Abstract
The role of tubular injury in diabetic nephropathy is relatively unknown, despite that apoptosis of tubular epithelial cells is commonly observed in human renal biopsies. The GTPase Ras-proximate-1 (Rap1b) is upregulated in the hyperglycemic state and is known to increase B-Raf, an antiapoptotic effector protein. In this study, the effects of high glucose on renal tubular apoptosis and the potential ability for Rap1b to ameliorate these effects were investigated. In the kidneys of diabetic mice, apoptotic tubular cells and dysmorphic mitochondria were observed, Bcl-2 expression was decreased, and Bax expression was increased. Total Rap1b expression was slightly increased, but its associated GTPase activity was significantly decreased. In vitro, high extracellular glucose led to decreased Bcl-2 expression, reduced Rap1b GTPase activity, and increased levels of both Bax and GTPase activating protein in a proximal tubular cell line (HK-2). These changes were accompanied by increased DNA fragmentation, decreased high molecular weight mitochondrial DNA, altered mitochondrial morphology and function, disrupted Bcl-2–Bax and Bcl-2–Rap1b interactions, and reduced cell survival. Overexpression of Rap1b partially prevents these abnormalities. Furthermore, the BH4 domain of Bcl-2 was found to be required for successful protein–protein interaction between Bcl-2 and Rap1b. In summary, these data suggest that Rap1b ameliorates glucose-induced mitochondrial dysfunction in renal tubular cells.
Diabetic nephropathy is a widely known complication of type 1 and 2 diabetes that affects glomerular, tubular, and interstitial cells of the kidney. The mechanisms of its pathogenesis have been mainly derived from studies carried out on glomerular cells (i.e., mesangial, endothelial, and podocytes1–6). Typical events that can be ascribed to the renal cells include accentuated activity of polyol and hexosamine pathways and formation of advanced glycation end products, which apparently leads to an increased activity of protein kinase C and generation of reactive oxygen species (ROS).2,7 Both protein kinase C and ROS, directly or indirectly, are capable of initiating CTGF/TGF-β–Smad–mitogen-activated protein kinase signaling pathway with a final outcome of increased synthesis and amassing of extracellular matrix.2,7 In addition, altered expression of various extracellular matrix–degrading enzymes, cyclins, and GTP-binding proteins has been observed. In this intricate scenario of criss-cross signaling, the ROS are regarded as central to adversely affecting a wide variety of cellular processes, including apoptosis.2,8 The latter is frequently seen in renal biopsies of patients with diabetes, especially in the tubular cells.9 Apoptosis is reflective of marked DNA fragmentation, and the processes leading to such nuclear damage are initiated in the mitochondria by ROS.10 The latter increases mitochondrial membrane permeability, release of cytochrome C, altered expression of pre- (Bax) and antiapoptotic (Bcl-2) proteins, and activation of caspases, which cause nuclear condensation, decreased cell survival, and ultimately cell death.11–13 Conceivably, these events may occur in hyperglycemic states but need to be investigated in an integrated, comprehensive manner.
Previously, we observed that genes affecting renal tubular pathobiology (inner mitochondrial membrane translocase), glycolytic pathway (myo-inositol oxygenase), and a GTPase (Ras-proximate-1 [Rap1b]) were differentially upregulated in hyperglycemic state.14–16 The Rap1b is a homolog of a well-characterized small GTPase, Ras, which has received much attention because it regulates cellular proliferation, differentiation, and apoptosis.17,18 Increasing evidence suggests that some of the Ras family members are endowed with antiapoptotic properties that are mediated via their effectors (e.g., Raf).19 Interestingly, Rap1b is known to stimulate B-Raf activity,16–19 and, thus, it could serve as a modulator of cell proliferation and apoptosis. In view of this, studies were initiated to delineate the mechanisms of renal tubular apoptosis and mitochondrial dysfunctions and whether Rap1b could ameliorate such cellular injury induced by high-glucose ambience.
RESULTS
Apoptosis, Altered Mitochondrial Morphology, Expression of Bcl-2 and Bax, and Rap1b Activity in Diabetic Mouse Kidneys
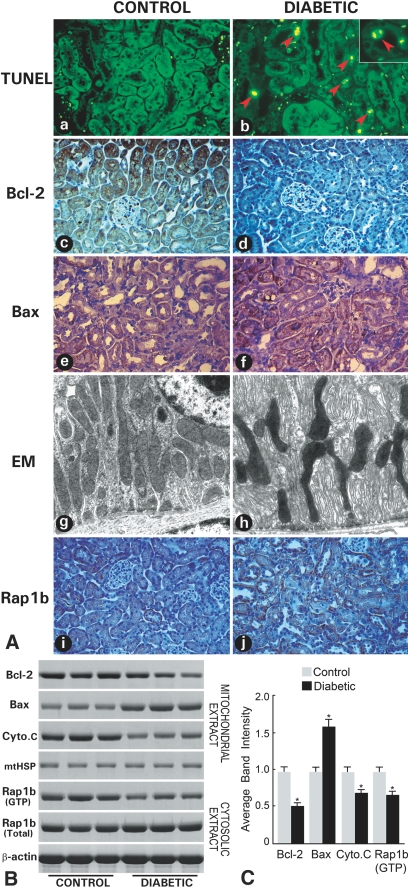
An accentuated apoptosis was observed in the kidney, as assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) procedure. Apoptotic cells were mainly confined to cortical tubules (Figure 1A-b, arrowheads) and were not seen in renal glomeruli. Bcl-2 protein expression was notably decreased, as assessed by immunohistochemistry (Figure 1A-d) and immunoblotting (Figure 1, B and C). Cytochrome C was decreased in the mitochondria (Figure 1, B and C). Conversely, the Bax protein was increased (Figure 1, A-f, B, and C). By electron microscopy, some of the tubular mitochondria exhibited deformations, such as attenuation and angulation along their longitudinal axis with focal enlargement and swelling of their cristae (Figure 1A-h). Because Rap1b is upregulated during hyperglycemia20 and the Ras family of GTPases are known to modulate cellular proliferation and apoptosis,17,18 its status was investigated. A mild increase in the total Rap1b protein expression was observed (Figure 1A-j); however, its associated GTP activity was notably decreased (Figure 1, B and C). The expression of β-actin and mitochondrial heat-shock protein 70 (mtHSP-70) serving as loading controls was unchanged. In view of these in vivo observations, cell culture studies using a proximal tubular epithelial cell line, HK-2, were initiated to delineate the relevance of Rap1b in mitochondrial dysfunction and apoptosis in a diabetic milieu.
