing 100 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO) to kill any extracellular bacteria. After rinsing in PBS, cells were then lysed in 0.1% Triton X-100 in PBS and plated on LB-agar plates. The percentage of intracellular bacteria was calculated as the number of CFU recovered after cell lysis following incubation in the presence of gentamicin divided by the total number of CFU counted before addition of gentamicin.
Translocation Assay and Transepithelial Electrical Resistance Measurement
mpkIMCD cells and primary cultures of MCD dissected from the kidneys of C3H/HeJ or C3H/HeOuJ mice (referred to as Lpsd MCD and Lpsn MCD, respectively) were seeded and grown in antibiotic-free DM on 1-μm-pore-size permeable Transwell filters (apical medium 200 μl; basal medium 600 μl). A total of 100 μl of apical medium was removed and replaced by 100 μl of bacterial suspension (5 × 105 bacteria in DM). Thereafter, samples of the apical and basal medium were taken at various times and plated on LB-agar plates to count the number of bacteria present in the basal medium and in the apical medium for each selected incubation time, to take into account bacterial growth during the time course of experiments. In parallel, RT was measured using dual silver/silver chloride (Ag/AgCl) electrodes connected to a Millicel-ERS voltohmmeter (Millipore, Billerica, MA).
Preparation of Detergent-Insoluble Membrane Fractions and Flotation Centrifugation on Sucrose Gradient
Confluent mpkIMCD cells were incubated with or without HT7 (5 × 105 bacteria/well), then rinsed three times in ice-cold PBS, scraped off the plates, and centrifuged (300 × g, 5 min) at 4°C. Pelleted cells were incubated in 167 μl of PBS containing protease inhibitors and 1% Triton X-100 by rotary shaking at 4°C for 45 min. The lysates were adjusted to 40% sucrose (333 μl of 60% sucrose in PBS) in a final volume of 500 μl in polyallomer centrifuge tubes (Beckman Coulter, Fullerton, CA) and overlaid with 3.5 ml of 30% sucrose in PBS, followed by 1 ml of 5% sucrose in PBS. The gradients were centrifuged at 150,000 × g for 18 h at 4°C in a Beckman Coulter SW55Ti rotor. Fractions (500 μl) were collected from the top of the tubes, and proteins were precipitated by addition of 700 μl of 10% trichloracetic acid in the presence of 375 μg of Na deoxycholate as a carrier and then centrifuged (19,000 × g, 30 min) at 4°C. Resuspended pellets from total cell lysates of gradient fractions were heated at 70°C for 10 min and run on 10% SDS-PAGE before being transferred onto nitrocellulose membranes and subjected to Western blotting using a rabbit polyclonal anti–caveolin-1 (1:200; Santa Cruz Biotechnology, Santa Cruz, Ca) and anti–β-actin (1:1000; Sigma-Aldrich) antibodies.
Cytotoxicity Assay
Cell cytotoxicity was determined using the LIVE/DEAD viability assay (Molecular Probes, Eugene, OR). mpkIMCD cells grown on permeable Transwell filters and incubated without or with the various E. coli strains for 3 h were then incubated with the LIVE/DEAD reagents according to the manufacturer's instructions. The filters were then cut from their holders, mounted on glass slides, examined using a LEIZ DMIRB fluorescence video microscope (Leica Microsystems, Bensheim, Germany), and photographed.
siRNA Studies
Experiments were performed using predesigned HP GenomeWide (Qiagen) siRNA using a target DNA sequence from the tlr4 gene (5′-AATTCTCCGAACGTGTCACGT-3′; sense UUCUCCGAACGUGUCACGUdTdT; antisense ACGUGACACGUUCGGAGAAdTdT) or from the caveolin-1 gene (5′-AAAGGTGATGATGTCATACAA-3′; sense AGGUGAUGAUGUCAUACAAdTdT; antisense UUGUAUGACAUCAUCACCUdTdT). A universal negative control siRNA (target DNA sequence AATTCTCCGAACGTGTCACGT; sense UUCUCCGAACGUGUCACGUdTdT; antisense ACGUGACACGUUCGGAGAAdTdT) was also used. Single-strand sense and antisense RNA nucleotides were annealed (90°C for 1 min and then 37°C for 1 h) to generate an RNA duplex according to the manufacturer's instructions. Cells were trypsinized and transferred to 24-well plates (35,000 cells per well), and 24 h later, 0.6 pmol of siRNA duplex and 2 μl of INTERFERin (Polyplus-Transfection, San Marcos, CA) preincubated in serum-free medium for 10 min was added to each well. No cytotoxic effects were observed during the incubation period. Cells were then incubated for 48 h at 37°C before addition of bacteria. Gene silencing was monitored by reverse transcription–PCR. The caveolin-1 primers were NM_007616: nt 249 to 269, nt 693 to 673. The TLR4 primers and the thermal cycling program were the same as described previously.22 Amplification products were run on a 2% agarose gel, then stained with ethidium bromide and autoradiographed.
