Abstract
Environmental pathogens are suspected to aggravate renal injury in IgA nephropathy (IgAN), but neither underlying mechanisms nor specific exogenous antigens have been identified. In this study, a genome-wide scan of ddY mice, which spontaneously develop IgAN, was performed, and myeloid differentiation factor 88 (MyD88) was identified as a candidate gene for progression of renal injury (χ2 = 21.103, P = 0.00017). For evaluation of the potential influence of environmental pathogens on progression of renal injury, ddY mice were housed in either conventional or specific pathogen-free conditions. Expression of genes encoding toll-like receptors (TLR) and the signaling molecule MyD88 were quantified by real-time reverse transcription–PCR in splenocytes. Although the housing conditions did not affect the prevalence of IgAN, the severity of renal injuries was higher in the conventionally housed group. Mice that had IgAN and were housed in conventional conditions had higher levels of TLR9 and MyD88 transcripts than mice that had IgAN and were housed in specific pathogen-free conditions. Furthermore, nasal challenge with CpG-oligodeoxynucleotides, which are ligands for TLR9, aggravated renal injury, led to strong Th1 polarization, and increased serum and mesangial IgA. For investigation of whether these results may be generalizable to humans, single-nucleotide polymorphisms in the TLR9 and MyD88 genes were analyzed in two cohorts of patients with IgAN; an association was observed between TLR9 polymorphisms and disease progression. In summary, these findings suggest that activation of the TLR9/MyD88 pathway by common antigens may affect the severity of IgAN.
IgA nephropathy (IgAN) is the most common form of progressive glomerulonephritis, although its pathogenesis is unclear. Clinical findings suggest that upper respiratory tract infections and tonsillitis are risk factors for IgAN.1,2 Microbial infections affect various immune and autoimmune responses.3,4 Mucosal exposure to exogenous antigens of fungi, bacteria, or viruses was suggested to be involved in the pathogenesis of IgAN; however, it remains unclear how all of the various antigens interact with IgA immune system to trigger or aggravate this disease.3,5–7 Genetic background may influence the disease susceptibility in general8–10; several candidate genes are associated with progression of IgAN.11–16 Consequently, one can speculate that the amplitude of immune responses to common exogenous antigens in IgAN may be partly dependent on the genetic background and contribute to the disease susceptibility.
Toll-like receptors (TLR) are a family of pathogen pattern recognition receptors that sense different classes of pathogen-related structures and activate defense mechanisms, particularly in innate immunity.17,18 Myeloid differentiation factor 88 (MyD88) is a common adaptor molecule required for the signaling mediated by TLR. The immunostimulatory effects by exogenous antigens are sensed by different members of the MyD88-dependent or -independent TLR family.19,20 For example, cell wall components of Gram-positive bacteria are recognized by TLR2.21 TLR4 recognizes LPS of Gram-negative bacteria.22 TLR9 has been identified as a receptor for bacterial and viral DNA containing a specific sequence pattern including unmethylated cystein-guanosine dinucleotide (CpG) motif.23 The classical TLR9-triggered signaling is strictly MyD88 dependent.24
The ddY mouse is a widely known model of spontaneous IgAN with variable incidence and extent of glomerular injury mimicking human IgAN.25,26 We recently reported that the ddY mice could be classified into three groups—early-onset, late-onset, and quiescent groups—on the basis of serial histologic confirmation of glomerular lesions and IgA deposits.26 The ddY mice with commencing IgAN exhibit strong polarization toward Th1, whereas those with quiescent disease are Th2 polarized. Furthermore, serum levels of IgA-IgG2a immune complexes (IC) correlate with severity of the glomerular lesions.27 Genome-wide association study in early-onset and quiescent mice showed that the susceptibility of the murine IgAN is partly regulated by specific locus syntenic to the IGAN1,28 known as a candidate locus of human familial IgAN. These observations suggest that this mouse model shares common susceptibility factors with human IgAN and is thus suitable for examining the pathogenesis of IgAN.
Furthermore, we hypothesized that microbial antigens may induce excessive mucosal IgA responses and subsequent development of IgAN. Genome-wide scan using the grouped ddY mouse model identified association of MyD88 with IgAN progression. We further investigated underlying molecular mechanisms by which MyD88 may be implicated in the pathogenesis of IgAN and explored the genetic association in human IgAN populations.
RESULTS
MyD88 Represents a Susceptibility Gene Predisposing to the Progression of Murine IgAN
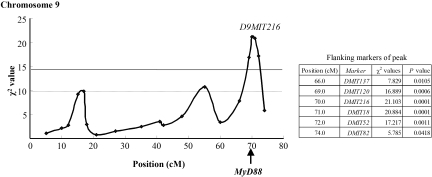
We determined the onset and progression of glomerular injury by serial renal biopsies in 361 female ddY mice. The ddY mice could be classified into three groups on the basis of the onset of glomerular injury: Early-onset (n = 115), late-onset (n = 137), and quiescent (n = 109) groups.26 The early- and late-onset ddY mice were grouped into severe (n = 114; 61 were from the early-onset group, and 53 were from the late-onset group) and mild glomerulonephritis (GN; n = 138; 54 were from the early-onset group, and 84 were from the late-onset group) groups on the basis of the severity of renal injuries at 60 wk of age. A genome-wide scan using 270 microsatellite markers was performed for each ddY mouse. In this study, we analyzed the association between the severe and mild GN groups to detect the candidate gene associated with progression of IgAN. The marker loci with association between the severe and mild GN groups were distributed on chromosome 9. The marker locus with the highest χ2 value was D9MIT216 at 70.0 cM on chromosome 9 (χ2 = 21.103, genome-wide significant P = 0.0027; Figure 1) that is located close to MyD88 gene. The flanking markers of peak and their positions are shown in Figure 1.
Figure 1.
Susceptibility locus for the progression of renal injury on murine chromosome 9. Solid line at χ2 = 14.4 indicates the significant threshold, and the lower dotted line at χ2 = 10.0 indicates the suggestive thresholds, which were calculated by permutation analysis. On chromosome 9, the maximal χ2 value was 21.103 (P = 0.00017, genome-wide significant P = 0.0027) at the D9MIT216 marker locus, which lies in very close proximity to the MyD88 gene. The flanking markers of peak and their positions are shown in the inset.
Renal Injury Index Increased in Mice in Conventional Environment
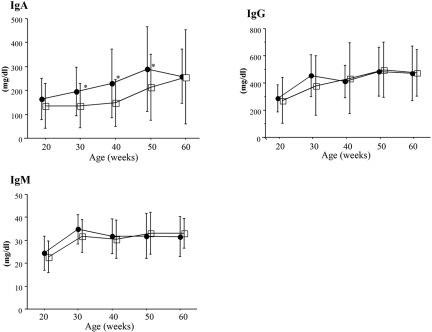
For evaluation of potential effect of pathogens on the incidence of murine IgAN, ddY mice were maintained under conventional and specific pathogen-free (SPF) conditions. There were no differences in the prevalence of IgAN between conventional and SPF groups (Table 1). Prevalence of renal injury increased with age in both the conventional and SPF groups; however, scores of renal injury in the conventional group were higher than those in the SPF group at 60 wk of age (P < 0.001; Table 1). Moreover, serum IgA levels in the conventional group were higher than those in the SPF group (Figure 2).