Figure 1.
Apoptosis, gene expression, morphologic, and GTP activity studies in diabetic mouse kidneys. (A) Increased apoptosis is seen tubular in cells of diabetic mouse kidneys (A-b versus A-a). Immunohistochemical studies revealed a decreased in situ expression of Bcl-2 (A-d versus A-c) and increased expression of Bax (A-f versus A-e) and of Rap1b (A-j versus A-i). By electron microscopy, notable deformations are seen in the tubular mitochondria (A-h versus A-g). (B) By Western blotting, an altered expression of Bcl-2, Bax, and cytochrome C in mitochondrial fraction and of the total Rap1b in the cytosolic fraction is seen, whereas the Rap1b GTP activity is decreased in diabetic state. No change in the expression of mtHSP-70 and β-actin is observed. (C) Quantification of average band intensity from four separate Western blots. *P < 0.01.
Inhibition of Apoptosis, Normalization of Altered Expression of Apoptotic Proteins, and DNA Laddering by Overexpression of Rap1b in HK-2 Cells Subjected to High-Glucose Ambience
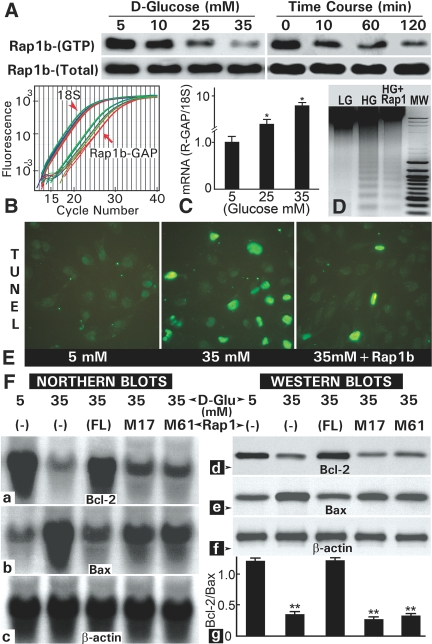
A glucose concentration- and duration-dependent decrease in the GTP-bound Rap1b (Rap1b-GTP) was observed, whereas a mild increase in its total Rap1b expression was noted (Figure 2A). The decreased activity was associated with increased gene expression of Rap1b-GTP–activating protein (GAP) that hydrolyzes Rap1b-GTP (Figure 2, B and C). Like in vivo, high glucose–induced apoptosis associated with DNA laddering (Figure 2, D and E). Interestingly, Rap1b transfection notably reduced high glucose–induced DNA fragmentation and apoptosis (Figure 2, D and E). The Rap1b overexpression also normalized the high glucose–induced altered mRNA and protein expression of Bax and Bcl-2 (Figure 2F). The l-glucose did not affect the gene expression or GTPase activity. To define the specificity of Rap1b modulation of apoptotic genes, we performed transfection of mutant Rap1b constructs (M17: S17N, M61:T61R). Neither of them normalized expression of Bcl-2 and Bax (Figure 2F), thus suggesting the Rap1b specificity and interrelationship with apoptotic proteins. Because during apoptosis there is a translocation of cytochrome C, the next set of studies were conducted to assess its distribution in cells treated with high glucose.
Figure 2.
Apoptosis, gene expression, morphologic, and GTP activity studies in HK-2 cells exposed to high-glucose ambience. (A) A decrease in the Rap1b GTP activity is observed in a dosage- and time-dependent manner. (B and C) The decrease was associated with an increased expression of Rap1b-GAP, as assessed by real-time PCR. (D and E) High-glucose ambience (35 mM) also induced increased DNA fragmentation and apoptosis, which were reduced with the transfection of HK-2 cells with Rap1b-pcDNA. (F) The overexpression of full-length Rap1b-pcDNA (FL) normalized the altered expression of Bcl-2 and Bax, whereas transfection with Rap1b mutant constructs (M17 and M61) failed to correct the expression. F-g reflects quantification of Bcl-2/Bax ratio of the band density from four Western blots. **P < 0.001.