FACS Analysis
TLR4 intracellular staining was visualized using a rat monoclonal anti-TLR4/MD2 (MTS510) antibody provided by K. Miyake (University of Tokyo, Tokyo, Japan) and a secondary goat anti-rat Cy5-conjugated antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) after fixation (Cytofix; BD Biosciences, Erembodegem, Belgium) with or without permeabilization in Ca2+- and Mg2+-free PBS containing or not 0.5% saponin and 2% FCS. Rat anti-hemagglutinin mAb (Roche Diagnostics, Meylan, France) was used as isotype control.
Western Blot Analysis
Western blot analyses were performed on mpkIMCD cell lysates as described previously.22 Antibodies against TLR4,21 active caspase-3 (Promega Corp., Madison, WI), claudin-4 (Zymed Laboratories, South San Francisco, CA), E-cadherin (BD Transduction Laboratories, Lexington, KY), intercellular adhesion molecule 1 (Santa Cruz Biotechnology), or β-actin (Sigma-Aldrich) were used to detect the corresponding antigens. Protein bands were revealed using peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (Jackson Immunoresearch Laboratories) and detected using the ECL+ Western Blotting Detection System (GE Healthcare, Buckinghamshire, UK).
Immunohistochemical Studies
Cells were fixed in 2% paraformaldehyde and permeabilized with 0.25% NP40 for 5 min. Immunohistochemical studies were performed on nonpermeabilized or permeabilized cells using antibodies raised against murine TLR4 (1:200),21 E. coli (1:200; Interchim, Montluçon, France), ZO-1 (1:200; Zymed Laboratories), or the p58K Golgi marker (1:200; Sigma-Aldrich) and species-specific Alexa 488–and Cy3-conjugated IgG as secondary antibodies (Jackson ImmunoResearch Laboratories). Cells were stained with rhodamine-conjugated phalloidin to visualize the F-actin network, and, in some cases, nuclei were counterstained using Hoechst 33258 (Pierce Chemical, Rockford, IL). Cells were also labeled using active caspase-3 antibody (Promega Corp.) to detect apoptotic cells. The number of active caspase-3–positive cells was counted over three different surface areas of three independent cultures for each of the experimental conditions tested. Specimens were examined using a video microscope for cells grown on filters or a confocal laser-scanning microscope (CLSM-510-META; Zeiss, Mannheim, Germany) for cells grown on glass slides and were photographed.
Transmission Electron Microscopy
Confluent mpkIMCD cells grown on filters and incubated with HT7 added to the apical side of the filters for 3 h were fixed for 30 min in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, embedded in Epon, and processed for transmission electron microscopy by standard procedures.
Quantitative Real-Time PCR
Total RNA was extracted from cultured cells using the RNeasy mini kit (Qiagen) and reverse-transcribed using Moloney murine leukemia virus reverse transcriptase (Invitrogen, Cergy-Pontoise, France). cDNA was subjected to real-time PCR, using a Chromo IV sequence detector (MJ Research, Waltham, MA). The mouse primers and TaqMan probes used for MIP-2, monocyte chemoattractant protein-1, RANTES, TNF-α, and β-actin were the same as described previously.22 The mouse primers and Taqman probes for keratinocyte-derived chemokine, and IFN-γ were as follows: KC (NM_008176): nt 282 to 303, nt 350 to 331 and probe, nt 307 to 329; IFN-γ (NM_008337): nt 224 to 247, nt 315 to 295 and probe, nt 268 to 294. PCR data were reported as the relative increase in mRNA transcripts versus that found in untreated cultured MCD and corrected by the levels of β-actin mRNA, used as the internal standard.
ELISA
MIP-2 and TNF-α secreted in cell supernatants from mpkIMCD cells were determined using ELISA kits (R&D Systems, Lille, France) according to the manufacturer's instructions.
Statistical Analysis
Results are expressed as means ± SEM. The significance of the differences was analyzed by an unpaired t test or a one-way ANOVA, using the Bonferroni or Dunn test for comparisons of two or more groups. P < 0.05 was considered to be significant.
DISCLOSURES
None.