Table 1.
Prevalence and severity of IgAN distributed in conventional and SPF environmentsa
| Parameter | Age
|
|||||
|---|---|---|---|---|---|---|
| 20 wk
|
40 wk
|
60 wk
|
||||
| Conventional | SPF | Conventional | SPF | Conventional | SPF | |
| No. of mice with renal injuries/total mice (%) | 29/100 (29.0) | 31/100 (31.0) | 65/98 (66.3) | 64/99 (64.6) | 69/92 (75.0) | 68/95 (71.6) |
| Scores of renal injuries | 4.241 ± 0.511 | 4.161 ± 0.374 | 5.046 ± 1.205 | 4.703 ± 0.937 | 6.232 ± 1.682b | 5.441 ± 1.429 |
Data are means ± SD.
P < 0.001.
Figure 2.
Changes of serum IgA, IgG, and IgM levels in the conventional and SPF groups. Although the mean IgA levels increased with age in both groups, IgA levels in the conventional group were higher than those in the SPF group at 30 to 50 wk of age. •, conventional group; □, SPF group. Data are means ± SD. *P < 0.05.
Transcriptional Levels of TLR9 and MyD88 Were Elevated in Mice with Commencing IgAN in the Conventional Group
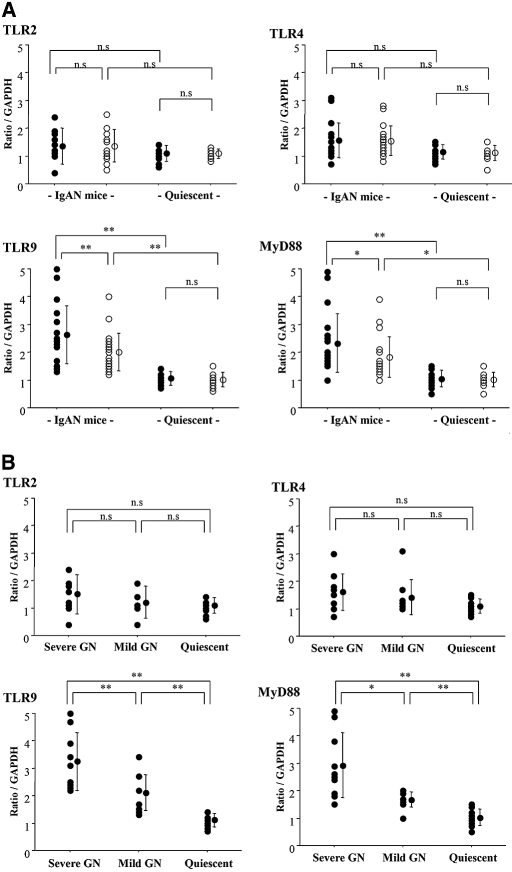
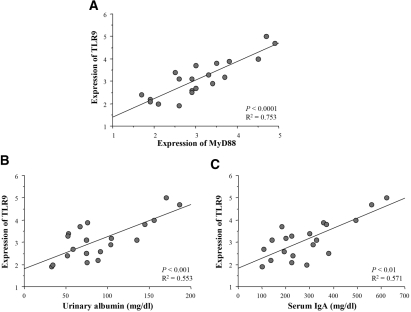
Splenocyte transcript levels of TLR2, 4, and 9 and MyD88 in the conventional and SPF groups at 60 wk of age were quantified by real-time reverse transcription–PCR (RT-PCR). The quiescent mice in both groups were used as controls. Transcript levels of TLR9 (P < 0.01) and MyD88 (P < 0.05) in mice with commencing IgAN of the conventional group were higher than those in the SPF group (Figure 3A). In contrast, quiescent mice did not show any differences between the conventional and SPF groups. Furthermore, levels of TLR2 and 4 transcripts were similar between the conventional and SPF groups. Next, the mice with commencing IgAN in the conventional group at 60 wk of age were grouped into severe and mild GN groups on the basis of their renal injury scores. To determine the cutoff criterion of the histology score, we defined severe GN group at 60 wk of age with a histology score of more than the mean + 2 SD (7.456) of the score of ddY mice with renal injuries at 40 wk of age. Importantly, levels of TLR9 and MyD88 transcripts were elevated depending on the severity of renal injury (Figure 3B). Furthermore, we analyzed the correlation between the TLR9/MyD88 transcript levels and serum IgA levels or the amount of albuminuria in the mice with severe GN (n = 20). The transcript levels of TLR9 correlated with levels of MyD88 transcripts (P < 0.0001; Figure 4A), and the scores of renal injuries were associated with the amount of urinary albumin (P < 0.001). Levels of TLR9/MyD88 transcripts correlated not only with the histologic severity but also with the degree of albuminuria (P < 0.001; Figure 4B). Moreover, the levels of TLR9/MyD88 transcripts correlated with serum IgA levels (P < 0.01; Figure 4C).
Figure 3.
(A) Splenic transcriptional levels of TLR quantified by real-time RT-PCR in mice with renal injuries at 60 wk of age. Levels of TLR9 and MyD88 transcription in the conventional group (n = 20) were higher than those in the SPF group (n = 20). The quiescent mice in both groups were used for controls (each n = 10). Transcriptional levels of TLR2 and TLR4 did not show any differences among the groups. (B) The ddY mice with commencing IgAN were divided into severe GN (n = 10) and mild GN (n = 10) groups by the severity of renal injuries. Levels of TLR9 and MyD88 transcriptions were elevated in mice with severe GN. •, conventional group; ○, SPF group. Each circle shows individual datum standardized as a ratio of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcription. Data are means ± SD. *P < 0.05; **P < 0.01.
Figure 4.
(A) The transcript levels of TLR9 correlated with those of MyD88 in conventional group mice with severe GN (regression analysis; n = 20; P < 0.0001). (B and C) In these mice, transcript levels of TLR9 were associated with the degree of urinary albumin as well as levels of serum IgA (P < 0.001 and P < 0.01, respectively). Transcript levels of TLR9 and MyD88 were standardized relative to GAPDH.