Suppression of Mitochondrial Cytochrome C Release in Cells Overexpressing Rap1b
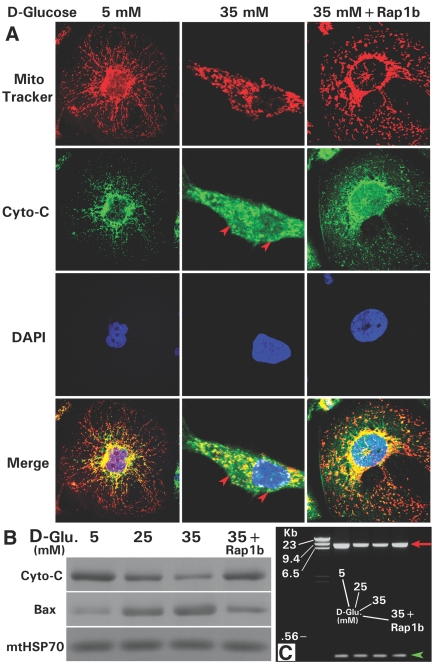
Confocal microscopy delineated mitochondria and nuclei stained with Mitotracker (red) and 4′-6-diamidino-2-phenylindole (DAPI; blue) dyes. The distribution of cytochrome C was monitored by immunofluorescence (green; Figure 3A, left). High glucose (35 mM) induced release of cytochrome C from mitochondria into the cytosolic compartment (Figure 3A, middle, arrowheads). With Rap1b transfection, an inhibition of its release occurred and cell morphology was restored (Figure 3A, right). Associated with the release, cytochrome C levels were reduced and expression of Bax was increased in the mitochondrial compartment (Figure 3B), suggesting a significant high glucose–induced mitochondrial dysfunction, which led us to investigate their morphology and membrane potential. Expression of mtHSP-70, serving as a loading control, was unchanged.
Figure 3.
Effect of Rap1b transfection on the high glucose–induced cytochrome C release and mtDNA fragmentation. (A) At a basal concentration of d-glucose (5 mM), the cytochrome C is localized in the mitochondria, as assessed by Mitotracker dye (red) and anti–cytochrome C antibody (green) by confocal microscopy (left subpanels). With the treatment of 35 mM of d-glucose, the cytochrome C is released into the cytosol (red arrowheads in the middle subpanels), and the release is inhibited by overexpression of Rap1b-pcDNA (right panels). The nuclei (blue) were delineated by DAPI staining. (B) Rap1b transfection also normalized the high glucose–induced altered cytochrome C and Bax expression in the mitochondrial fractions. The mtHSP-70 served as a loading control. (C) The overexpression of Rap1b partially corrected the reduced expression of high molecular weight mtDNA (7883 bp, red arrow), whereas the low molecular weight DNA (420 bp, green arrowhead) was unaffected by high-glucose ambience or transfection.
Overexpression of Rap1b Restores Altered Mitochondrial Morphology, Membrane Potential, and High Molecular Weight Mitochondrial DNA Damage
Like in vivo diabetic state (Figure 1A-h), the mitochondrial morphology was altered in cells exposed to high d-glucose. Some of the mitochondria were angulated and attenuated along their longitudinal axis, whereas others were somewhat swollen and had dilated cristae. In addition, frequent “cristolysis” with focal disruption of the inner mitochondrial membranes was observed (Figure 4A, middle, arrows). The regions with cristolysis appeared as small vesicles. With the Rap1b transfection, mitochondrial morphology was partially restored, even though cells had swollen mitochondria and waviness along their longitudinal axis; however, aberrant cristae were infrequently observed (Figure 4A, right). In view of these abnormalities, mitochondrial membrane potential was assessed. Normally, tetramethylrhodamine ethyl ester (TMRE) is diffusely localized to the mitochondria (Figure 4B, left). A loss of TMRE was observed with the treatment of high glucose. The loss of fluorescence was especially noted in apoptotic cells with fragmented nuclei, as outlined by DAPI staining (Figure 4B, middle, arrowheads). Cells transfected with Rap1b and subjected to high-glucose ambience had TMRE fluorescence similar to those treated with 5 mM d-glucose (Figure 4B, right). By FACS analysis, the cells treated with high glucose and loss of TMRE had decreased survival, whereas the Rap1b-transfected cells had similar survival rate as the control (Figure 4, C and D). Along with the decreased survival and increased apoptosis of HK-2 cells, high-glucose treatment led to a reduction of high molecular weight DNA (7883 bp; Figure 3C). Similarly, a decrease in the intensity of the high molecular weight mitochondrial DNA (mtDNA; 8636 bp) of tubules in diabetic mouse kidneys was observed (data not shown). Rap1b transfection of HK-2 cells partially restored the mtDNA damage (Figure 3C, red arrow). No significant change in the low molecular weight DNA (420 bp) was observed (Figure 3C, green arrowhead).
Figure 4.