Acknowledgments
This work was funded by INSERM and in part by grants from the Agence Nationale de la Recherche (ANR-08-MIE-30 grant to A.V.) and the German Research Foundation (DFG grant Ho 2236/5-2 to M.W.H.). Dr. Chassin was supported by a PhD student's grant from the French Ministère de la Défense (Délégation Générale de l’Armement/Mission pour la Recherche et l’Innovation Scientifique). Dr. Vandewalle received an Interface INSERM-AP-HP fellowship.
We thank N. Quellard for expert assistance. We also thank E. Tourneur for help in ELISA assay.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Foxman B, Brown P: Epidemiology of urinary tract infections: Transmission and risk factors, incidence, and costs. Infect Dis Clin North Am 17: 227–241, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Johnson JR, Russo TA: Molecular epidemiology of extraintestinal pathogenic (uropathogenic) Escherichia coli. Int J Med Microbiol 295: 383–404, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bergsten G, Wullt B, Svanborg C: Escherichia coli, fimbriae, bacterial persistence and host response induction in the human urinary tract. Int J Med Microbiol 295: 487–502, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Connell I, Agace W, Klemm P, Schembri M, Marild S, Svanborg C: Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci U S A 93: 9827–9832, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ: Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282: 1494–1497, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Leffler H, Svanborg-Edén C: Chemical identification of a glycosphingolipid receptor for Escherichia coli attaching to human urinary tract epithelial cells and agglutinating human erythrocytes. FEMS Microbiol Lett 8: 117–182, 1980 [Google Scholar]
- 7.Roberts JA, Marklund BI, Ilver D, Hasla D, Kaack MB, Baskin G, Louis M, Mollby R, Winberg J, Normark S: The Gal(alpha 1–4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc Natl Acad Sci U S A 91: 11889–11893, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowicki B, Moulds J, Hull R, Hull S: A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect Immun 56: 1057–1060, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B, Finberg RW, Ingalls RR, Golenbock DT: Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest 105: 497–504, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez JJ, Mulvey MA, Schilling JD, Pinkne JS, Hultgren SJ: Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J 19: 2803–2812, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ: Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol 166: 1148–1155, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Wullt B, Bergsten G, Connell H, Rollano P, Gebratsedik N, Hang L, Svanborg C: P-fimbriae trigger mucosal responses to Escherichia coli in the human urinary tract. Cell Microbiol 3: 255–264, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Simons K, Ikonen E: Functional rafts in cell membranes. Nature 387: 569–572, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Brown DA, London E: Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 275: 17221–17224, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Laude AJ, Prior IA: Plasma membrane microdomains: Organization, function and trafficking. Mol Membr Biol 21: 193–205, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manes S, del Real G, Martinez-A C: Pathogens: Raft hijackers. Nat Rev Immunol 3: 557–568, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Duncan MJ, Li G, Shin JS, Carson JL, Abraham SN: Bacterial penetration of bladder epithelium through lipid rafts. J Biol Chem 279: 18944–18951, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Anderson RG: The caveolae membrane system. Annu Rev Biochem 67: 199–225, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Kurzchalia TV, Parton RG: Membrane microdomains and caveolae. Curr Opin Cell Biol 11: 424–431, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K: Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci 115: 2603–2611, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Hornef MW, Normark BH, Vandewalle A, Normark S: Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med 198: 1225–1235, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chassin C, Goujon JM, Darche S, du Merle L, Bens M, Cluzeaud F, Werts C, Ogier-Denis E, Le Bouguenec C, Buzoni-Gatel D, Vandewalle A: Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J Immunol 177: 4773–4784, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Van Huyen JP, Bens M, Teulon J, Vandewalle A: Vasopressin-stimulated chloride transport in transimmortalized mouse cell lines derived from the distal convoluted tubule and cortical and inner medullary collecting ducts. Nephrol Dial Transplant 16: 238–245, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg CM, Ricciardi-Castagnoli P, Layton B, Beutler B: Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 282: 2085–2088, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Johnson JR: Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev 4: 80–128, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strömberg N, Marklund BI, Lund B, Ilver D, Hamers A, Gaastra W, Karlsson KA, Normark S: Host-specificity of uropathogenic Escherichia coli depends on differences in binding specificity to Gal α1–4Gal-containing isoreceptors. EMBO J 9: 2001–2010, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills M, Meysick KC, O’Brien AD: Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect Immun 68: 5669–5680, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyer DM, Radulovic S, Jones FE, Mobley HL: Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect Immun 70: 