Polymorphisms in Murine TLR9 and MyD88 Genes Were Detected between Mice with Severe and Mild GN
We compared sequences of entire MyD88 gene between mice with severe and mild GN (each n = 50). A single-nucleotide polymorphism (SNP; ATC[G/A]GC) at 644 bp of this gene located in the exon segment was identified. This mutation corresponds to amino acid change, arginine to glutamine, in TIR motif of MyD88 protein. The frequency of A allele differed between mice with severe and mild GN, 38 and 18%, respectively (P = 0.02). For the polymorphism, higher frequency of allele A was associated with high score of renal injury as well as high degree of albuminuria (Supplemental Table 1). To assess whether the TLR9 gene is also involved in the disease progression in ddY mice, we analyzed sequences of entire TLR9 gene in mice with severe and mild GN. We found an SNP (CTG[C/G]AG) at 159 bp of this gene located in the exon segment. This mutation corresponds to amino acid change, alanine to glycine, just before the leucine-rich repeat of TLR9 protein. The frequency of G allele was increased in mice with severe GN compared with mice with mild GN, 40 and 14%, respectively (P < 0.01). This polymorphism was associated with the scores of renal injuries as well as with the degree of albuminuria (P < 0.01, each).
Th1 Polarization Was Strongly Enhanced under Conventional Environment
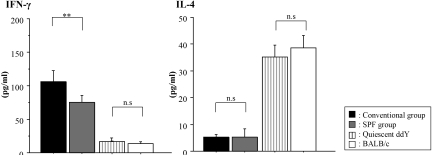
In this study, we investigated whether the Th1/Th2 balance is affected by pathogen-mediated immune responses. The mice with commencing IgAN in the conventional group showed higher levels of IFN-γ produced by their splenic T cells than those in the SPF group at 60 wk of age (P < 0.01; Figure 5).
Figure 5.
Levels of cytokines produced by splenic T cells. Levels of IFN-γ in culture supernatants of spleen cells from mice with commencing IgAN in the conventional group (n = 10) were higher than those in the SPF group (n = 10), although levels of IL-4 did not change between those two groups. Data are means ± SD. **P < 0.01. Age-matched quiescent ddY mice (n = 10) and BALB/c mice (n = 10) showed low production of IFN-γ and high production of IL-4 compared with the ddY mice with commencing IgAN.
CpG Oligodeoxynucleotides Aggravated Renal Injury and Induced Transcriptional Activation of TLR9 and MyD88
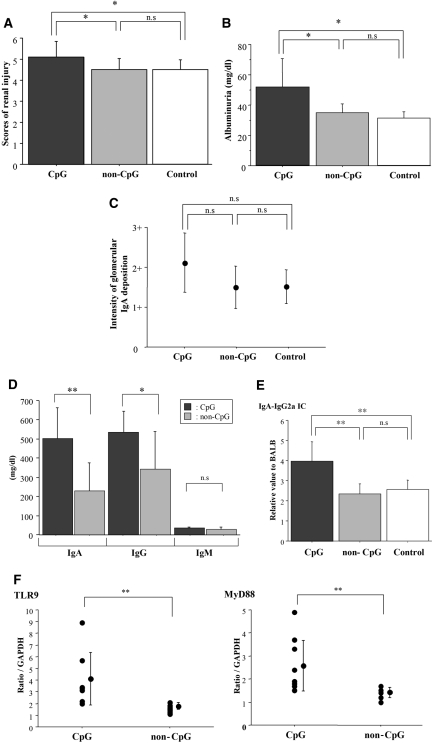
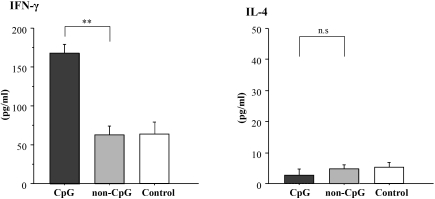
To assess whether activation of TLR9 with CpG oligodeoxynucleotides (CpG-ODN) affects progression of IgAN in the SPF mice, we performed nasal challenge with CpG-ODN in the early-onset ddY mice of the SPF group (n = 10). Renal histology was analyzed 5 wk after the first CpG challenge. In control experiment, we confirmed that CpG-ODN did not induce any renal injury in BALB/c mice (data not shown). Scores of renal injuries and degree of albuminuria increased in the mice challenged with CpG-ODN (P < 0.05) but not in mice treated with non–CpG-ODN (Figure 6, A and B). There was a tendency for the intensity of glomerular IgA deposition to increase after treatment with CpG-ODN (Figure 6C). In the mice treated with CpG-ODN, levels of serum IgA, IgG, and IgA-IgG2a IC increased (Figure 6, D and E). Furthermore, transcript levels of TLR9 and MyD88 also increased (P < 0.01; Figure 6F). We showed that the Th1 activity of CpG-ODN was specific to the CpG motif; splenic production of IFN-γ was enhanced by CpG-ODN but not by non–CpG-ODN treatment (Figure 7).
Figure 6.
(A) Evaluation of renal injuries by semiquantitative scoring after challenge with CpG-ODN. The ddY mice with commencing IgAN in the SPF group were treated with CpG-ODN (n = 10) or non–CpG-ODN (n = 10). Untreated ddY mice with commencing IgAN were evaluated as control (n = 10). Renal injuries in mice challenged with CpG-ODN were exacerbated. The degree of renal injuries in mice treated with non–CpG-ODN was similar to those in control. (B) CpG-ODN enhanced the degree of urinary albumin. (C) Intensity of glomerular IgA deposition was evaluated semiquantitatively. There was a tendency for the intensity of glomerular IgA deposition to increase after treatment with CpG-ODN. (D) Serum levels of IgA and IgG were elevated in the mice treated with CpG-ODN. (E) Serum levels of IgA-IgG2a IC expressed relative to those of BALB/c mice. CpG-ODN increased serum IgA-IgG2a IC levels. (F) Transcript levels of TLR9 and MyD88 were quantified by real-time RT-PCR. Levels of TLR9 and MyD88 transcription were enhanced by treatment with CpG-ODN. Data are means ± SD. *P < 0.05; **P < 0.01. CpG, mice immunized with CpG-ODN; non-CpG, mice immunized with non–CpG-ODN; control, age-matched ddY mice with commencing IgAN without immunization.
Figure 7.
Levels of cytokines produced by splenic T cells after treatment with CpG-ODN. After treatment with CpG-ODN, the level of IFN-γ in culture supernatant of spleen cells was elevated. Data are means ± SD. **P < 0.01. CpG, early-onset mice in the SPF group treated with CpG-ODN (n = 10); non-CpG, early-onset ddY mice of the SPF group treated with CpG-ODN (n = 10); control, untreated early-onset ddY mice of the SPF group (n = 10).