Effect of Rap1b transfection on the high glucose–induced altered mitochondrial morphology, membrane potential, and cell survival. (A) High-glucose (35 mM) treatment induced marked deformation of the mitochondria with dilation of the cristae (arrows), as observed by electron microscopy. With Rap1b overexpression, the aberrant cristae were not observed, but the swelling of the mitochondria persisted to a certain extent. (B) By confocal microscopy, a loss of TMRE staining, indicative of mitochondrial ΔΨm, was observed under high-glucose ambience in cells undergoing apoptosis (green arrowheads, middle subpanels). DAPI staining highlights the fragmented nuclei (blue staining) of apoptotic cells (arrowheads). Rap1b transfection restored the TMRE staining and ΔΨm (right subpanels). (C) FACS analyses revealed decreased cell survival under high-glucose (HG) ambience, which was restored to baseline with overexpression of Rap1b as in cells with low glucose (LG; 5 mM) culture media. (D) Quantification of FACS analyses of four experiments. *P < 0.01.
Normalization of Altered Protein–Protein Interactions between Apoptotic Proteins by Overexpression of Rap1b GTPase
Bcl2–Bax interactions were investigated because such heterodimeric associations may be necessary for normal cell survival.11,13,21 A sequential combined approach (immunoprecipitation [IP] followed by Western blotting), along with the reciprocal use of anti–Bcl-2 and -Bax antibodies was used (see the Concise Methods section). A decrease in the intensity of bands in lysates of cells treated with high glucose and sequentially subjected to precipitation and blotting was observed (Figure 5, A and B). In addition, similarly, a decreased density of bands was observed in experiments in which anti–Bcl-2 and -Rap1b antibodies were used, suggesting a perturbation in Bcl-2–Rap1b interactions (Figure 5, A and B).
Figure 5.
Effect of Rap1b transfection on the high glucose–perturbed Bcl-2–Bax and Bcl-2–Rap1b interactions. (A) The protein–protein interactions were studied using IP followed by Western blotting. The antibodies were switched for each of the procedures, and perturbed interactions under high-glucose ambience (25 to 35 mM) were reflected by a decrease in the band density. These interactions seem to be normalized with restoration of the band intensity after Rap1b overexpression. (B) Band density averaged from duplicate experiments is included. (C and D) Rap1b interactions with mutant Bcl-2 GST proteins, reflecting deletion of BH1 through BH4 domains, were investigated. The deletion of BH4 domain of Bcl-2 leads to a loss of binding with Rap1b protein (lane 3), whereas it was unaffected with other mutant proteins, suggesting specificity of Bcl-2–Rap1b interactions.
Characterization of Potential Bcl-2 Domains’ Interactions with Rap1b GTPase
To confirm the specificity of Bcl-2–Rap1b interactions, we prepared Bcl-2 deletion constructs and mutant GST fusion proteins. The latter were used to study interactions with in vitro translated radiolabeled Rap1b probe by GST pull-down assay. Among various BH domains of Bcl-2, deletion of BH-4 led to a loss of binding with Rap1b (Figure 5, C and D, lane 3). The binding was unaffected by deletion of BH1, BH2, or BH3 domain, suggesting specificity of interactions and relevance of Rap1b in the pathobiology of mitochondria, apoptosis, and cell survival.
DISCUSSION
Tubulointerstitial injury is observed in a wide variety of kidney diseases, including diabetic nephropathy, and it is often reflected by declining renal function.22,23 These studies with streptozotocin (STZ)-induced diabetes in mice indicate that there is a substantial injury to the tubular compartment as well. The readily noticeable injury seems to be apoptosis of tubular cells (Figures 1 and 2), which evidently is also seen in the rat model of acute ischemia-reperfusion injury or after intravenous loading of high concentration of glucose (55 mM).24,25 That moderately high blood glucose levels or in vitro exposure of 15 to 35 mM d-glucose also induced apoptosis in tubular cells suggests that mechanisms other than osmotic injury are involved. In this regard, many studies indicate that a number of genes modulating various cellular events and pathways are affected in cells undergoing apoptosis in a wide variety of model systems.10–13 To begin with, an altered expression of Bcl-2, Bax, and cytochrome C along with mitochondrial dysfunctions, increased oxidant stress, and activation of caspases has been reported.24–26 In line with such observations, altered expression of apoptogenic molecules along with mitochondrial deformation were observed in this study (Figures 1 through 4). Although changes in the expression of Bcl-2 or Bax have been described in high glucose–induced injury in various renal cell types in vitro,8,9 the apoptosis or mitochondrial deformations have not been documented. This study encompasses various events associated with apoptosis and alludes to mechanisms involved in an integrated manner and elucidates the protective role of a small GTPase, Rap1b, in hyperglycemic injury.