4539–4546, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korhonen TK, Virkola R, Holthofer H: Localization of binding sites for purified Escherichia coli P fimbriae in the human kidney. Infect Immun 54: 328–332, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leach JL, Garber SA, Marcon AA, Prieto PA: In vitro and in vivo effects of soluble, monovalent globotriose on bacterial attachment and colonization. Antimicrob Agents Chemother 49: 3842–3846, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyes JL, Lamas M, Martin D, del Carmen Namorado M, Islas S, Luna J, Tauc M, González-Mariscal L: The renal segmental distribution of claudins changes with development. Kidney Int 62: 476–487, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Boyd AW, Wawryk SO, Burns GF, Fecondo JV: Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci U S A 85: 3095–3099, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dekker LV, Segal AW: Signals to move cells. Science: 287: 982–985, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Cowan DB, Noria S, Stamm C, Garcia LM, Poutias DN, del Nido PJ, McGowan FX Jr: Lipopolysaccharide internalization activates endotoxin-dependent signal transduction in cardiomyocytes. Circ Res 88: 491–498, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Guillot L, Medjane S, Le-Barillec K, Balloy V, Danel C, Chignard M, Si-Tahar M: Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J Biol Chem 279: 2712–2718, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WG, Phillips MC, Rothblat GH: Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem 270: 17250–17256, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Blau L, Bittman R: Interaction of filipin with cholesterol in vesicles of saturated phospholipids. Biochemistry 16: 4139–4144, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Carrière B, Le Grimellec C: Effects of benzyl alcohol on enzyme activities and D-glucose transport in kidney brush-border membranes. Biochim Biophys Acta 857: 131–138, 1986 [DOI] [PubMed] [Google Scholar]
- 39.Prié D, Ronco P, Baudouin B, Geniteau-Legendre M, Antoine M, Piedagnel R, Estrade S, Lelongt B, Verroust PJ, Cassingena R, Vandewalle A: Activation of the simian virus 40 (SV40) genome abrogates sensitivity to AVP in a rabbit collecting tubule cell line by repressing membrane expression of AVP receptors. J Cell Biol 113: 951–962, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakrabarti G, McClane BA: The importance of calcium influx, calpain and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin. Cell Microbiol 7: 129–146, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Wu JH, Billings BJ, Balkovetz DF: Hepatocyte growth factor alters renal epithelial cell susceptibility to uropathogenic Escherichia coli. J Am Soc Nephrol 12: 2543–2553, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Chassin C, Hornef MW, Bens M, Lotz M, Goujon JM, Vimont S, Arlet G, Hertig A, Rondeau E, Vandewalle A: Hormonal control of the renal immune response and antibacterial host defense by arginine vasopressin. J Exp Med 204: 2837–2852, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frendeus B, Wachtler C, Hedlund M, Fischer H, Samuelsson P, Svensson M, Svanborg C: Escherichia coli P fimbriae utilize the Toll-like receptor 4 pathway for cell activation. Mol Microbiol 40: 37–51, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ: Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol 176: 3070–3079, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Dunzendorfer S, Lee HK, Soldau K, Tobias PS: Toll-like receptor 4 functions intracellularly in human coronary artery endothelial cells: roles of LBP and CD14 in mediating LPS responses. FASEB J 18: 1117–1119, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Jury EC, Kabouridis PS, Flores-Borja F, Mageed RA, Isenberg DA: Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J Clin Invest 113: 1176–1187, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G: Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology 128: 1258–1267, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Hagberg L, Hull R, Hull S, McGhee JR, Michalek SM, Svanborg-Eden C: Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun 46: 839–844, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer H, Yamamoto M, Akira S, Beutler B, Svanborg C: Mechanism of pathogen-specific TLR4 activation in the mucosa: Fimbriae, recognition receptors and adaptor protein selection. Eur J Immunol 36: 267–277, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Welch RA, Burland V, Plunkett G 3rd, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DC, Perna NT, Mobley HL, Donnenberg MS, Blattner FR: Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A 99: 17020–17024, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blattner FR, Plunkett G 3rd, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y: The complete genome sequence of Escherichia coli K-12. Science 277: 1453–1474, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Johnson JR, Stell AL: Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis 181: 261–272, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Ruiz J, Navia MM, Vila J, Gascon J: Prevalence of the SAT gene among clinical isolates of Shigella spp. causing traveler's diarrhea: Geographical and specific differences. J Clin Microbiol 40: 1565–1566, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim E, O’Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E: 2004. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol 24: 10703–10717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elsinghorst EA: Measurement of invasion by gentamicin resistance. Methods Enzymol 236: 405–420, 1994 [DOI] [PubMed] [Google Scholar]