Polymorphisms of the TLR9 Gene Are Associated with Histologic Severity of Japanese Patients with IgAN
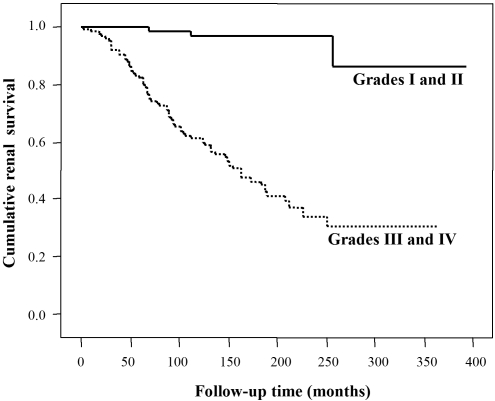
Five common single nucleotide polymorphisms (SNP) in the TLR9 gene and two SNP in the MyD88 gene were genotyped in patients with IgAN (n = 89) and healthy control subjects (n = 94; cohort 1). Because the rs5743836 and rs199396 were not polymorphic in this population, these SNP were excluded from this analysis. There was no association of either TLR9 or MyD88 polymorphisms between patients with IgAN and healthy control subjects (data not shown). Next, patients with IgAN were divided into two subgroups on the basis of the histopathologic severity (Table 2), nonprogressive group (grades I and II; n = 41) and progressive group (grades III and IV; n = 48), respectively. Clinical manifestations, such as serum creatinine concentration and urinary protein excretion at the time of renal biopsy, supported this classification on the basis of histologic severity (P < 0.001 and P < 0.0001, respectively; Table 3). As shown in Table 3, we found a possible association with the histologic severity of IgAN in the two SNP in the TLR9 gene (rs352139 and rs352140). To reproduce these findings, we used another nonprogressive group (grades I and II; n = 151) and progressive group (grades III and IV; n = 177) of Japanese patients with IgAN (cohort 2). Kaplan-Meier analysis of cumulative renal survival between these two groups and clinical manifestations demonstrated the suitability of cohort 2 for genetic association study (Figure 8). In cohort 2, we found a strong association with the progression of IgAN in the TLR9 gene (Table 3). Logistic regression analysis suggested that CC or CT genotypes in rs352139 and TT genotypes in rs352140 have an increased risk for the progression of IgAN (Table 4).
Table 2.
Prognostic grading for patients with IgAN
| Parameter | Grade I (Good Prognosis) | Grade II (Relatively Good Prognosis) | Grade III (Relatively Poor Prognosis) | Grade IV (Poor Prognosis) |
|---|---|---|---|---|
| Mesangial proliferation and increased matrix | Slight | Slight | Moderate, diffuse | Severe, diffuse |
| Glomerulosclerosis, crescent formation, or adhesion to Bowman's capsule | – | <10% | 10 to 30% | >30% |
| Interstitial cellular infiltration | – | – | Slight | + |
| Tubular atrophy | – | – | Slight | + |
| Changes of blood vessels | – | – | Mild sclerosis | Hyperplasia or degeneration |
Table 3.
Relationship between TLR9 polymorphisms and histologic severity in patients with IgAN
| Parameter | Nonprogressive Group (Grades I and II; n = 41) | Progressive Group (Grades III and IV; n = 48) | χ2 | Pa |
|---|---|---|---|---|
| In the first cohort genotype frequencies | ||||
| rs352139 (TLR9) | ||||
| CC | 8 (19.5%) | 14 (29.2%) | ||
| CT | 18 (43.9%) | 27 (56.3%) | 5.831 | 0.1084 |
| TT | 15 (36.6%) | 7 (14.6%) | ||
| rs352140 (TLR9) | ||||
| TT | 7 (17.1%) | 14 (29.2%) | ||
| CT | 17 (41.5%) | 26 (54.2%) | 6.949 | 0.0620 |
| CC | 17 (41.5%) | 8 (16.6%) | ||
| Allele frequencies | ||||
| rs352139 (TLR9) | ||||
| C | 34 (41.5%) | 55 (57.3%) | 4.451 | 0.0698 |
| T | 48 (58.5%) | 41 (42.7%) | ||
| rs352140 (TLR9) | ||||
| T | 31 (37.8%) | 54 (56.3%) | 6.072 | 0.0274b |
| C | 51 (62.2%) | 42 (43.7%) | ||
| clinical data at the time of renal biopsy | ||||
| age (yr) | 31.0 ± 11.1 | 34.6 ± 10.9 | NS | |
| gender (% male) | 63.4 | 62.5 | NS | |
| serum creatinine (mg/dl) | 0.70 ± 0.17 | 1.01 ± 0.39 | <0.0010b | |
| urinary protein (g/d) | 0.55 ± 0.74 | 1.85 ± 1.71 | <0.0001b | |
| In the second cohort genotype frequencies | (n = 151) | (n = 177) | ||
| rs352139 (TLR9) | ||||
| CC | 21 (13.9%) | 61 (34.5%) | ||
| CT | 80 (53.0%) | 80 (45.2%) | 19.855 | <0.0001b |
| TT | 50 (33.1%) | 36 (20.3%) | ||
| rs352140 (TLR9) | ||||
| TT | 24 (15.9%) | 62 (35.0%) | ||
| CT | 79 (52.3%) | 80 (45.2%) | 16.878 | 0.0004b |
| CC | 48 (31.8%) | 35 (19.8%) | ||
| allele frequencies | ||||
| rs352139 (TLR9) | ||||
| C | 122 (40.4%) | 202 (57.1%) | 18.197 | <0.0001b |
| T | 180 (59.6%) | 152 (42.9%) | ||
| rs352140 (TLR9) | ||||
| T | 127 (42.1%) | 204 (57.6%) | 15.876 | <0.0001b |
| C | 175 (57.9%) | 150 (42.4%) | ||
| clinical data at the time of renal biopsy | ||||
| age (yr) | 34.6 ± 14.7 | 39.7 ± 13.0 | 0.0003b | |
| gender (% male) | 40.5 | 47.7 | NS | |
| serum creatinine (mg/dl) | 0.77 ± 0.22 | 1.15 ± 0.71 | <0.0001b | |
| urinary protein (g/d) | 0.77 ± 0.93 | 1.74 ± 0.77 | <0.0001b |
P values were adjusted by Bonferroni correction.
P < 0.05.
Figure 8.
Kaplan-Meier analysis of cumulative renal survival between nonprogressive group (grades I and II; n = 151) and progressive group (grades III and IV; n = 177) of patients with IgAN. χ2 = 52.014, P < 0.0001.
Table 4.