It is conceivable that the early events that ultimately lead to apoptosis are initiated in the mitochondria and are related to dysfunctions of their cristae. In support of this contention, the mitochondrial cristae were found to be dilated with a certain degree of cristolysis, especially in cultured cells subjected to high-glucose ambience (Figures 1 and 4), which has been described in other states, such as ischemia and oxidant stress.27 The cristolysis perhaps may be reflective of an extreme degree of oxidant stress in the mitochondrial compartment. Inevitably, such changes would likely perturb voltage potential (ΔΨm) between the inner and outer mitochondrial membranes of cristae with increased permeability, as observed in this investigation (Figure 4). This may lead to altered mitochondrial levels of Bcl-2 and Bax and consequential leakage of cytochrome C into the cytosol followed by downstream events, such as activation of caspases and induction of apoptosis, DNA fragmentation, and decreased cell survival. Similar observations were made in these studies (Figure 3), in which in addition to altered levels of Bcl-2 and Bax and their ratio, perturbed protein–protein interactions were observed (Figures 3 and 5). Such perturbations with accumulation of Bax in the mitochondria and unavailability of Bcl-2 to heterodimerize with Bax most likely led to translocation of cytochrome C, a pivotal finding described in this investigation (Figure 3). These high glucose–induced changes are typically seen in various other cell systems during the induction of apoptosis, in which the ROS play a central role in mitochondrial dysfunctions.28,29 Certainly, the role of ROS in pathogenesis of diabetic nephropathy is known,1–7 but these studies document and highlight the events leading to high-glucose ambience–induced tubular cell injury that are correlative with mitochondrial dysfunctions, including the damage to mitochondrial DNA (Figure 3). The latter has been observed in other model systems in which ROS seem to play a role, such as in aging or immune complex–mediated injury.30 The other interesting aspect of this study relates to the role of Rap1b, a small GTPase, in the amelioration of high glucose–induced injury and normalization of mitochondrial functions.
Rap1b belongs to the Ras family of proteins that cycle between an active GTP-bound form and an inactive GDP-bound form.16–18 The latter state, GTP → GDP, is achieved after catalytic hydrolysis by GAP.16–18 Interestingly, although there was a mild increase in the expression of total Rap1b, its GTP activity was reduced under high-glucose ambience, which probably was due to increased expression of GAP (Figures 1 and 2). Because Rap1b-related oncogenic Ras and Raf suppress the apoptotic gene PTEN via the Raf-MEK–extracellular signal–regulated kinase–c-Jun pathway to induce cellular transformation and growth,31 it may mean that Rap1b may exert a protective role in the scenario of glucose-induced injury leading to apoptosis. This seems to be the case because transfection of Rap1b resulted in the normalization of Bcl-2 and Bax expression, mitochondrial ΔΨm with reduction in the leakage of cytochrome C, mtDNA damage, and apoptosis (Figures 2 through 4). Although mitochondria were somewhat swollen, the morphology of the cristae was restored after transfection (Figure 4). The protective effect of Rap1b seems to be specific because its mutant constructs (Rap1b/S17N and T61A) that negatively regulate its activity16 failed to normalize Bax or Bcl-2 expression (Figure 2). Here, the question that needs to be addressed is the mechanism(s) by which Rap1b GTPase ameliorates high glucose–induced injury, and, in doing so, does it interact with Bcl-2 or Bax? In this regard, Ras-related protein, R-Ras p23, has been shown to associate with Bcl-2 and to regulate apoptosis.32 Also, another Bcl-2–interacting protein, BAG, binds and activates the Raf-1, a kinase affected by GTPase.33 In light of such reports, Bcl-2–Rap1b interactions were investigated, and they are quite plausible because Rap1b has been reported to be localized in the mitochondria.34 The Western and IP experiments indeed indicated that there is a Bcl-2–Rap1b association that is perturbed by high-glucose ambience (Figure 5). These interactions were normalized by an overexpression of Rap1b, suggesting that it plays a critical role in amelioration of high glucose–induced mitochondrial dysfunctions. As to the specificity of interactions, various Bcl-2 deletion constructs were generated to tease out functions of its domains. The Bcl-2 has four domains, BH1 through BH4, that are conserved among its homologues.35 These domains have been described to be involved in Bcl-2, Bax, and other interacting proteins’ hetero- and homodimerizations that consequently modulate apoptosis.35,36 For instance, the conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis.37 Our studies indicate that deletion of BH4 domain led to a loss of binding of Bcl-2 with Rap1b, whereas it was unaffected by deletion of other domains, suggesting that the binding is specific (Figure 5); and conceivably Rap1b stabilizes the antiapoptotic effect of Bcl-2. Whether Rap1b–Bcl-2 association negatively regulates the activity of Bax remains to be investigated.
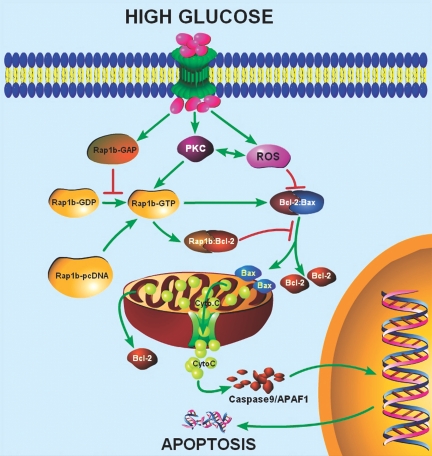
In conclusion, it seems that hyperglycemia leads to mitochondrial dysfunctions, notably the aberrations in their cristae with “cristolysis” of the inner membrane, which apparently perturbs ΔΨm, followed by a series of events leading to induction of apoptosis (Figure 6). These changes are reversed by overexpression of Rap1b, which conceivably stabilizes the antiapoptotic activity of Bcl-2 by interacting with its BH4 domain.
Figure 6.
Schematic sketch of conceivable cellular events after exposure to high-glucose ambience and overexpression of Rap1b-GTPase in renal tubular cells. Under high-glucose ambience, there is an activation of protein kinase C (PKC) and generation of ROS that apparently perturb Bcl2–Bax heterodimerization leading to the release of mitochondrial cytochrome C, activation of caspases, fragmentation of DNA, and ultimately apoptosis. Conceivably, upon transfection of GTPase Rap1b, there is a stabilization of Bcl2–Bax and Rap1b–Bcl2 interactions that would inhibit the release of cytochrome C, oxidant stress, DNA fragmentation, and apoptosis.
CONCISE METHODS
Animal Model
A diabetic state was induced in 8 wk-old ICR mice (Harlan Co., Indianapolis, IN) by an injection of STZ (200 mg/kg body wt; Sigma Chemical, St. Louis, MO). After 1 wk, the mice with blood glucose levels >250 mg/dl were selected for various studies. The mice were killed 6 wk after the STZ injection.
In Vivo Studies with Kidney Tissues in Diabetic Mice
First, apoptosis was assessed by TUNEL method.10 A second set of studies included evaluation of in situ protein expression by immunohistochemical methods of known pro- and antiapoptotic proteins (Bax, Bcl-2, and cytochrome C) and Rap1b, a Ras-related (Ras-proximate) GTPase that modulates cellular proliferation, differentiation, and apoptosis.16–18 Primary and secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). In addition, kidney tissues were processed for electron microscopy to assess mitochondrial alterations in tubules.14,30 A third set of studies were to measure protein expression in cellular and mitochondrial tubular fractions. Tubular enriched fraction was prepared by separating glomeruli using a sieving method at 4°C.16 The cytosolic proteins of tubular cells were extracted with 50 mM Tris-HCl buffer (pH 7.5) containing 50 mM NaCl, 10 mM EDTA, 0.5% NP-40, and a cocktail of protease inhibitors,15,16,30 and protein concentration was adjusted to 1 mg/ml. Mitochondria were harvested from renal tubular fraction using a Mitochondrial Isolation kit (Pierce, Rockford, IL) following the vendor's instructions. The protein expression of various genes was evaluated by Western blotting, and the integrated density of each band from four blots was quantified. The cytosolic extracts of renal tubules were also used to determine the specific Rap1b-GTPase activity.16
In Vitro Cell Culture Studies under High-Glucose Ambience
A proximal tubular cell line, HK-2 (ATCC, Rockville, MD), was maintained in a defined medium (3:1 mixture of DMEM and Ham's F-12 containing 10% FBS, penicillin [100 U/ml], streptomycin [100 μg/ml], and HEPES [14 mM]) at 37°C. For overexpression of Rap1b and its mutants (Rap1bS17N and Rap1bT61R), cells were transfected with full-length Rap1b-pcDNA3.1/Hygro and mutant plasmid constructs using LIPOFECTAMINE 2000 reagent (Invitrogen, Carlsbad, CA).14,16 After selection of stable transfectants, cells were maintained in the defined medium. After achieving 80% confluence, the medium was changed to FBS-free DMEM. Varying concentrations of d-glucose (5 to 35 mM) was added, and cell culture was maintained for 48 to 72 h. l-Glucose served as a control. Like the in vivo tissue studies, the cells of stable transfectants, including the mutants, subjected to high-glucose ambience were used for determining Rap1b activity and protein expression in the cytosolic and mitochondrial fractions and to assess the status of apoptosis and mitochondrial morphology.
Gene Expression Studies
Expression of Bcl-2 and Bax was investigated in HK-2 cells and transfectants exposed to d-glucose (5 to 35 mM) by Northern blotting procedures using Bcl-2 and Bax [32P] dCTP-labeled cDNA probes.14–16 The membrane blots were stripped and rehybridized with radiolabeled β-actin cDNA probe. The mRNA expression of Rap1b GAP was assessed by real-time PCR using SYBR green PCR reagent kit (Applied Biosystems, Foster City, CA).38 The primers were as follows: Sense 5′-AGCGTGTCATCCTCAGGAAC-3′ and antisense 5′-CATGTGCTGCTCAGATGCTT-3′. The 18S rRNA was used for normalization, and the primers were as follows: 5′-AAACGGCTACCACATCCAAG-3′ and antisense 5′-CCTCCAATGGATCCTCGTTA-3′.
Nuclear DNA and mtDNA Studies
Nuclear DNA fragmentation was assessed by gel electrophoresis in HK-2 cells and transfectants overexpressing Rap1b subjected to high-glucose ambience.30 The mtDNA damage to long and short DNA was evaluated as described previously.30 For sense primer of long PCR were as follows: 5′-AGTGCATACCGCCAAAAGA-3′ and antisense 5′-TCTAGAGCCCACTGTAAAG-3′; sense primer of short PCR 5′-ATGGTCTGAGCTATGATATCAA-3′ and antisense 5′-GATTTTGGCGTAGGTTGG-3′. PCR products of 7883 and 420 bp were visualized after gel electrophoresis. Similarly, mtDNA damage was assessed in kidney tubules.30
Cellular Distribution of Cytochrome C
Confocal microscopy was used to delineate the distribution of cytochrome C. Cells were incubated with 25 nM of mitotracker dye (Mitotracker red; Molecular Probes, Eugene, OR) at 37°C for 10 min. Slides were washed with PBS and fixed in 4% formaldehyde. They were then incubated with primary anti–cytochrome C antibody followed by incubation with FITC-labeled secondary antibody. The cells were also subjected to DAPI staining to visualize the nuclei. Images were taken using different excitation filters and merged. Expression of cytochrome C and Bax in isolated mitochondria was also assessed by Western blotting. Anti–mtHSP-70 was obtained from Novus Biologicals (Littleton, CA).