Logistic regression analysis for the progression of IgANa
| Parameter | Nonprogressive Group (Grades I and II; n = 41) | Progressive Group (Grades III and IV; n = 48) | χ2 | Pb | OR | 95% CI |
|---|---|---|---|---|---|---|
| In the first cohort genotypes | ||||||
| rs352139 (TLR9) | ||||||
| CC or CT | 26 | 41 | 5.752 | 0.0330c | 3.38 | 1.22 to 9.40 |
| TT | 15 | 7 | ||||
| rs352140 (TLR9) | ||||||
| TT | 7 | 14 | 1.794 | 0.3610 | 2.00 | 0.72 to 5.57 |
| CC or CT | 34 | 34 | ||||
| In the second cohort genotypes | (n = 151) | (n = 177) | ||||
| rs352139 (TLR9) | ||||||
| CC or CT | 101 | 141 | 6.873 | 0.0176c | 1.94 | 1.18 to 3.19 |
| TT | 50 | 36 | ||||
| rs352140 (TLR9) | ||||||
| TT | 24 | 62 | 15.422 | <0.0001c | 2.85 | 1.67 to 4.87 |
| CC or CT | 127 | 115 |
CI, confidence interval; OR, odds ratio.
P values were adjusted by Bonferroni correction.
P < 0.05.
DISCUSSION
The genome-wide association study between the mice with severe and mild GN demonstrated that MyD88 gene is a susceptibility gene for the development of murine IgAN (Figure 1). Because MyD88 is a common adaptor molecule required for signaling by TLR, microbial-mediated mechanisms and innate immunity may be involved in the progression of IgAN. Although bacterial and viral infections in mucosa have been considered a risk factor for the progression of human IgAN,1,2,5,6 Kawasaki et al.29 reported that viral infection can provoke renal injury also in murine IgAN. For assessment of potential influence of exogenous pathogens on the disease progression, the IgAN-prone ddY mice were maintained separately under the conventional and SPF conditions in this study. There was no significant difference in prevalence of disease between both groups, whereas the degree of renal injury in the conventional group was more severe than that in the SPF group at 60 wk of age. The conventional group showed higher serum IgA levels and stronger Th1 polarization that correlated with the severity of the disease.27 These findings strongly suggest that exogenous pathogen may contribute to production of nephritogenic IgA and formation of IgA-IgG2a IC,27 subsequently leading to the aggravation of renal injury. Importantly, transcriptional levels of TLR9 and MyD88 in splenocytes from the mice with commencing IgAN in the conventional group were higher than those in the SPF group and correlated with severity of renal injury; however, gene expressions of TLR2 and 4, known to be associated with renal injury in other models,30 were not different between the conventional and SPF groups. Therefore, we hypothesized MyD88 signaling after TLR9 binding of microbial components may be one of the major pathways in the pathogen-mediated aggravation of IgAN.
Gene polymorphisms of murine TLR9 and MyD88 genes were associated with the severity of murine IgAN. Both mutations cause amino acid change in the corresponding proteins. The transcript levels of TLR9 correlated with levels of MyD88 transcription (P < 0.0001). Importantly, the levels of TLR9/MyD88 transcription correlated not only with the severity of renal injury but also with serum IgA levels in the mice with severe GN (Figure 4). These findings were confirmed in the mice treated with CpG in the SPF group. Several studies have suggested that CpG-ODN aggravates IC-mediated GN via the shift toward the Th1 dominant immune response.31,32 In fact, this study showed that CpG-ODN aggravated the renal injury with increase of TLR9/MyD88 transcription and Th1 polarization. Mucosal challenge with CpG-ODN enhanced IgAN-specific phenomena, such as glomerular IgA deposition and increase of serum IgA and IgA-IgG2a IC27; therefore, TLR9/MyD88-mediated immune activation by exogenous antigens may play an important role in the pathogenesis of IgAN. Human TLR9 is exclusively expressed by plasmacytoid dendritic cells (DC) and B cells, whereas in mice, TLR9 is also expressed by macrophages and myeloid DC.24 Antigen-presenting cells, such as DC and macrophages, activate the adaptive immune response by migrating from the infection site to the regional lymph node, where they present microbe-derived antigens to naive CD4+ T cells. Moreover, activated DC express co-stimulatory molecules essential to the T cell activation and can prime naive CD4+ T cells into Th1/Th2 differentiation.33,34 Previous studies postulated that microbial antigens are involved in the pathogenesis of IgAN,1,3,35 partly mediated via DC function.36 CpG-ODN activates B cells directly, resulting in cellular proliferation and antibody production.37 In fact, in human IC-mediated GN, IC-containing CpG-ODN have been demonstrated to induce T cell–independent B cell activation, proliferation, and autoantibody production.38 In murine model, CpG-ODN activates glomerular and interstitial DC and macrophages via TLR932; however, renal pathologic analysis of the ddY mice did not show any distinct intrarenal monocyte/macrophage infiltration. Therefore, in the ddY mouse, aggravation was associated with immune modulation toward Th1 and enhanced IgA-IgG2a IC production, consistent with the expression of TLR9 on plasmacytoid DC and B cells. Further investigation is needed to address this hypothesis.
These genetic analyses in human IgAN further support the relevance of the pathogen-mediated signaling through TLR9 in the pathogenesis of this disease. An association of TLR9 polymorphisms and disease progression but not disease incidence was observed, suggesting that similar mechanisms involving TLR9/MyD88 may operate in both human and murine IgAN; however, we did not find any association of MyD88 polymorphism with disease progression in human IgAN. This discrepancy may suggest that regulation of TLR9/MyD88 signaling pathway is different between mice and humans. In this study, we used two SNP in MyD88 gene because of lack of heterozygosity in the Japanese population; therefore, genetic analysis of MyD88 gene polymorphisms using larger numbers of patients and different ethnic populations is desired. Although we detected TLR9 gene as a possible candidate gene for the progression of IgAN, the functional consequences of those polymorphisms remain unclear, and, thus, further studies are required.
In summary, TLR9/MyD88 activation by exogenous antigens may lead to deterioration of murine IgAN, at least in part, in an IgAN-specific manner. In addition, specific polymorphisms of TLR9 gene may have a significant influence on the progression of IgAN in Japanese patients. These findings indicate that immune responses to exposure of unmethylated CpG motif from common microbial antigens may play a role in the pathogenesis of this disease. Furthermore, amplitude of these responses, partly caused by gene polymorphisms, may affect severity of renal injury and thus contribute to disease progression; therefore, TLR9/MyD88 signaling pathway may represent a possible new target for a novel therapeutic strategy in IgAN.
CONCISE METHODS
Mice and Breeding Environment
A total of 361 female ddY mice at 10 wk of age (SLC Japan, Shizuoka, Japan) were maintained with regular chow (MF; Oriental Yeast, Tokyo, Japan) and water ad libitum in an SPF room at the animal facility of Juntendo University and used for genetic study. For assessment of the exogenous pathogen–mediated immune responses, the ddY mice were maintained under conventional condition (conventional group; n = 100) and SPF condition (SPF group; n = 100). Age-matched 22 female BALB/c mice were used for control. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Juntendo University School of Medicine.
Serum and Urinary Analyses
Blood samples were obtained from the orbital venous plexus using capillary tubes. Serum IgA, IgG, and IgM were measured by single radioimmunodiffusion (SRL, Tokyo, Japan). Urinary albumin was measured using an ELISA kit (Albuwell, Exocell, Philadelphia, PA). Serum IgA-IgG2a IC was detected by sandwich ELISA, as we previously reported.27 Purified rat anti-mouse IgG2a antisera (BD Biosciences, Pharmingen, San Diego, CA) and horseradish peroxidase–conjugated goat anti-mouse IgA (Zymed Laboratories, San Francisco, CA) were used. Data were expressed relative to the value of BALB/c mice.
Evaluation of Renal Injuries of ddY Mice
Serial renal biopsies were performed under general anesthesia at 20, 40, and 60 wk of age.26,27 For light microscopy, the specimens were fixed in 10% neutral phosphate-buffered formalin, embedded in paraffin, and sliced at 2 μm. The slices were stained with hematoxylin/eosin and periodic acid-Schiff and AZAN. Snap-frozen 4-μm-thick renal sections were used for immunofluorescence with FITC-conjugated goat anti-mouse IgA (BD Biosciences, Pharmingen). The biopsy specimens were evaluated in a triple-blind manner by two nephrologists and one pathologist. The biopsy specimens that contained more than 30 glomeruli were used for histopathologic analysis. All biopsy specimens were quantitatively analyzed to determine the percentages of glomeruli with (1) segmental and global sclerosis and/or (2) mesangial cell proliferation and/or (3) increase in mesangial matrix. Each specimen was scored semiquantitatively for the percentages of glomeruli with aforementioned lesions (0, 0%; 1, 1 to 24%; 2, 25 to 49%; and 3, >50% of all glomeruli).26,27,39,40 Total maximal score for each specimen was 9 using our scoring system. We defined renal injury as renal lesion whose histology score is more than the mean + 2 SD of the score for BALB/c mice. Because the average score for BALB/c mice was 1.424 ± 0.969, histology scores that were ≧4 were regarded as renal injury in this study. Then ddY mice were divided into three groups by the onset time: Early-onset, late-onset, and quiescent groups.26
Genome-Wide Association Study
Genomic DNA was obtained from mouse tails by the QIAamp DNA mini kit (Qiagen, Valencia, CA). Genotyping using 270 microsatellite markers was performed via PCR with fluorescence-labeled oligonucleotides (Perkin Elmer-Cetus, Foster City, CA) and the subsequent analysis with ABI 3700 sequencer (Applied Biosystems, Foster City, CA). Electrophoretic profiles were analyzed by Genescan and Genotyper software (Applied Biosystems). We grouped the ddY mice with commencing IgAN at 40 wk of age into severe and mild GN groups on the basis of scores of renal injuries. To determine the cutoff criterion of the histology score, we defined the severe GN group with a histology score of more than the mean ± 2 SD (>4.909) of the scores of early-onset ddY mice at 20 wk of age. The association value in each marker was tested for χ2 statistics. For the chromosomal regions with a significant association, allele frequencies were also compared between the severe and mild GN groups. To obtain the empirical significance levels associated with the χ2 statistics observed in the genome-wide association studies, we conducted permutation tests by SIMULATE41,42 and BINOM program,43 as we previously reported.26
Transcriptional Levels of TLR and MyD88
Spleens were removed under aseptic conditions. Total RNA was extracted using Trizol solution (Invitrogen, Tokyo, Japan) according to the manufacturer's instruction. Real-time RT-PCR was performed on the Applied Biosystems 7500 Real Time PCR System using SYBR Green PCR Master Mix (Applied Biosystems, Tokyo, Japan) and specific primers (Table 5). PCR conditions were as follows: Samples were preheated for 15 min at 95°C and then subjected to denaturing conditions at 95°C for 5 s. After annealing, genes were amplified for 15 s per cycle (Table 5).
Table 5.
Sequences of primers and PCR conditions
| Gene | Sense 5′-3′ | Antisense 5′-3′ | Amplification Conditions |
|---|---|---|---|
| TLR2 | TGGTTCTTTTCCCAAACTGG | ATAGGAGTTCGCAGGAGCAA | 40 cycles, 70°C |
| TLR4 | GCTTTCACCTCTGCCTTCAC | GCAATGGCTACACCAGGAAT | 40 cycles, 70°C |
| TLR9 | AAGAGCCTGAAGCTGCTGAG | CAGGTTGGGTAGGAAGGACA | 40 cycles, 70°C |
| MyD88 | TGATGACCCCCTAGGACAAA | TCATCTCCTGCACAAACTCG | 40 cycles, 70°C |
| GAPDH | TGCACCACCAACTGCTTA | GGATGCAGGGATGTTC | 25 cycles, 63°C |
Gene Sequencing
Genomic DNA specimens from mice with severe GN (n = 50) and mild GN (n = 50) were analyzed for gene sequence of TLR9 and MyD88 using several primer sets that covered the entire genes. PCR products were purified and diluted in molecular biology–grade water using QIAquick PCR purification kit (Qiagen) and subsequently directly sequenced at the DNA Sequencing Core at the University of Alabama at Birmingham (Birmingham, AL).
Reagents and Immunization
CpG-ODN and non–CpG-ODN were chemically synthesized (Invitrogen). Sequences of the ODN are TCCATGACGTTCCTGACGTT or TCCAATGAGCTTCCTGAGTCT (5′→3′) for CpG or non-CpG, respectively. Both ODN have been previously used for both parenteral44,45 and mucosal challenges.46 The early-onset ddY mice of the SPF group at 23 wk of age were nasally challenged at weekly intervals for 3 consecutive weeks with 10 μg CpG-ODN (n = 10) and non–CpG-ODN (n = 10), respectively. For evaluation of the toxicity for renal damage, the same dosage of CpG-ODN was administered to age-matched BALB/c mice (n = 3). After 5 wk from the first challenge, serum and urine samples were collected and kidney specimens were histopathologically evaluated.
Measurement of Cytokines Produced by Splenic T Cells
For assessment of the production of IFN-γ and IL-4 by spleen cells, spleen cell suspensions were prepared by compression with the handle of a syringe in RPMI 1640 1% FCS medium followed by passage through a 100-μm nylon mesh under aseptic conditions. Red blood cells were removed by ACK lysing buffer (0.15 mol/L NH4Cl, 1.0 mmol/L KHCO3, and 0.1 mmol/L Na2EDTA [pH 7.2]) for 5 min at room temperature and washed in RPMI 1640 1% FCS medium. Spleen cells (1 × 106 cells/ml) were cultured in RPMI 1640 medium with 10% FCS for 48 h at 37°C. Culture supernatants were removed after 48 h, and IFN-γ and IL-4 were measured using ELISA kit (Endogen; Pierce Biotechnology, Rockford, IL).