Assessment of Mitochondrial ΔΨm
Loss of ΔΨm was assessed by FACS and confocal microscopy of cells stained with TMRE (Molecular Probes).39 For confocal analysis, HK-2 cells and those overexpressing Rap1b were plated on 35-mm glass-bottom culture dishes (MatTak Corp., Ashland, MA) and maintained at 37°C in the defined medium. After high-glucose treatment for 72 h, cells were placed in a phenol red–free DMEM (Life-Tech, Carlsbad, CA) containing 50 nM TMRE for 30 min; after DAPI staining, cells were examined. For FACS analysis, cells were stained with TMRE for 30 min and then harvested after trypsinization. The fluorescence intensity of TMRE was monitored at 582 nm, and the data from four different experiments were analyzed.
IP and Western Blotting Binding Studies with Bcl-2, Bax, and Rap1b
Cellular proteins were extracted with the Tris-HCl buffer and protein concentration adjusted to 200 μg/ml (vide supra). Each sample was divided into two aliquots, containing 100 μg of protein, and used for IP with two different antibodies.15,16,30 Samples were first individually incubated with various antibodies in an IP buffer (25 mM Tris-HCl buffer [pH 7.5] containing 25 mM NaCl, 5 mM EDTA, and 0.5% Triton-X 100) for 12 h at 4°C with gentle rotation. Then 50 μl of protein A–Sepharose beads were added, and the incubation was extended for another 12 h. The beads were washed with the IP buffer, resuspended in SDS loading buffer, and boiled for 3 min, and the entire sample was subjected to 15% SDS-PAGE followed by Western blotting. Experiments were performed in duplicate, and density of each band was averaged.
In Vitro Characterization of Bcl-2 Domains and Interactions with Rap1b
A full-length pGEX-4T-1/GST-Bcl-2 plasmid was used as a template to create deletion in various domains. Four mutant constructs were generated by PCR using a Quick Change II XL site-directed mutagenesis kit (Stratagene, Milwaukee, WI) and the following primers: For ΔBH1/Bcl-2 5′-CGGTGGTGGAGGAACTCTTCAGTGGAGAGCGTCAACAGGGAGAT-3′; for ΔBH2/Bcl-2 5′-CCGGCATCTGCACACCTGGATCCAGTGTGAGGCCTCTGTTTGATTTC-3′; for ΔBH3/Bcl-2 5′-CCACCTGTGGTCCACCTGAGACTTCGCGGAGATGTCCAGTCAG-3′; and for ΔBH4/Bcl-2 5′-CTGGGGAGAACAGGGTATGATGTGGGAGATGTGGACGCC-3′. The full-length Bcl-2/GST and deletion constructs were used to generate fusion proteins for pull-down assay.16 A 35S-labeled Rap1b protein was generated using Rap1b/pBluescript II KS(+) plasmid and TNT Coupled Reticulocyte Lysate System (Promega, Madison, WI).15
For the GST pull-down assay, 10 μl of the in vitro translated Rap1b protein was incubated with 20 μl of glutathione-agarose beads adsorbed with 5 μg of various Bcl-2/GST fusion proteins in a total volume of 400 μl of binding buffer (20 mM Tris [pH 7.5], 25 mM NaCl, 60 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 10% glycerol, 0.5% NP-40, and 1% powder milk) for 4 h at 4°C. The agarose beads were then washed four times with the buffer. The bound proteins were eluted with 5 mM glutathione/10 mM Tris (pH 8.0) and subjected to SDS-PAGE. The gels were dried, and autoradiograms were prepared.
DISCLOSURES
None.
Acknowledgments
This study was supported by National Institutes of Health grants DK28492 and DK60635.
Published online ahead of print. Publication date available at www.jasn.org.
L.S. and P.X. contributed equally to this work.
REFERENCES
- 1.Susztak K, Bottinger EP: Diabetic nephropathy: A frontier for personalized medicine. J Am Soc Nephrol 17: 361–367, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Susztak K, Raff AC, Schiffer M, Bottinger EP: Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 3.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R: MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-β induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A 104: 3432–3437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato M, Yuan H, Xu ZG, Lanting L, Li SL, Wang M, Hu MC, Reddy MA, Natarajan R: Role of the Akt/Fox03a pathway in TGF-β-mediated mesangial cell dysfunction: A novel mechanism related to diabetic kidney disease. J Am Soc Nephrol 17: 3325–3335, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Gorin Y, Block K, Hernandez J, Bjandari B, Wagner B, Banrnes JL, Abboud HE: Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Abboud HE: Role of platelet-derived growth factor in renal injury. Annu Rev Physiol 57: 297–309, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M: Biochemistry and molecular biology of diabetic complications. Nature 414: 813–820, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Orbitz A, Ziyadeh FN, Neilson EG: Expression of apoptosis-regulatory genes in renal proximal tubular epithelial cells exposed to high ambient glucose in diabetic kidneys. J Investig Med 45: 50–56, 1997 [PubMed] [Google Scholar]
- 9.Kumar D, Robertson S, Burns KD: Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem 259: 67–70, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Lee HC, Wei YH: Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Exp Biol Med 232: 592–606, 2007 [PubMed] [Google Scholar]
- 11.Vander Heiden MG, Thompson CB: Bcl-2 proteins: Regulators of apoptosis or of mitochondrial homeostasis. Nat Cell Biol 8: E209–E216, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Wang X: The expanding role of mitochondria in apoptosis. Genes Dev 15: 2922–2933, 2001 [PubMed] [Google Scholar]
- 13.Kakkar P, Singh BK: Mitochondria: A hub of redox activities and cellular distress control. Mol Cell Biochem 305: 235–253, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Wada J, Kanwar YS: Characterization of mammalian translocase of inner mitochondrial membrane isolated from diabetic mouse kidney. Proc Natl Acad Sci U S A 95: 144–149, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nayak B, Xie P, Akagi S, Yang Q, Sun L, Wada J, Thakur A, Danesh FR, Chugh SS, Kanwar YS: Modulation of renal-specific oxidorectase/myo-inositol oxygenase by high-glucose ambience. Proc Natl Acad Sci U S A 102: 17952–17957, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S, Sahai A, Chugh SS, Pan X, Wallner EI, Danesh FR, Lomasney JW, Kanwar YS: High glucose stimulates synthesis of fibronectin via a novel protein kinase C, Rap1b and B-Raf signaling pathway. J Biol Chem 277: 41725–41735, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Altschuler DL, Ribeiro-Neto F: Mitogenic and oncogenic properties of small G protein Rap1b. Proc Natl Acad Sci U S A 95: 7475–7479, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bos JL, de Rooij J, Reedquist KA: Rap1 signalling: Adhering to new models. Nat Rev Mol Cell Biol 2: 369–377, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Erhardt P, Schremser EJ, Cooper GM: B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol 19: 5308–5315, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun S, Chugh S, Pan X, Wallner EI, Wada J, Kanwar YS: Identification of up-regulated Ras-like GTPase, Rap1b, by suppression subtractive hybridization. Kidney Int 60: 2129–2141, 2001 [DOI] [PubMed] [Google Scholar]
- 21.St Clair EG, Anderson SJ, Oltvai ZN: Bcl-2 counters apoptosis by Bax heterodimerization-dependent and -independent mechanisms in the T-cell lineage. J Biol Chem 272: 29347–29355, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Nath KA: The tubulointerstitium in progressive renal disease. Kidney Int 54: 992–998, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Phillips AO: The role of renal proximal tubular cells in diabetic nephropathy. Curr Diab Rep 3: 491–496, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Saikumar P, Venkatachalam MA: Role of apoptosis in hypoxic/ischemic changes in the kidney. Semin Nephrol 23: 511–521, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Ishii N, Ogawa Z, Suzuki K, Numakami K, Saruta T, Itoh H: Glucose loading induces DNA fragmentation in rat proximal tubular cells. Metabolism 45: 1348–1353, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Shen Y, White E: p53-dependent apoptosis pathways. Adv Cancer Res 82: 55–84, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Cheung EC, Joza N, Steenaart NA, McClellan KA, Neuspiel M, McNamara S, MacLaurin JG, Rippstein P, Park DS, Shore GC, McBride HM, Penninger JM, Stacks RS: Dissociating the dual roles of apotosis-inducing factor in maintaining mitochondrial structure and apoptosis. EMBO J 25: 4061–4073, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, Feldman EL: High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J 16: 1738–1748, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Schmeichal AM, Schmelcher JD, Low PA: Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic nephropathy. Diabetes 52: 165–171, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Saski T, Fujimori T, Xie P, Kanwar YS: Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A 104: 2331–2336, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasudevan KM, Burikhanov R, Goswami A, Rangnekar VM: Suppression of PTEN expression is essential for antiapoptosis and cellular transformation by oncogenic Ras. Cancer Res 67: 10343–10350, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Sarabia MJ, Bischoff JR: Bcl-2 associates with ras-related protein R-ras p23. Nature 366: 274–275, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Wang HG, Takayama S, Rapp UR, Reed JC: Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc Natl Acad Sci U S A 93: 7063–7068, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S, Mizoguchi A, Kikuchi A, Takai Y: Tissue and subcellular distributions of the smg-21/rap1/Krev-1 proteins which are partly distinct from those of c-ras p21. Mol Cell Biol 10: 2645–2652, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed JC, Zha H, Aime-Sempe C, Takayama S, Wang HG: Structure-function analysis of Bcl-2 family proteins. Adv Exp Med Biol 406: 99–112, 1996 [PubMed] [Google Scholar]
- 36.Denis GV, Yu Q, Ma P, Deeds L, Faller DV, Chen CY: Bcl-2, via its BH4 domain, blocks apoptotic signaling mediated by mitochondrial Ras. J Biol Chem 278: 5775–5785, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang DC, Adams JM, Cory S: The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4. EMBO J 17: 1029–1039, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu G, Clement LC, Kanwar YS, Avila-Casado C, Chugh SS: ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J Biol Chem 281: 39681–39692, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Grimm S, Brdiczka D: The permeability transition pore in cell death. Apoptosis 12: 841–855, 2007 [DOI] [PubMed] [Google Scholar]