Human DNA Isolation for SNP Genotyping
Blood samples were collected from 89 Japanese patients with IgAN proved by renal biopsy and 94 healthy volunteers as control subjects. As a second cohort, we recruited 328 patients with IgAN proved by renal biopsy. The control subjects were also native Japanese and unrelated to the patients but born in the same geographic area. Genomic DNA from whole blood samples was purified using a QIAmp Blood Mini Kit (Qiagen, Hilden, Germany). From all patients and volunteers who participated in this study, informed consent was obtained according to the protocol approved by the research ethics committee in our institutions and their hospitals.
Classification of the Severity of IgAN
The severity of IgAN was classified into four groups by “Clinical Guidelines for Diagnosis and Treatment of Patients with Immunoglobulin A (IgA) Nephropathy in Japan.”47 The prognostic grading in this guideline using renal biopsy specimens was done by the Special Study Group on Progressive Glomerular Disease in the Ministry of Health and Welfare of Japan and the Japanese Society of Nephrology, with retrospective clinical data. Each prognostic grade is based on light microscopic histologic findings as summarized in Table 2. The renal prognosis by these criteria is divided into the following four groups: Good prognosis group (group I) who will probably never fall into end-stage renal failure (ESRF), relatively good prognosis group (group II) who have low possibility to progress to ESRF, relatively poor prognosis group (group III) who have high possibility to progress to ESRF within 5 to 20 yr, and poor prognosis group (group IV) who will progress to ESRF within 5 yr.47 This grading was evaluated by one pathologist and at least three nephrologists in a blind manner. To avoid the possibility that the duration between the onset of IgAN and the time of renal biopsy may influence the pathologic severity, we also analyzed the duration in each pathologic grade of patients. The disease onset was defined as the time of the first manifestation of urinary abnormality.
SNP Genotyping
Four common SNP at different locations of the TLR9 gene that have been reported to have higher heterozygous frequencies were selected: rs187084 and rs5743836 (promoter), rs352139 (intron), and rs352140 (exon).48–50 The SNP probes used for the genotyping are purchased from Applied Biosystems. In addition, we used data generated by the Haplotype Map (HapMap) project (http://www.hapmap.org/index.html.en) and SNP database of the National Institutes of Health (http://www.ncbi.nlm.nih.gov/SNP). Because only a few SNP have high frequencies in MyD88 gene, two SNP in this gene were selected: rs199396 (exon) and rs7744 (3′ untranslated region). PCR was performed using the Applied Biosystems 7500 RealTime PCR System using TaqMan Universal PCR Master Mix, No AmpErase UNG (Applied Biosystems). PCR program was 50°C for 2 min, 95°C for 10 min, and 40 cycles at 92°C for 15 s and 60°C for 1 min. Then, allelic discrimination plate read was performed. χ2 test was used for contingency table to analyze the association genotype and allele frequencies between patients with IgAN and control subjects, and Bonferroni correction was used for compensation of each datum. The odds ratio and 95% confidence interval for the progression of IgAN were calculated using logistic regression analysis.
Statistical Analysis
Data are expressed as means ± SD. Comparison of groups was performed using univariate ANOVA, and post hoc Bonferroni correction was used for multiple comparisons. Unpaired t test was used for comparison of single groups. P < 0.05 was considered significant. All statistical analyses were performed using the Windows version of StatView 5.0 software (Abacus Concepts, Berkeley, CA).
DISCLOSURES
None.
Acknowledgments
This work was supported by grants from the Special Study Group on Progressive Glomerular Disease, Ministry of Health, Labor and Welfare of Japan; the Japan Intractable Diseases Research Foundation; and the study group on IgA nephropathy. J.N. and S.H. were supported in part by National Institutes of Health grants DK078244, DK080301, and DK064400.
We thank S. Hirose, H. Nishimura, J. Toei, T. Shigihara, T. Shibata, and all members of the laboratory for technical support and discussions.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Tomino Y, Sakai H: Exacerbating factors in patients with IgA nephropathy. Semin Nephrol 7: 315–317, 1987 [PubMed] [Google Scholar]
- 2.Xie Y, Chen X, Nishi S, Narita I, Gejyo F: Relationship between tonsils and IgA nephropathy as well as indications of tonsillectomy. Kidney Int 65: 1135–1144, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Hricik DE, Chung-Park M, Sedor JR: Glomerulonephritis. N Engl J Med 339: 888–899, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Pawar RD, Patole PS, Wornle M, Anders HJ: Microbial nucleic acids pay a Toll in kidney disease. Am J Physiol Renal Physiol 291: 509–516, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Tomino Y, Yagame M, Omata F, Nomoto Y, Sakai H: A case of IgA nephropathy associated with adeno- and herpes simplex viruses. Nephron 47: 258–261, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Koyama A, Sharmin S, Sakurai H, Shimizu Y, Hirayama K, Usui J, Nagata M, Yoh K, Yamagata K, Muro K, Kobayashi M, Ohtani K, Shimizu T: Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int 66: 121–132, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, Tomino Y: The mucosa-bone-marrow axis in IgA nephropathy. Contrib Nephrol 157: 70–79, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Schena FP: Immunogenetic aspects of primary IgA nephropathy: Nephrology Forum. Kidney Int 48: 1998–2013, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Hsu SI, Ramirez SB, Winn MP, Bonventre JV, Owen WF: Evidence for genetic factors in the development and progression of IgA nephropathy. Kidney Int 57: 1818–1835, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Scolari F: Inherited forms of IgA nephropathy. J Nephrol 16: 317–320, 2003 [PubMed] [Google Scholar]
- 11.Narita I, Goto S, Saito N, Song J, Ajiro J, Sato F, Saga D, Kondo D, Akazawa K, Sakatsume M, Gejyo F: Interaction between ACE and ADD1 gene polymorphisms in the progression of IgA nephropathy in Japanese patients. Hypertension 42: 304–309, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Song J, Narita I, Goto S, Saito N, Omori K, Sato F, Ajiro J, Saga D, Kondo D, Sakatsume M, Gejyo F: Gender specific association of aldosterone synthase gene polymorphism with renal survival in patients with IgA nephropathy. J Med Genet 40: 372–376, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto S, Narita I, Saito N, Watanabe Y, Yamazaki H, Sakatsume M, Shimada H, Nishi S, Ueno M, Akazawa K, Arakawa M, Gejyo F: A(-20)C polymorphism of the angiotensinogen gene and progression of IgA nephropathy. Kidney Int 62: 980–985, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Narita I, Saito N, Goto S, Jin S, Omori K, Sakatsume M, Gejyo F: Role of uteroglobin G38A polymorphism in the progression of IgA nephropathy in Japanese patients. Kidney Int 61: 1853–1858, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Akiyama F, Tanaka T, Yamada R, Ohnishi Y, Tsunoda T, Maeda S, Takei T, Obara W, Ito K, Honda K, Uchida K, Tsuchiya K, Nitta K, Yumura W, Nihei H, Ujiie T, Nagane Y, Miyano S, Suzuki Y, Fujioka T, Narita I, Gejyo F, Nakamura Y: Single-nucleotide polymorphisms in the class II region of the major histocompatibility complex in Japanese patients with immunoglobulin A nephropathy. J Hum Genet 47: 532–538, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka Y, Suzuki Y, Tsuge T, Kanamaru Y, Horikoshi S, Monteiro RC, Tomino Y: FcγRIIa-131R allele and FcγRIIIa-176V/V genotype are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant 20: 2439–2445, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Janeway CA Jr, Medzhitov R: Innate immune recognition. Annu Rev Immunol 20: 197–216, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Takeda K, Kaisho T, Akira S: Toll-like receptors. Annu Rev Immunol 21: 335–376, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Akira S, Uematsu S, Takeuchi O: Pathogen recognition and innate immunity. Cell 124: 783–801, 2006 [DOI] [PubMed] [Google Scholar]
- 20.O'Neill LA, Bowie AG: The family of five: TIR-domain-containing adaptors in Toll-like receptor signaling. Nat Rev Immunol 7: 353–364, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D: Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol 163: 1–5, 1999 [PubMed] [Google Scholar]
- 22.Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B, Finberg RW, Ingalls RR, Golenbock DT: Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest 105: 497–504, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akira S, Takeda K, Kaisho T: Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol 2: 675–680, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Wagner H: The immunobiology of the TLR9 subfamily. Trends Immunol 25: 381–386, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Imai H, Nakamoto Y, Asakura K, Miki K, Yasuda T, Miura AB: Spontaneous glomerular IgA deposition in ddY mice: An animal model of IgA nephritis. Kidney Int 27: 756–761, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Suzuki H, Suzuki Y, Yamanaka T, Hirose S, Toei J, Nishimura H, Horikoshi S, Tomino Y: Genome-wide scan in novel IgA nephropathy model identifies susceptibility locus on murine chromosome 10, in a region syntenic to human IGAN1 on chromosome 6q22–23. J Am Soc Nephrol 16: 1289–1299, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Suzuki Y, Aizawa M, Yamanaka T, Kihara M, Pang H, Horikoshi S, Tomino Y: Th1 polarization in murine IgA nephropathy directed by bone marrow-derived cells. Kidney Int 72: 319–327, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Gharavi AG, Yan Y, Scolari F, Schena FP, Frasca GM, Ghiggeri GM, Cooper K, Amoroso A, Viola BF, Battini G, Caridi G, Canova C, Farhi A, Subramanian V, Nelson-Williams C, Woodford S, Julian BA, Wyatt RJ, Lifton RP: IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22–23. Nat Genet 26: 354–357, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki Y, Mitsuaki H, Isome M, Nozawa R, Suzuki H: Renal effects of Coxsackie B4 virus in hyper-IgA mice. J Am Soc Nephrol 17: 2760–2769, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Anders HJ, Banas B, Schlondorff D: Signaling danger: Toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15: 854–867, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Anders HJ, Banas B, Linde Y, Weller L, Cohen CD, Kretzler M, Martin S, Vielhauer V, Schlondorff D, Grone HJ: Bacterial CpG-DNA aggravates immune complex glomerulonephritis: Role of TLR9-mediated expression of chemokines and chemokine receptors. J Am Soc Nephrol 14: 317–326, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Anders HJ, Vielhauer V, Eis V, Linde Y, Kretzler M, Perez de Lema G, Strutz F, Bauer S, Rutz M, Wagner H, Grone HJ, Schlondorff D: Activation of toll-like receptor-9 induces progression of renal disease in MRL-Fas(lpr) mice. FASEB J 18: 534–536, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ: Reciprocal control of T helper cell and dendritic cell differentiation. Science 283: 1183–1186, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Shortman K, Liu YJ: Mouse and human dendritic cell subtypes. Nat Rev Immunol 2: 151–161, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Suzuki S, Nakatomi Y, Sato H, Tsukada H, Arakawa M: Haemophilus parainfluenzae antigen and antibody in renal biopsy samples and serum of patients with IgA nephropathy. Lancet 343: 12–16, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Eijgenraam JW, Woltman AM, Kamerling SW, Briere F, de Fijter JW, Daha MR, van Kooten C: Dendritic cells of IgA nephropathy patients have an impaired capacity to induce IgA production in naive B cells. Kidney Int 68: 1604–1612, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM: CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374: 546–549, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A: Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416: 603–607, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Katafuchi R, Kiyoshi Y, Oh Y: Glomerular score as a prognosticator in IgA nephropathy: Its usefulness and limitation. Clin Nephrol 1: 1–8, 1998 [PubMed] [Google Scholar]
- 40.Ballardie FW, Roberts IS: Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol 13: 142–148, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Terwilliger JD, Speer M, Ott J: Chromosome-based method for rapid computer simulation in human genetic linkage analysis. Genet Epidemiol 10: 217–224, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Terwilliger JD, Ott J: Handbook of Human Genetic Linkage, Baltimore, Johns Hopkins University Press, 1994
- 43.Ott J: Analysis of Human Genetic Linkage, 3rd Ed., Baltimore, Johns Hopkins University Press, 1999
- 44.Davis HL, Weeratna R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM: CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol 160: 870–876, 1998 [PubMed] [Google Scholar]
- 45.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV: CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med 186: 1623–1631, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallichan WS, Woolstencroft RN, Guarasci T, McCluskie MJ, Davis HL, Rosenthal KL: Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J Immunol 166: 3451–3457, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Tomino Y, Sakai H: Special Study Group (IgA Nephropathy) on Progressive Glomerular Disease: Clinical guidelines for IgAN in Japan. Clin Exp Nephrol 7: 93–97, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Lazarus R, Klimecki WT, Raby BA, Vercelli D, Palmer LJ, Kwiatkowski DJ, Silverman EK, Martinez F, Weiss ST: Single-nucleotide polymorphisms in the Toll-like receptor 9 gene (TLR9): Frequencies, pairwise linkage disequilibrium, and haplotypes in three U.S. ethnic groups and exploratory case-control disease association studies. Genomics 81: 85–91, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Berghofer B, Frommer T, Konig IR, Ziegler A, Chakraborty T, Bein G, Hackstein H: Common human Toll-like receptor 9 polymorphisms and haplotypes: Association with atopy and functional relevance. Clin Exp Allergy 35: 1147–1154, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Hur JW, Shin HD, Park BL, Kim LH, Kim SY, Bae SC: Association study of Toll-like receptor 9 gene polymorphism in Korean patients with systemic lupus erythematosus. Tissue Antigens 65: 266–270, 2005 [DOI] [PubMed] [Google Scholar]